Abstract
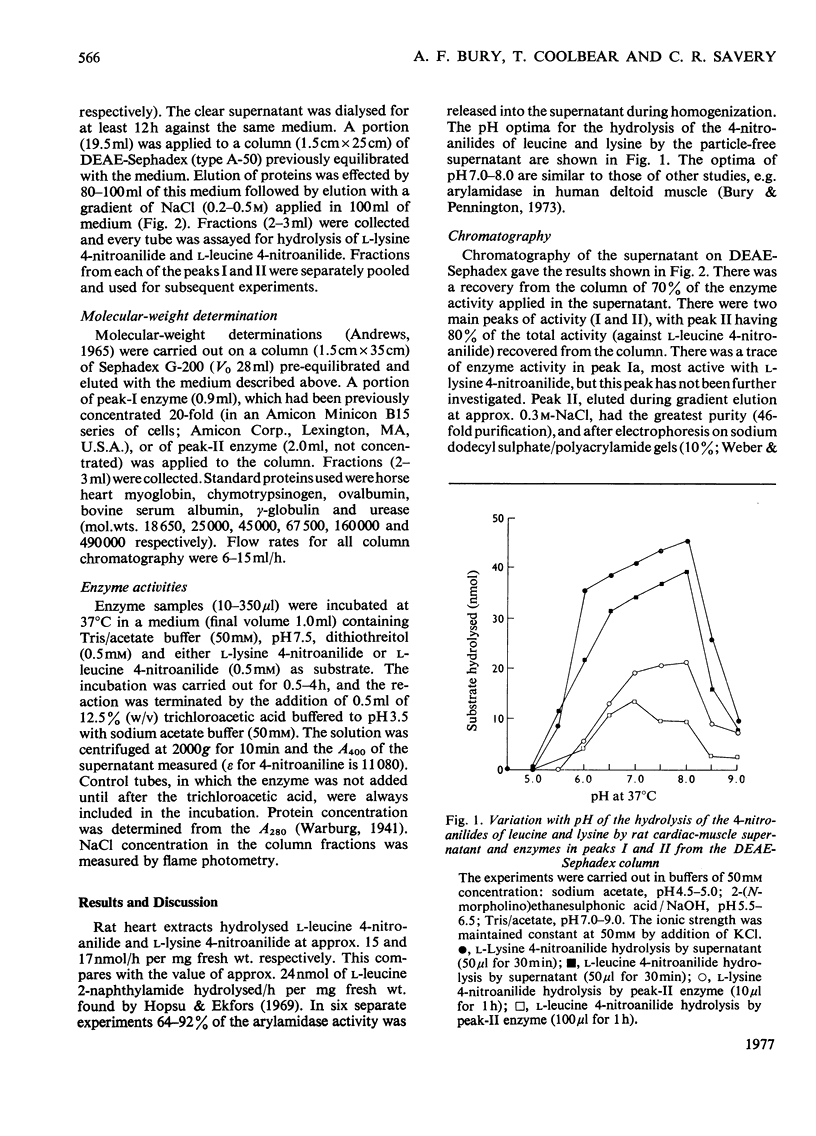
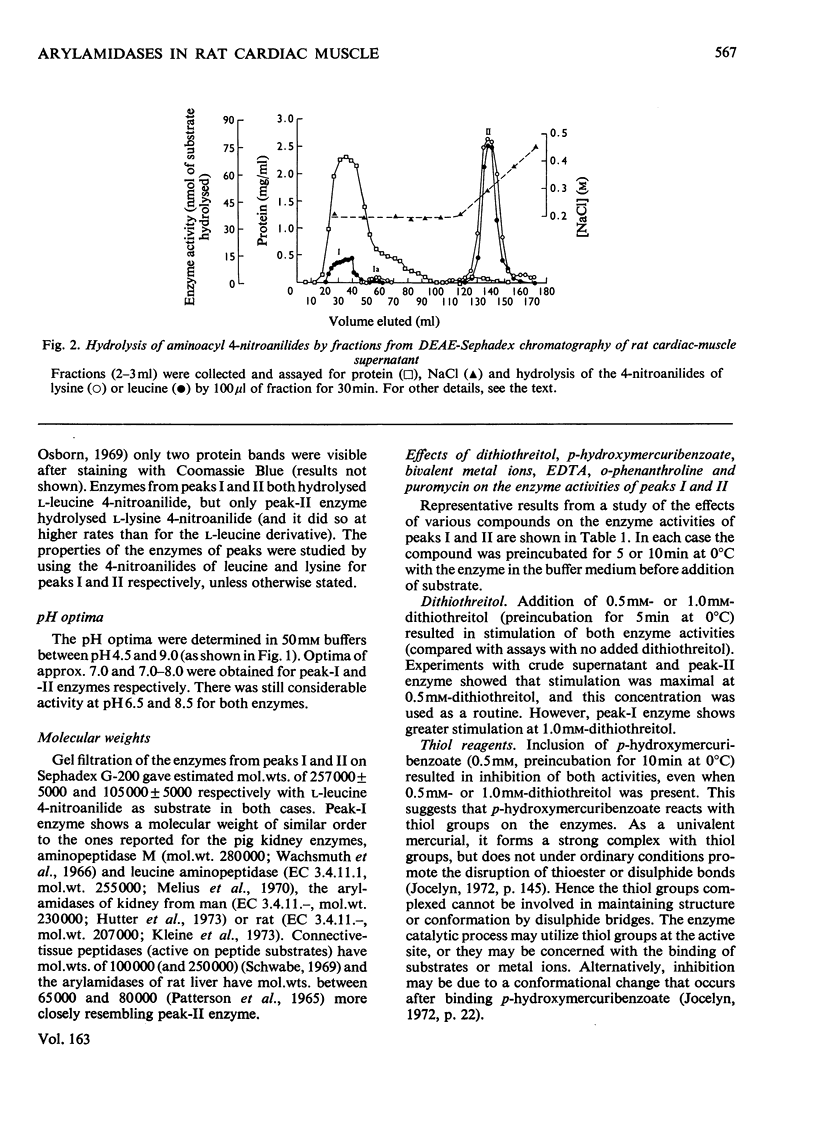
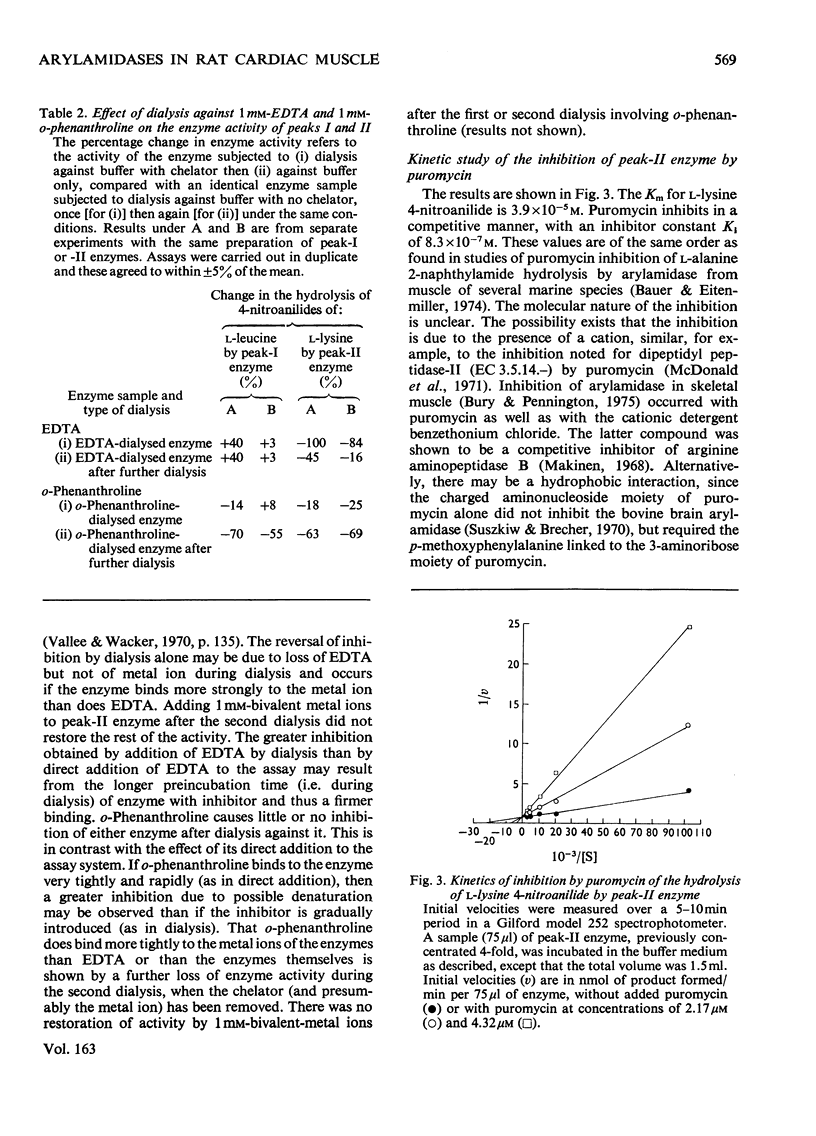
Two main arylamidase activities were separated from a particle-free supernatant of rat heart by chromatography on DEAE-Sephadex. Although both enzymes hydrolysed L-leucine 4-nitroanilide, only peak-II enzyme hydrolysed L-lysine 4-nitroanilide. A third minor peak (Ia) contained an enzyme that was active mainly on the L-lysine 4-nitroanilide. The mol.wts. of the enzymes in peaks I and II were approx. 257000 and 105000 respectively. The pH optimum was approx. pH7.0 for peak-I enzyme and 7.0-8.0 for peak-II enzyme. Both enzymes were inhibited by addition of puromycin, p-hydroxymercuribenzoate, o-phenanthroline and bivalent metal ions. Addition of dithiothreitol resulted in stimulation of both activities. Dialysis against o-phenanthroline resulted in inhibition of peak-I and -II enzymes, but after dialysis against EDTA only peak-II enzyme was inhibited.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury A. F., Pennington R. J. Hydrolysis of diepptide 2-naphthylamides by human muscle enzymes. Biochem J. 1975 Feb;145(2):413–416. doi: 10.1042/bj1450413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS S. A THIOL-ACTIVATED AMINOPEPTIDASE OF THE PITUITARY. Biochem Biophys Res Commun. 1963 Aug 20;12:452–456. doi: 10.1016/0006-291x(63)90314-0. [DOI] [PubMed] [Google Scholar]
- Ellis S., Perry M. Pituitary arylamidases and peptidases. J Biol Chem. 1966 Aug 25;241(16):3679–3686. [PubMed] [Google Scholar]
- Femfert U., Pfleiderer G. The effect of cations and complexing agents on the hydrolysis of L-Alanine-4-nitroanilide by aminopeptidase M. FEBS Lett. 1971 Apr;14(2):89–91. doi: 10.1016/0014-5793(71)80107-2. [DOI] [PubMed] [Google Scholar]
- Hopsu-Havu V. K., Ekfors T. O. Distribution of a dipeptide naphthylamidase in rat tissues and its localisation by using diazo coupling and labeled antibody techniques. Histochemie. 1969;17(1):30–38. doi: 10.1007/BF00306327. [DOI] [PubMed] [Google Scholar]
- Marks N., Datta R. K., Lajtha A. Partial resolution of brain arylamidases and aminopeptidases. J Biol Chem. 1968 Jun 10;243(11):2882–2889. [PubMed] [Google Scholar]
- Melius P., Moseley M. H., Brown D. M. Characterization of the subunits of swine kidney leucine aminopeptidase. Biochim Biophys Acta. 1970 Oct 20;221(1):62–68. doi: 10.1016/0005-2795(70)90197-2. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K. Inhibition of arylaminopeptidases and proline iminopeptidases of human whole saliva by benzethonium chloride (benzyldimethyl [2-[2-(p-1,1,3,3-tetramethylbutylphenoxy)ethoxy]-ethyl] ammonium chloride). FEBS Lett. 1968 Dec;2(2):101–104. doi: 10.1016/0014-5793(68)80113-9. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Mäkinen P. L. Evidence on erythrocyte aminopeptidase B. Int J Protein Res. 1971;3(1):41–47. doi: 10.1111/j.1399-3011.1971.tb01691.x. [DOI] [PubMed] [Google Scholar]
- PATTERSON E. K., HSIAO S. H., KEPPEL A., SOROF S. STUDIES ON DIPEPTIDASES AND AMINOPEPTIDASES. II. ZONAL ELECTROPHORETIC SEPARATION OF RAT LIVER PEPTIDASES. J Biol Chem. 1965 Feb;240:710–716. [PubMed] [Google Scholar]
- PATTERSON E. K., HSIAO S. H., KEPPEL A. STUDIES ON DIPEPTIDASES AND AMINOPEPTIDASES. I. DISTINCTION BETWEEN LEUCINE AMINOPEPTIDASE AND ENZYMES THAT HYDROLYZE L-LEUCYL-BETA-NAPHTHYLAMIDE. J Biol Chem. 1963 Nov;238:3611–3620. [PubMed] [Google Scholar]
- Parsons M. E., Pennington R. J. Separation of rat muscle aminopeptidases. Biochem J. 1976 May 1;155(2):375–381. doi: 10.1042/bj1550375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld N., Peters J. E., Giesecke H., Beier L., Haschen R. J. Untersuchungen über Aminosäure-arylamidasen. I. Verteilung und Isoenzyme der Aminosäure-arylamidase im menschlichen Organismus. Acta Biol Med Ger. 1967;19(6):809–818. [PubMed] [Google Scholar]
- SMITH E. L., SPACKMAN D. H. Leucine aminopeptidase. V. Activation, specificity, and mechanism of action. J Biol Chem. 1955 Jan;212(1):271–299. [PubMed] [Google Scholar]
- Schwabe C. Peptide hydrolases in mammalian connective tissue. II. Leucine aminopeptidase. Purification and evidence for subunit structure. Biochemistry. 1969 Mar;8(3):783–794. doi: 10.1021/bi00831a005. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., Brecher A. S. Brain aminoacyl arylamidase. Further purification of the soluble bovine enzyme and studies on substrate specificity and possible active-site residues. Biochemistry. 1970 Sep 29;9(20):4008–4017. doi: 10.1021/bi00822a021. [DOI] [PubMed] [Google Scholar]
- Szewczuk A., Kochman M., Baranowski T. Dipeptide nitriles as substrates for colorimetric determination of aminopeptidases. Acta Biochim Pol. 1965;12(4):357–367. [PubMed] [Google Scholar]
- TUPPY H., WIESBAUER U., WINTERSBERGER E. [Amino acid-p-nitroanilide as a substrate for aminopeptidases and other proteolytic enzymes]. Hoppe Seylers Z Physiol Chem. 1962 Nov 15;329:278–288. doi: 10.1515/bchm2.1962.329.1.278. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D., Fritze I., Pfleiderer G. An aminopeptidase occurring in pig kidney. II. A study on the mechanism of the hydrolysis. Biochemistry. 1966 Jan;5(1):175–182. doi: 10.1021/bi00865a023. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


