Abstract
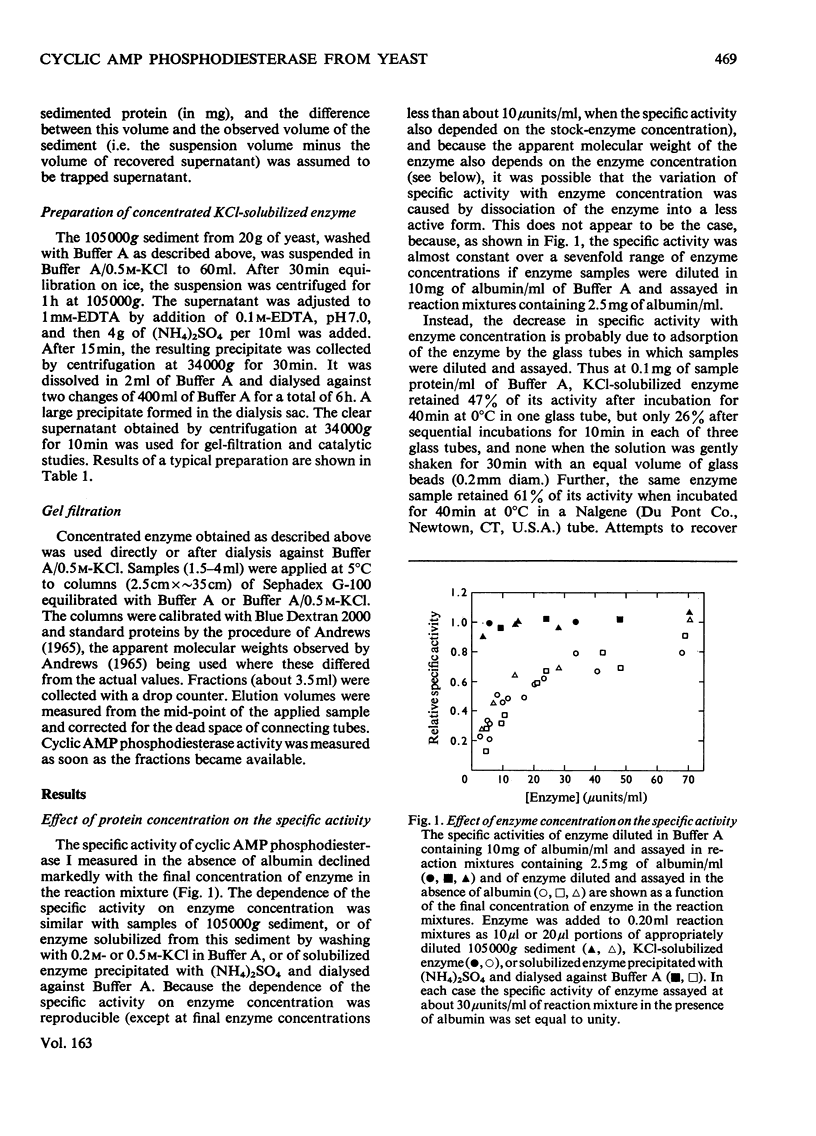
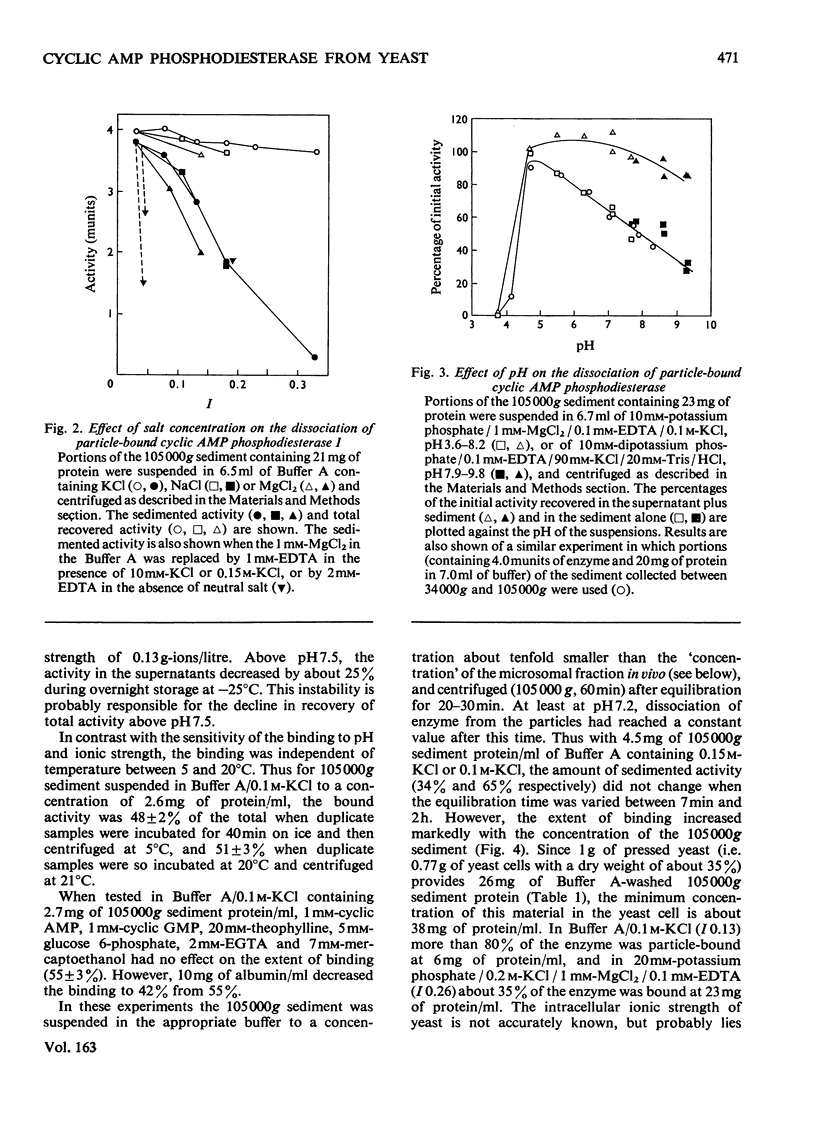
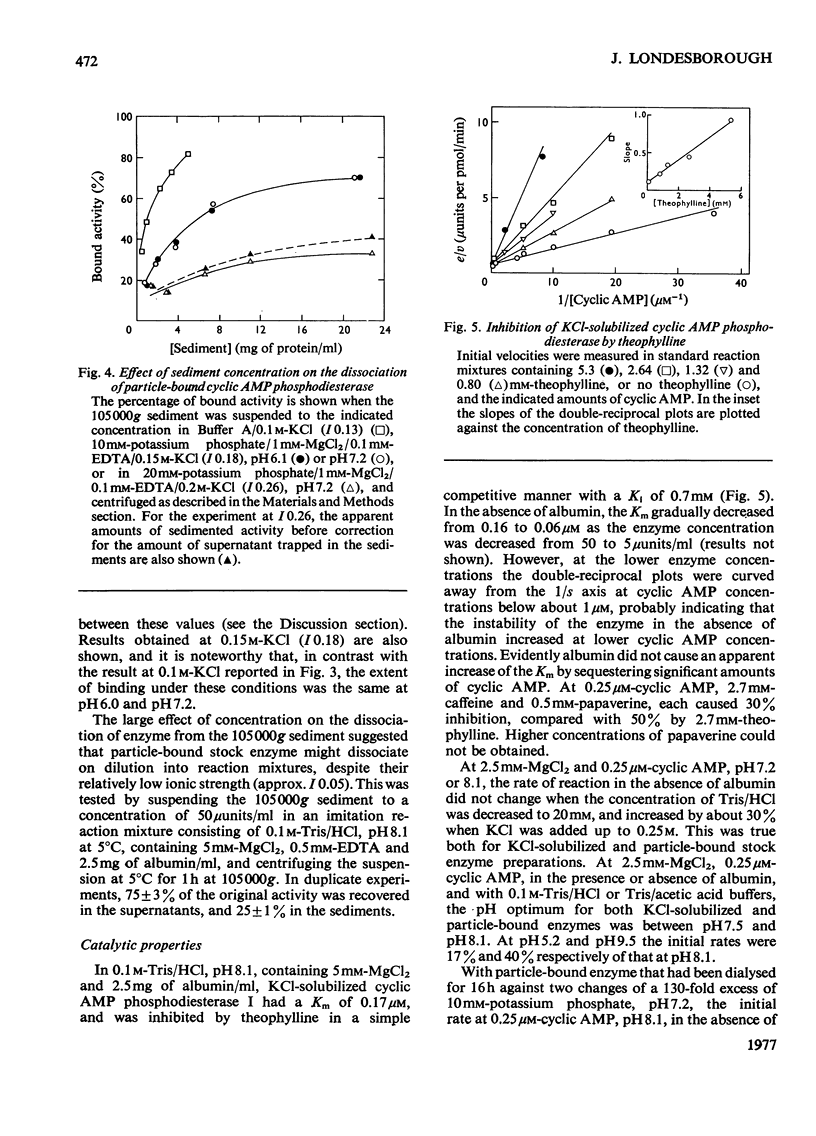
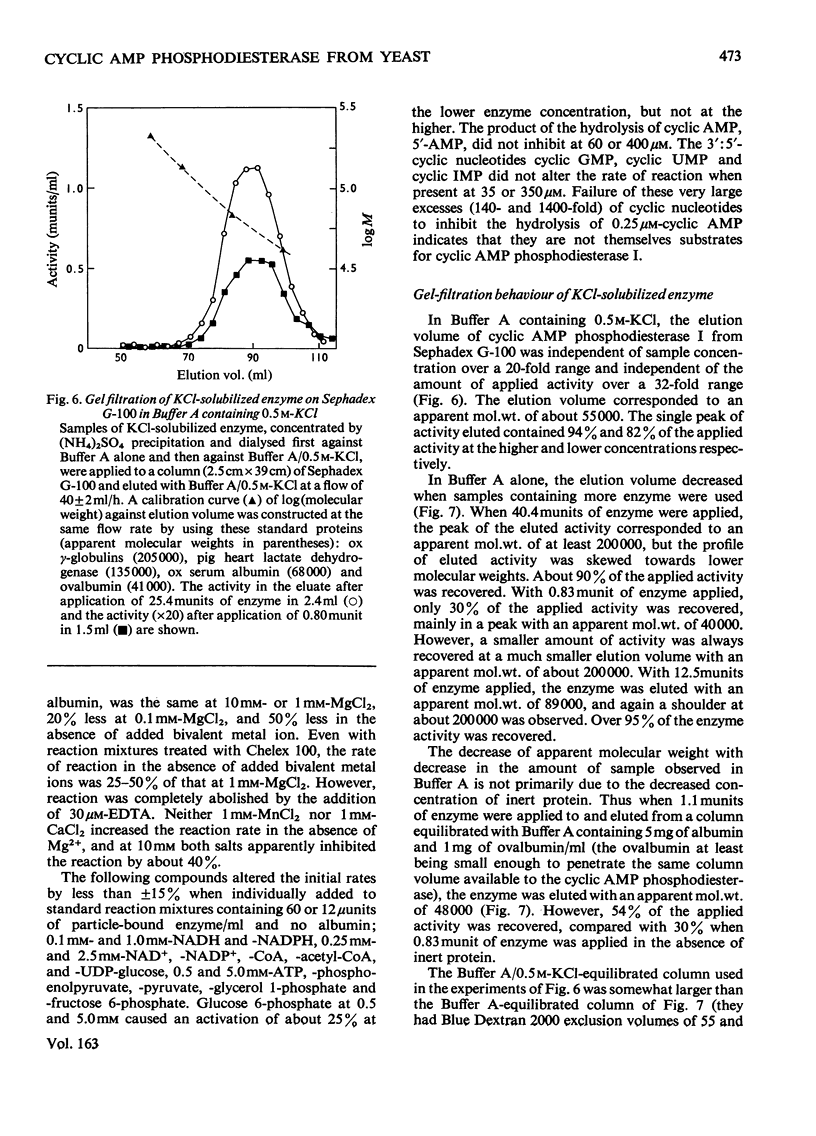
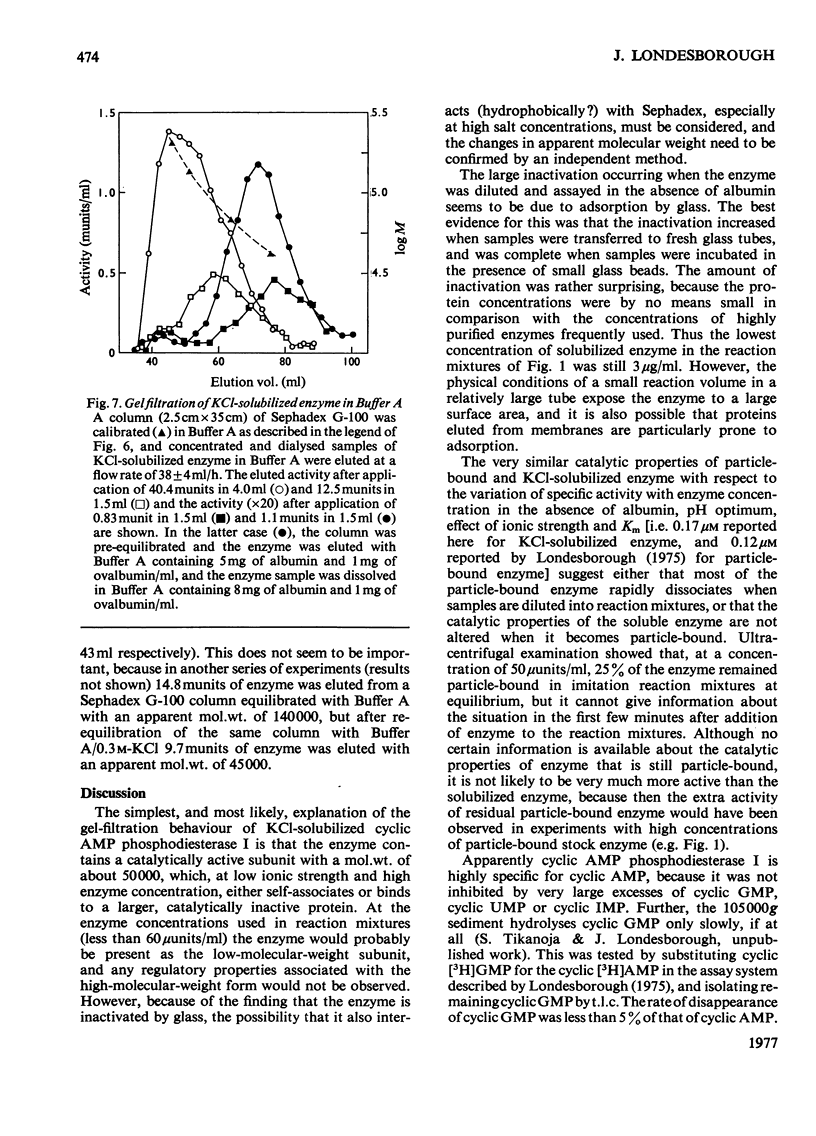
1. The 3':5'-cyclic AMP phosphodiesterase in the microsomal fraction of baker's yeast is highly specific for cyclic AMP, and not inhibited by cyclic GMP, cyclic IMP or cyclic UMP. Catalytic activity is abolished by 30 micrometer-EDTA. At 30 degrees C and pH8.1, the Km is 0.17 micrometer, and theophylline is a simple competitive inhibitor with Ki 0.7 micrometer. The pH optimum is about 7.8 at 0.25 micrometer-cyclic AMP, so that over the physiological range of pH in yeast the activity changes in the opposite direction to that of adenylate cyclase [PH optimum about 6.2; Londesborough & Nurminen (1972) Acta Chem. Scand. 26, 3396-3398].2. At pH 7.2, dissociation of the enzyme from dilute microsomal suspensions increased with ionic strength and was almost complete at 0.3 M-KCl. MgCl2 caused more dissociation than did KCl or NaCl at the same ionic strength, but at low KCl concentrations binding required small amounts of free bivalent metal ions. In 0.1 M-KCl the binding decreased between pH 4.7 and 9.3. At pH 7.2 the binding was independent of temperature between 5 and 20 degrees C. These observations suggest that the binding is electrostatic rather than hydrophobic. 3. The proportion of bound activity increased with the concentration of the microsomal fraction, and at 22 mg of protein/ml and pH 7.2 was 70% at I0.18, and 35% at I0.26. Presumably a substantial amount of the enzyme is particle-bound in vivo. 4. At 5 degrees C in 10 mM-potassium phosphate, pH 7.2, the apparent molecular weight of KCl-solubilized enzyme decreased with enzyme concentration from about 200 000 to 40 000. In the presence of 0.5M-KCl, a constant mol.wt. of about 55 000 was observed over a 20-fold range of enzyme concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Masters C. J. On the reversible and selective adsorption of aldolase isoenzymes in rat brain. Arch Biochem Biophys. 1972 Nov;153(1):258–265. doi: 10.1016/0003-9861(72)90444-4. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- KOTYK A. Intracellular pH of Baker's yeast. Folia Microbiol (Praha) 1963 Jan;8:27–31. doi: 10.1007/BF02868762. [DOI] [PubMed] [Google Scholar]
- Kant J. A., Steck T. L. Specificity in the association of glyceraldehyde 3-phosphate dehydrogenase with isolated human erythrocyte membranes. J Biol Chem. 1973 Dec 25;248(24):8457–8464. [PubMed] [Google Scholar]
- Letko G., Bohnensack R. Investigations on the release of membrane-bound glyceraldehyde-3-phosphate dehydrogenase. FEBS Lett. 1974 Mar 1;39(3):313–316. doi: 10.1016/0014-5793(74)80138-9. [DOI] [PubMed] [Google Scholar]
- Londesborough J. C., Nurminen T. A manganese-dependent adenyl cyclase in baker's yeast, Saccharomyces cerevisiae. Acta Chem Scand. 1972;26(8):3396–3398. doi: 10.3891/acta.chem.scand.26-3396. [DOI] [PubMed] [Google Scholar]
- Londesborough J. C. Soluble and membrane-bound cyclic AMP diesterase activity with a low Michaelis constant in baker's yeast. FEBS Lett. 1975 Feb 1;50(2):283–287. doi: 10.1016/0014-5793(75)80509-6. [DOI] [PubMed] [Google Scholar]
- Londesborough J. Quantitative estimation of 3'5' cyclic AMP phosphodiesterase using anion exchange resin in a batch process. Anal Biochem. 1976 Apr;71(2):623–628. doi: 10.1016/s0003-2697(76)80037-1. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A., DEMIS C. The relationship of the cell surface to metabolism; the stimulation of fermentation by extracellular potassium. Arch Biochem Biophys. 1953 May;44(1):18–29. doi: 10.1016/0003-9861(53)90005-8. [DOI] [PubMed] [Google Scholar]
- Schlanderer G., Dellweg H. Cyclid AMP and catabolite repression in yeasts, In Schizosaccharomyces pombe glucose lowers both intracellular adenosine 3':5'-monophosphate levels and the activity of catabolite-sensitive enzymes. Eur J Biochem. 1974 Nov 1;49(1):305–316. doi: 10.1111/j.1432-1033.1974.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Sy J., Richter D. Content of cyclic 3',5'-adenosine monophosphate and adenylyl cyclase in yeast at various growth conditions. Biochemistry. 1972 Jul 18;11(15):2788–2791. doi: 10.1021/bi00765a009. [DOI] [PubMed] [Google Scholar]
- Van Wijk R., Konijn T. M. Cyclic 3', 5'-amp in Saccharomyces carlsbergensis under various conditions of catabolite repression. FEBS Lett. 1971 Mar 5;13(3):184–186. doi: 10.1016/0014-5793(71)80231-4. [DOI] [PubMed] [Google Scholar]
- Varimo K., Londesborough J. Solubilization and other studies on adenylate cyclase of baker's yeast. Biochem J. 1976 Nov;159(2):363–370. doi: 10.1042/bj1590363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3',5'-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem Biophys Res Commun. 1974 Feb 4;56(3):580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]


