Abstract
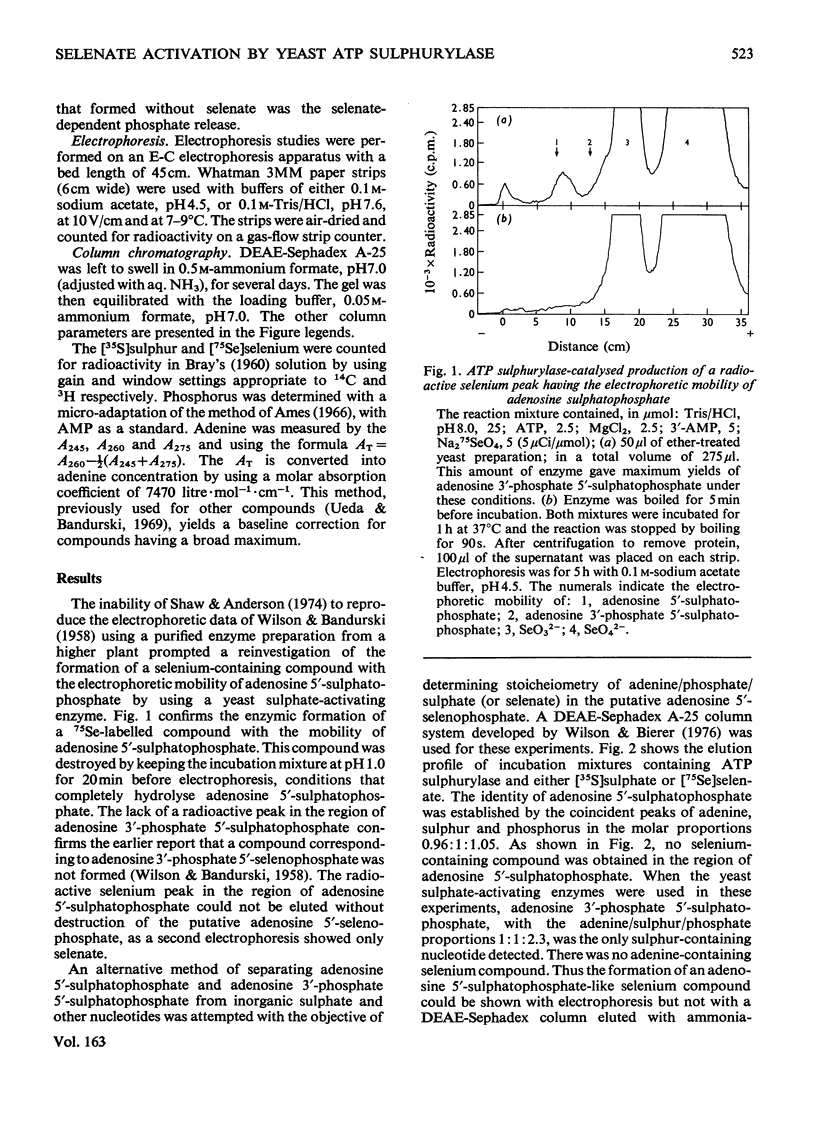
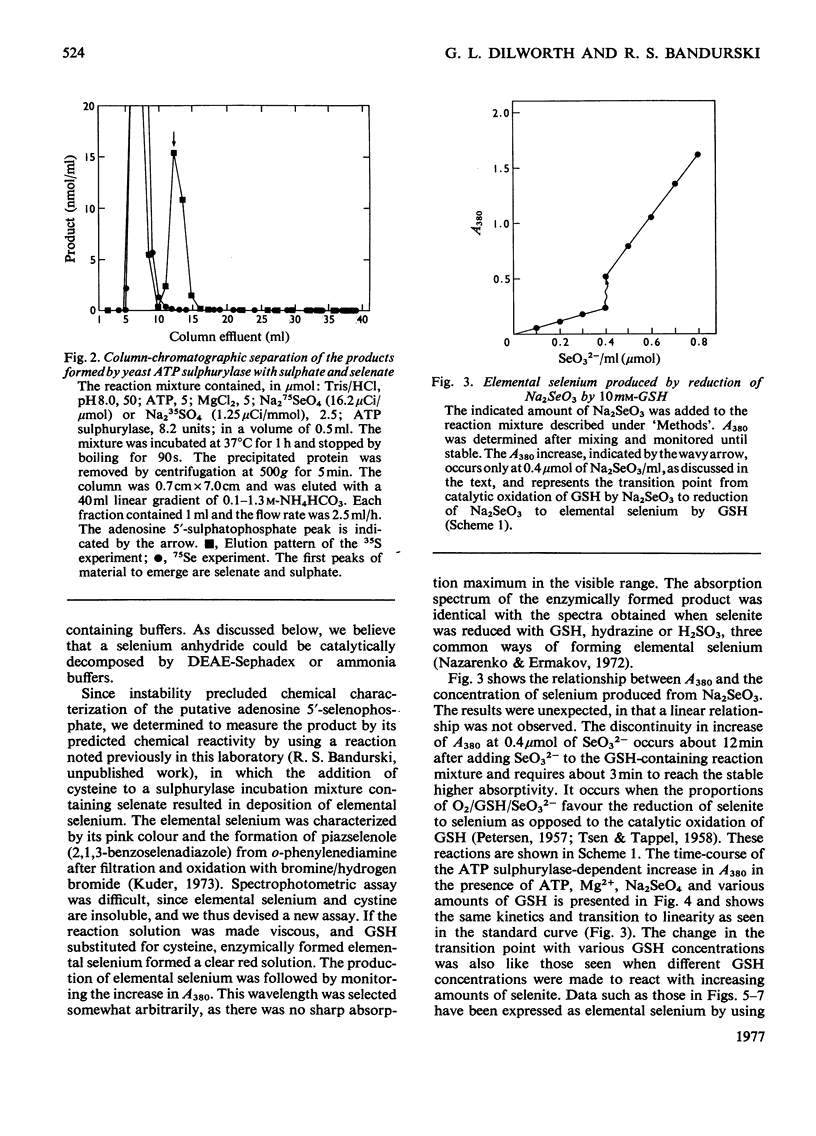
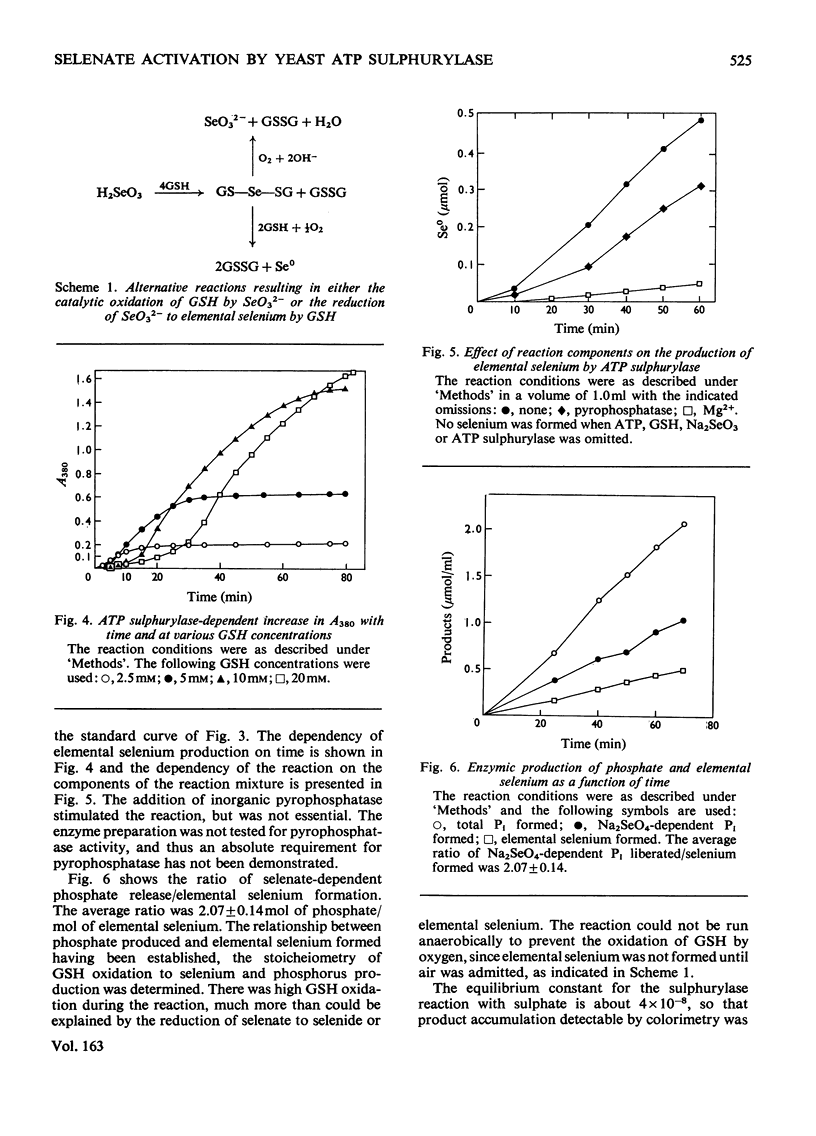
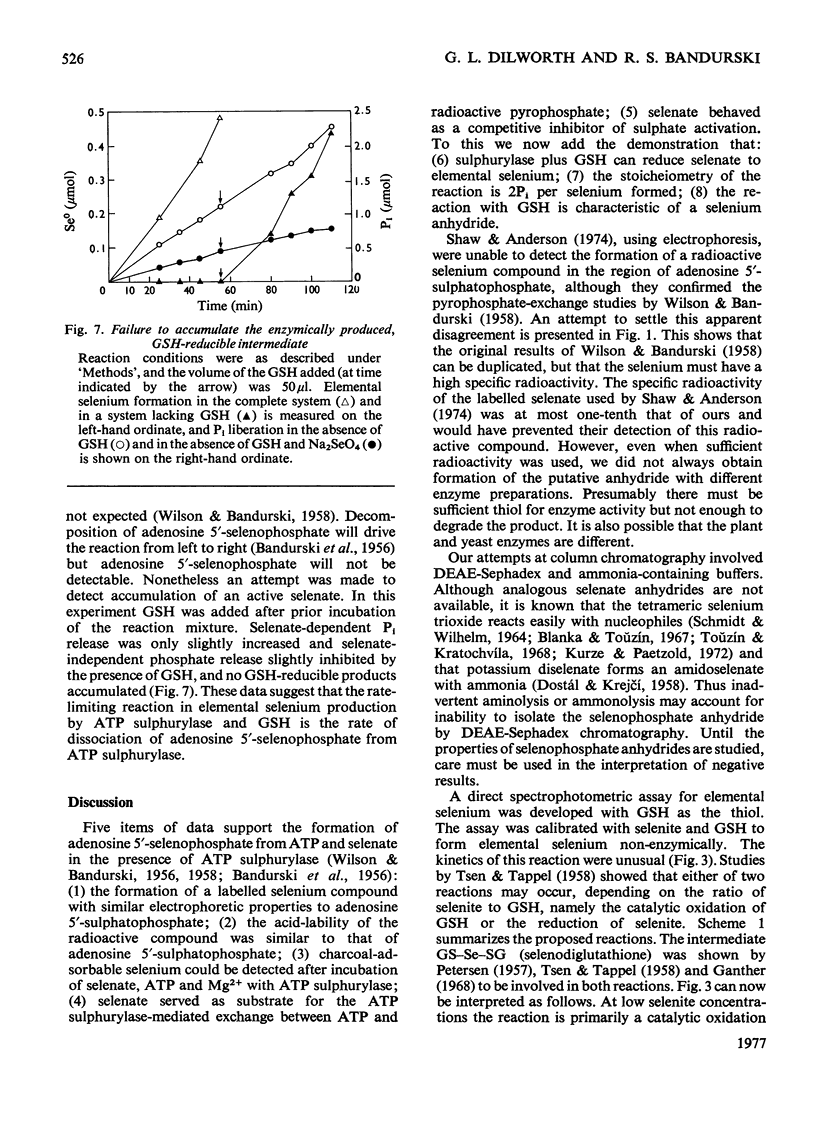
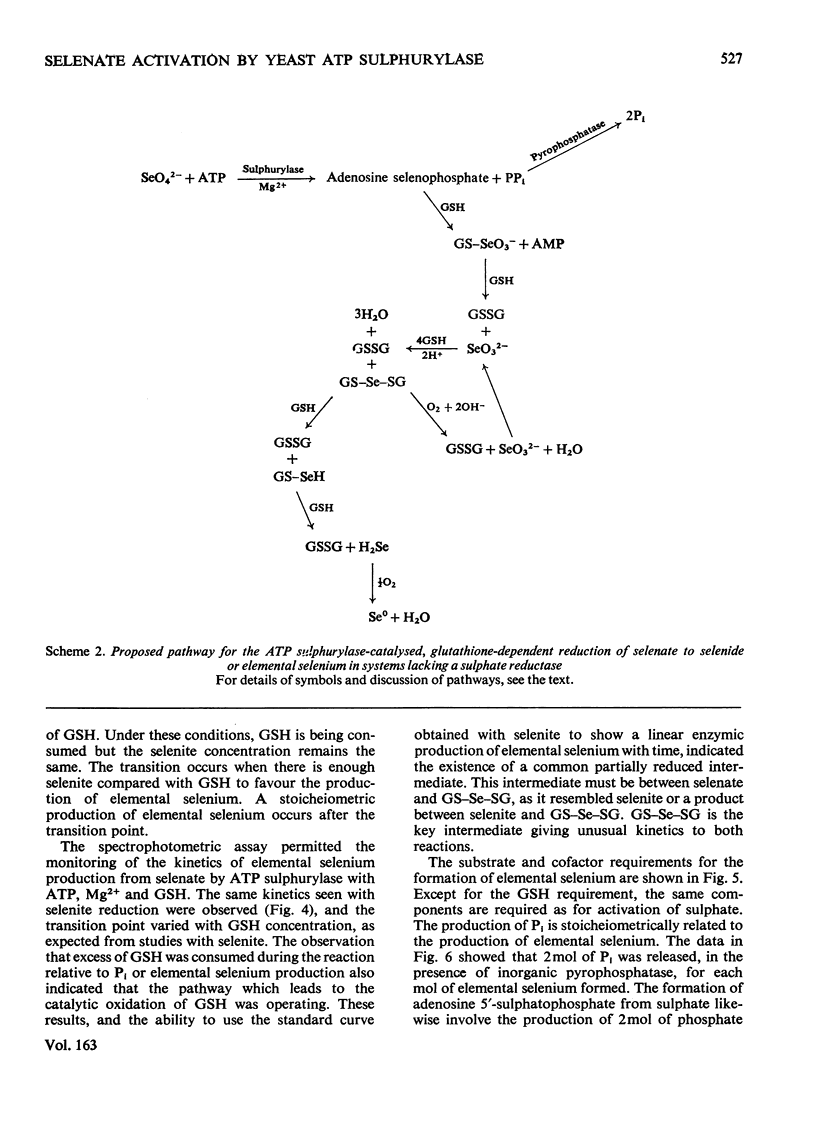
In the presence of ATP and Mg2+, ATP sulphurylase from Saccharomyces cerevisiae catalysed the conversion of selenate into a compound with the electrophoretic and acid-lability properties of adenosine 5'-sulphatophosphate. Structural characterization, involving extensive purification of adenosine 5'-selenophosphate, proved impossible. However, we showed ATP-, Mg2+- and ATP sulphurylase-dependent, and inorganic pyrophosphatase-stimulated, production of elemental selenium from selenate in the presence of GSH (reduced glutathione). Since selenate was not reduced by GSH, this reaction proved that ATP sulphurylase had formed an active selenate. The enzyme catalysed formation of elemental selenium had the same kinetics and GSH-dependency as the non-enzymic reduction of selenite to elemental selenium by GSH. In the presence of inorganic pyrophosphatase, 2 mol of Pi was released for each mol of 'active selenate' formed. This was shown by a spectrophotometric assay for elemental selenium. The observed reactivity with thiols and the instability of the enzymic product were those predicted for selenium anhydrides. By analogy with the chemistry of sulphur, the product of the thiolytic cleavage of a selenium anhydride would be converted into selenite. The selenite would then be reduced by the thiol to elemental selenium. We conclude that ATP sulphurylase can catalyse the formation of adenosine 5'-selenophosphate. The anhydride can be reduced by thiols in a manner similar to the reduction of selenite. These results probably explain the ability of mammals, lacking a sulphate reductase system, to incorporate selenium from selenate into seleno-amino acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr Genetic analysis of the first steps of sulphate metabolism in Aspergillus nidulans. Nature. 1968 Jul 20;219(5151):268–270. doi: 10.1038/219268a0. [DOI] [PubMed] [Google Scholar]
- Fleming R. W., Alexander M. Dimethylselenide and dimethyltelluride formation by a strain of Penicillium. Appl Microbiol. 1972 Sep;24(3):424–429. doi: 10.1128/am.24.3.424-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost D. V., Lish P. M. Selenium in biology. Annu Rev Pharmacol. 1975;15:259–284. doi: 10.1146/annurev.pa.15.040175.001355. [DOI] [PubMed] [Google Scholar]
- Ganther H. E. Enzymic synthesis of dimethyl selenide from sodium selenite in mouse liver extracts. Biochemistry. 1966 Mar;5(3):1089–1098. doi: 10.1021/bi00867a039. [DOI] [PubMed] [Google Scholar]
- Ganther H. E. Reduction of the selenotrisulfide derivative of glutathione to a persulfide analog by glutathione reductase. Biochemistry. 1971 Oct 26;10(22):4089–4098. doi: 10.1021/bi00798a013. [DOI] [PubMed] [Google Scholar]
- Ganther H. E. Selenotrisulfides. Formation by the reaction of thiols with selenious acid. Biochemistry. 1968 Aug;7(8):2898–2905. doi: 10.1021/bi00848a029. [DOI] [PubMed] [Google Scholar]
- Hsieh H. S., Ganther H. E. Acid-volatile selenium formation catalyzed by glutathione reductase. Biochemistry. 1975 Apr 22;14(8):1632–1636. doi: 10.1021/bi00679a014. [DOI] [PubMed] [Google Scholar]
- KEMP J. D., ATKINSON D. E., EHRET A., LAZZARINI R. A. EVIDENCE FOR THE IDENTITY OF THE NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC SULFITE AND NITRITE REDUCTASES OF ESCHERICHIA COLI. J Biol Chem. 1963 Oct;238:3466–3471. [PubMed] [Google Scholar]
- Leggett J. E., Epstein E. Kinetics of Sulfate Absorption by Barley Roots. Plant Physiol. 1956 May;31(3):222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCONNELL K. P., PORTMAN O. W. Toxicity of dimethyl selenide in the rat and mouse. Proc Soc Exp Biol Med. 1952 Feb;79(2):230–231. doi: 10.3181/00379727-79-19333. [DOI] [PubMed] [Google Scholar]
- NISSEN P., BENSON A. A. ABSENCE OF SELENATE ESTERS AND "SELENOLIPID" IN PLANTS. Biochim Biophys Acta. 1964 Feb 10;82:400–402. doi: 10.1016/0304-4165(64)90313-7. [DOI] [PubMed] [Google Scholar]
- SCHWARZ K., FOLTZ C. M. Factor 3 activity of selenium compounds. J Biol Chem. 1958 Jul;233(1):245–251. [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Comparative enzymology of the adenosine triphosphate sulphurylases from leaf tissue of selenium-accumulator and non-accumulator plants. Biochem J. 1974 Apr;139(1):37–42. doi: 10.1042/bj1390037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Sumner J. B. A METHOD FOR THE COLORIMETRIC DETERMINATION OF PHOSPHORUS. Science. 1944 Nov 3;100(2601):413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- TSEN C. C., TAPPEL A. L. Catalytic oxidation of glutathione and other sulfhydryl compounds by selenite. J Biol Chem. 1958 Nov;233(5):1230–1232. [PubMed] [Google Scholar]
- TUVE T., WILLIAMS H. H. Metabolism of selenium by Escherichia coli: biosynthesis of selenomethionine. J Biol Chem. 1961 Feb;236:597–601. [PubMed] [Google Scholar]
- Ueda M., Bandurski R. S. A Quantitative Estimation of Alkali-labile Indole-3-Acetic Acid Compounds in Dormant and Germinating Maize Kernels. Plant Physiol. 1969 Aug;44(8):1175–1181. doi: 10.1104/pp.44.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. An enzymatic reaction involving adenosine triphosphate and selenate. Arch Biochem Biophys. 1956 Jun;62(2):503–506. doi: 10.1016/0003-9861(56)90151-5. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wilson L. G., Bierer D. The formation of exchangeable sulphite from adenosine 3'-phosphate 5'-sulphatophosphate in yeast. Biochem J. 1976 Aug 15;158(2):255–270. doi: 10.1042/bj1580255. [DOI] [PMC free article] [PubMed] [Google Scholar]


