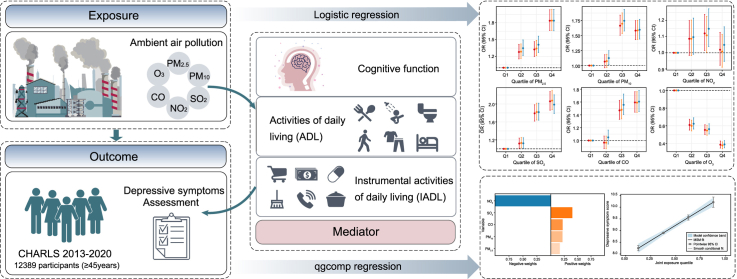
Abstract
Depression is a leading mental health disorder worldwide, contributing substantially to the global disease burden. While emerging evidence suggests links between specific air pollutants and depression, the potential interactions among multiple pollutants remain underexplored. Here we show the influence of six common air pollutants on depressive symptoms among middle-aged and older Chinese adults. In single-pollutant models, a 10 μg m−3 increase in SO2, CO, PM10, and PM2.5 is associated with increased risks of depressive symptoms, with odds ratios (95% confidence intervals) of 1.276 (1.238–1.315), 1.007 (1.006–1.008), 1.066 (1.055–1.078), and 1.130 (1.108–1.153), respectively. In two-pollutant models, SO2 remains significantly associated with depressive symptoms after adjusting for other pollutants. Multi-pollutant models uncover synergistic effects, with SO2, CO, NO2, PM10, and PM2.5 exhibiting significant interactions, identifying SO2 as the primary driver of these associations. Mediation analyses further indicate that cognitive and physical impairments partially mediate the relationship between air pollution and depressive symptoms. These findings underscore the critical mental health impacts of air pollution and highlight the need for integrated air quality management strategies. Targeted mitigation of specific pollutants, particularly SO2, is expected to significantly enhance public mental health outcomes.
Keywords: Air pollution, Long-term exposure, Depressive symptoms, Joint effect, Mediation analysis
Graphical abstract
Highlights
-
•
The adverse effects of PM₂.₅, PM₁₀, SO₂, and CO on depressive symptoms are observed.
-
•
For each quantile rise in combined air pollution, depression risk climbs by 38% (95% CI: 32%–44%).
-
•
SO₂ is the primary contributor to depressive symptoms, accounting for 40% of the risk.
-
•
Cognitive function, ADL, and IADL have significant mediating effects.
1. Introduction
Air pollution significantly contributes to the worldwide disease burden as a major environmental factor, causing the premature demise of more than 4.2 million individuals annually across the globe, of whom approximately 89% are from economically underdeveloped countries [1]. Previous studies have confirmed connections between exposure to air pollutants and various health issues, including cardiovascular, respiratory, and kidney diseases [[2], [3], [4]]. However, research into the possible associations between air pollution and psychiatric disorders remains nascent. Depression, a prevalent mental health disorder, has been estimated by the World Health Organization to affect approximately 5% of the global population. In addition, it is a major cause of suicide, which accounts for more than 700,000 deaths annually, highlighting its impact on public health [5]. Moreover, from 2010 to 2018, the financial impact of major depressive disorder (MDD) on adults from the United States increased by 37.9%, that is, $236.6–326.2 billion in 2020 dollars [6]. Considering the significant economic burden of depression, a deeper understanding of environmental factors, particularly those that can be modified, is crucial for effective disease control and prevention.
Epidemiologic research increasingly points to a correlation between air pollution and psychological health issues [[7], [8], [9]]. Short-term exposure to air pollutants has been linked to increased rates of depression-related hospitalization or outpatient visits [[10], [11], [12]]. However, evidence regarding its long-term effects is less conclusive, with conflicting results reported. A prospective cohort investigation in the United States revealed that exposures to PM2.5 and O3 might increase the likelihood of depression among middle-aged and older women [13]. A similar correlation was observed in another longitudinal study conducted among the insured elderly population in the United States [14]. However, a meta-analysis revealed that exposures to SO2, PM2.5, and PM10 showed no association with depression [15]. A scarcity of research has investigated the prolonged impacts of air contaminants on the prevalence of depressive disorder in the broader populace [[16], [17], [18]]. Furthermore, the bulk of previous investigations have focused on single air pollutants. Therefore, a comprehensive assessment that considers both particulate matter and gaseous pollutants is essential owing to the complex composition of ambient air pollution.
Although the exact mechanism that links air pollutants to depressive disorders remains largely unclear, experimental studies have suggested a possible connection between oxidative stress and inflammatory responses within the nervous system [[19], [20], [21], [22]]. Several epidemiological investigations have examined the potential mediating roles of fecal short-chain fatty acids, gut microbiota, metabolic risk factors, prefrontal cortex, and insula in the relationship between chronic exposure to air pollutants and depression [[23], [24], [25]]. However, the mediating effects of sleep duration, cognitive function, activities of daily living (ADL), and instrumental activities of daily living (IADL) on the development of depression caused by air pollution have been largely overlooked. Cognitive impairment is a common symptom in patients with depression, and research has indicated that it may be exacerbated by air pollution [26,27]. Exposure to air pollutants, both in the short and long term, may disrupt sleep patterns and cognitive functions via increased oxidative stress and neurotransmitter imbalances, which are mechanisms that parallel those implicated in the development of depressive disorders [[28], [29], [30]]. Furthermore, the capacity to perform daily living tasks is linked to mental health and can be affected by environmental factors such as air quality [[31], [32], [33]]. In light of the potential causal chain between the aforementioned factors and depressive mood caused by air pollution, further research, especially focusing on cognitive and physical performance, is necessary to identify potential mediators.
Given these literature gaps, our aims in this study were (1) to evaluate the associations between exposure to single, dual, and multiple air pollutants and depressive symptoms using a large representative national prospective cohort dataset; (2) to identify the primary pollutants that contribute to depressive symptoms; and (3) to investigate the underlying processes through mediation analysis. The insights gained from this study could provide valuable information on the relationship between exposure to air contaminants and the prevalence of depressive symptoms in China.
2. Methods
2.1. Study population
This study was based on data from the China Health and Retirement Longitudinal Study (CHARLS), a comprehensive and ongoing longitudinal survey that targets the Chinese population aged 45 years and older. CHARLS employs a multistage stratified probability-proportional-to-size sampling method to select participants from 150 districts or counties and 450 urban settlements or villages across 28 provinces, ensuring a representative sample. The sampling procedures and comprehensive study design are explained in detail in previous documentation [34]. The initial investigation, which involved 17,708 respondents, was performed between June 2011 and March 2012. Follow-ups were conducted every 2–3 years, and a limited number of new participants were recruited from each subsequent survey. Since the baseline, four follow-up surveys have been conducted in the years 2013, 2015, 2018, and 2020. The data used in this study is accessible at https://charls.pku.edu.cn/.
For the current analysis, we examined a seven-year dynamic cohort of CHARLS data ranging from the second wave in 2013 to the fifth wave in 2020. Participants were excluded from the study if they (1) attended fewer than two follow-up visits, (2) were younger than 45 years, (3) had a diagnosis of depressive symptoms at baseline, or (4) had missing data for the primary variables. Following these exclusion criteria, the final dataset for analysis encompassed 12,389 participants (for further details, see Supplementary Material Fig. S1).
2.2. Assessment of depressive symptoms
A 10-item questionnaire from the Center for Epidemiologic Studies-Depression (CESD) was used to evaluate symptoms of depression. The participants were requested to report the frequency with which they experienced each item during the week preceding the survey. Responses to the questionnaire were based on a 4-point Likert scale defined as follows: 0 (never or seldom, less than 1 day), 1 (some or a little of the time, 1–2 days), 2 (occasionally or a moderate amount of the time, 3–4 days), or 3 (most or all of the time, 5–7 days). The scale ranged from 0 to 30, with lower ratings corresponding to milder symptoms. For the purposes of this study, participants with scores of 10 or higher were classified as exhibiting depressed symptoms. Previous research has demonstrated that this threshold exhibits reasonable sensitivity and specificity in the Chinese elderly population [35,36].
2.3. Exposure assessment
Ground-level concentrations of CO, O3, NO2, SO2, PM10, and PM2.5 were sourced from the ChinaHighAirPollutants (CHAP) datasets, publicly available at https://weijing-rs.github.io/product.html. CHAP data are produced using the space-time extremely randomized trees model, which integrates large database sources such as satellite retrievers, model simulations such as emission inventory, land-use data, topographical information, meteorological data, and aerosol optical depth (AOD). Atmospheric correction techniques were used at multiple angles to extract AOD data from a moderate-resolution image spectroradiometer. In this study, the yearly mean PM10 [37] and PM2.5 levels [38,39] at a spatial resolution of 0.01° (approximately 1 km) and the yearly mean concentrations of SO2 [40], NO2 [41], CO [40], and O3 [42] at 0.1° (roughly 10 km) were used in the analysis. These datasets underwent rigorous cross-validation procedures, demonstrating high accuracy, with tenfold cross-validation reliability coefficients ranging from 0.80 to 0.92. Owing to privacy considerations, the participants' residential addresses were not made publicly available. Therefore, in our analysis, we estimated air pollution exposure at the city level by linking participants to 126 prefectural cities. For each participant, the exposure to air pollution was measured as the annual mean ambient air pollution level from the year the participant entered the cohort until the onset of depressive symptoms or the end of the follow-up period, whichever came first.
2.4. Mediator assessment
This study considered sleep hours (as a continuous variable), cognitive function (score range, 0–31), ADL (yes or no), and IADL (yes or no) as possible mediators in the mediation analyses. Cognitive function was evaluated using the following measures: immediate word recall (0–10 point), delayed word recall (0–10 point), identification of current date (month, day, year, and season) and day of the week (0–5 point), figure redrawing (0–1 point), and serial subtraction tasks (0–5 point). Higher scores reflect better cognitive function, with the total score ranging from 0 to 31. ADL encompassed basic self-care activities, including difficulty eating, bathing, dressing, toileting, walking, and transferring in and out of bed. IADL included more complex daily tasks such as performing domestic chores, cooking, shopping, making phone calls, managing finances, and administering medication. Responses to the questions on ADL and IADL were rated on a 4-point scale from 1 (no difficulty) to 4 (unable to perform), with the aggregate score ranging from 6 to 24. Participants who registered higher scores were deemed to have functional impairments.
2.5. Covariates
Drawing from previous research [43,44], the following covariates were selected for analysis. The demographic covariates encompassed sex (female and male) and age. The socioeconomic factors consisted of urbanicity (urban and rural), marital status (never married, divorced/separated/widowed, and married/partnered), educational level (primary school or lower, or middle school or higher), employment status (employed or unemployed), and income (income positive or income zero). The health behavior variables included drinking status (drinker or non-drinker) and smoking status (smoker or non-smoker). In addition, social activity participation (yes or no) was also included in the analysis.
2.6. Statistical analyses
Means and standard deviations (SDs) were used to describe continuous variables, while percentages and frequencies were applied to categorical variables. The chi-square test was used to compare the categorical variables, and the t-test was applied for the continuous variables to evaluate differences in the distributions of the variables between individuals with and without depressive symptoms. A Pearson correlation analysis was performed to investigate the associations between all exposure variables.
In the primary analysis, logistic regression was applied to examine the correlation between individual air contaminants and depressive symptoms. Odds ratios (ORs) were presented with their corresponding 95% CIs. Air pollutant concentrations were introduced into the logistic regression model as both continuous and categorical variables, categorized into quartiles as follows: Q1 (reference), Q2, Q3, and Q4. Three models were constructed: (1) Model 1, an unadjusted model; (2) Model 2, adjusted for age and sex; and (3) Model 3, a fully adjusted model, which included additional adjustments for educational level, marital status, alcohol consumption, urbanicity, smoking status, social activity, and employment status. To identify potential effect modifiers, stratified analyses were performed based on sex (males or females) and age group (45–59 years and 60 years or older). In addition, potential nonlinear correlations between exposure to each air pollutant and depressive symptoms were simulated using a restricted cubic spline. We then examined two pollutant models, each time adjusting for one additional air pollutant in the regression models. The variance inflation factor (VIF) was used to detect multicollinearity, revealing significant collinearity between PM2.5 and PM10, while the multicollinearity between the other pairs of pollutants was weak or nonexistent (Supplementary Material Table S1). The quantile-based g-computation (qgcomp) model [45], a multi-pollutant analysis approach, was used to evaluate the combined effect of air pollutants on depressive symptoms and determine each pollutant's individual contribution. The qgcomp model estimates the combined impact of raising all exposures by one quantile simultaneously, capturing a “mixture effect” that is particularly relevant for air pollution mixture exposure. This method overcomes the limitations of the traditional weighted quantile sum regression model by not assuming a unidirectional relationship and accommodating the nonlinear characteristics of joint exposures. CO, O3, SO2, NO2, PM10, and PM2.5 were incorporated into the multi-pollutant analysis, with adjustments made for all covariates, as in the primary analysis. Binomial distributions were specified as a link function. The quantile indicator was set to 4, and the 95% CIs were calculated using 500 bootstrap resamples. Similarly, the VIF was used again to test the collinearity in the multi-pollutant model, and the VIF results showed that PM2.5 and PM10 had considerable multicollinearity problems (Supplementary Material Table S2). To address this, we conducted mixture analyses, both with PM2.5 and PM10 included simultaneously and with each included individually. Finally, mediation analyses were performed using functions developed within the “mediation” R package, employing quasi-Bayesian Monte Carlo simulation techniques with 1000 iterations.
Four sensitivity analyses were performed to affirm the robustness of the main outcomes. First, using repeated measurements of depressive symptom scores as the dependent variable, the linear mixed-effects model was used to examine the impact of air pollutants on depression scores, with all covariates adjusted. Second, the same data were substituted into the qgcomp model for repeating the main analysis. Third, further adjustments were performed for medical history, including self-reported conditions at baseline, such as diabetes, hypertension, asthma, arthritis, heart problems, digestive disease, liver disease, kidney disease, lung disease, and stroke, to control potential confounders. The total number of conditions was calculated and grouped into categories of 0 condition, 1 condition, and ≥2 conditions. Finally, to mitigate the impact of reverse causation, cases of depressive symptoms occurring within the initial two years of follow-up were excluded from the analysis. All statistical analyses were conducted in R v4.2.1 (R Development Core Team), and a two-tailed P value < 0.05 was considered to indicate statistical significance.
3. Results
This study involved 12,389 participants from the second to the fifth wave of CHARLS, encompassing 28 provinces across China. The participants' geographic distribution is illustrated in Fig. S2 (Supplementary Material). Over a median follow-up period of five years, 5830 new cases of depressive symptoms were identified. The basic characteristics of the participants are presented in Table 1, with a mean (±SD) age of 57.77 ± 9.42 years. Among all participants, 46.96% were female, and 48.76% and 44.61% were current alcohol drinkers and smokers at baseline, respectively. During the follow-up period, individuals who developed depressive symptoms were more inclined to be female, reside in rural areas, be single or separated/divorced, be nonsmokers, be nondrinkers, show less engagement in social activities, and have lower educational levels.
Table 1.
Basic characteristics of participants according to depressive symptoms.
| Characteristics | Total (n = 12,389) | CES-D < 10 (n = 6559) | CES-D ≥ 10 (n = 5830) | Statistic | P value |
|---|---|---|---|---|---|
| Age, Mean ± SD | 57.77 ± 9.42 | 57.93 ± 9.70 | 57.59 ± 9.09 | t = 2.03 | 0.043 |
| Sex, n (%) | – | – | – | χ2 = 214.59 | <0.001 |
| Male | 6571 (53.04) | 3885 (59.23) | 2686 (46.07) | – | – |
| Female | 5818 (46.96) | 2674 (40.77) | 3144 (53.93) | – | – |
| Urbanicity, n (%) | – | – | – | χ2 = 140.75 | <0.001 |
| Urban | 5259 (42.45) | 3110 (47.42) | 2149 (36.86) | – | – |
| Rural | 7130 (57.55) | 3449 (52.58) | 3681 (63.14) | – | – |
| Education level, n (%) | – | – | – | χ2 = 36.00 | <0.001 |
| Primary school or below | 8015 (64.69) | 4084 (62.27) | 3931 (67.43) | – | – |
| Middle school or above | 4374 (35.31) | 2475 (37.73) | 1899 (32.57) | – | – |
| Marital status, n (%) | – | – | – | χ2 = 9.34 | 0.009 |
| Married/partnered | 11,264 (90.92) | 5981 (91.19) | 5283 (90.62) | – | – |
| Divorced/separated/widowed | 1056 (8.52) | 554 (8.45) | 502 (8.61) | – | – |
| Never married | 69 (0.56) | 24 (0.37) | 45 (0.77) | – | – |
| Employment status, n (%) | – | – | – | χ2 = 12.14 | <0.001 |
| Unemployed | 3578 (28.88) | 1982 (30.22) | 1596 (27.38) | – | – |
| Employed | 8811 (71.12) | 4577 (69.78) | 4234 (72.62) | – | – |
| Income, n (%) | – | – | – | χ2 = 39.20 | <0.001 |
| No | 8750 (70.63) | 4474 (68.21) | 4276 (73.34) | – | – |
| Yes | 3639 (29.37) | 2085 (31.79) | 1554 (26.66) | – | – |
| Smoking status, n (%) | – | – | – | χ2 = 79.92 | <0.001 |
| Non-smoker | 6862 (55.39) | 3386 (51.62) | 3476 (59.62) | – | – |
| Smoker | 5527 (44.61) | 3173 (48.38) | 2354 (40.38) | – | – |
| Drinking status, n (%) | – | – | – | χ2 = 62.06 | <0.001 |
| Non-drinker | 6348 (51.24) | 3142 (47.90) | 3206 (54.99) | – | – |
| Drinker | 6041 (48.76) | 3417 (52.10) | 2624 (45.01) | – | – |
| Social activity, n (%) | – | – | – | χ2 = 8.93 | 0.003 |
| No | 5399 (43.58) | 2776 (42.32) | 2623 (44.99) | – | – |
| Yes | 6990 (56.42) | 3783 (57.68) | 3207 (55.01) | – | – |
Note: CES-D, Center for Epidemiologic Studies Depression.
Fig. S3 (Supplementary Material) depicts the mean annual exposure concentrations of six pollutants for the study participants from 2013 to 2020. Overall, a general decreasing trend was observed in the mean exposure concentrations of all contaminants except for O3. Table S3 and Fig. S4 (Supplementary Materials) display the statistical descriptions of the assumed exposure concentrations of air pollutants for all participants and the results of the Pearson correlation analysis, respectively. The mean (±SD) exposure concentrations of PM2.5, PM10, SO2, NO2, CO, and O3 were 53.55 ± 18.89, 92.66 ± 34.42, 24.12 ± 12.26, 33.83 ± 10.60, 1.07 ± 0.33, and 89.92 ± 11.35 μg m−3, respectively. The annual means for PM2.5 and PM10 exceeded the guidelines established by the World Health Organization (Air Quality Guidelines 2021, PM2.5: 5 μg m−3, PM10: 10 μg m−3) and the secondary standard of the Chinese ambient air quality guideline (GB 3095–2012, PM2.5: 35 μg m−3, PM10: 70 μg m−3). The correlation between PM10 and PM2.5 was particularly strong, with a high coefficient of 0.94. By contrast, the correlations between O3 and the other five air pollutants were relatively weak. Moreover, the correlation coefficients between the other pollutants ranged from 0.09 to 0.74.
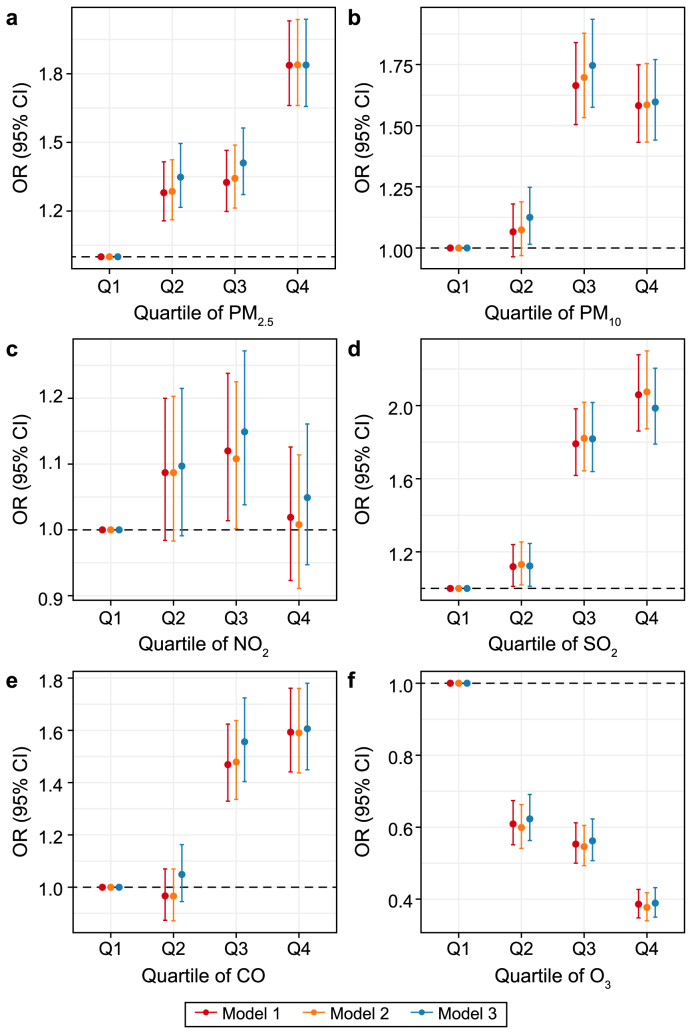
Table 2 presents the estimates of the single-pollutant models when air pollutant concentrations were treated as continuous variables. Statistically significant correlations were observed between PM2.5, PM10, SO2, and CO and the depressive symptoms. Although the relationship between NO2 and depressive symptoms was consistently positive across all models, it was not consistently statistically significant. However, O3 demonstrated a negative relationship with the occurrence of depressive symptoms. In the primary model, the ORs (95% CIs) for depressive symptoms associated with a 10 μg m−3 increase in PM2.5, PM10, SO2, NO2, CO, and O3 were 1.130 (1.108–1.153), 1.066 (1.055–1.078), 1.276 (1.238–1.315), 1.019 (0.985–1.054), 1.007 (1.006–1.008), and 0.732 (0.708–0.757), respectively. The risk of depressive symptoms corresponding to the air pollutant quartiles is illustrated in Fig. 1. Consistently, compared with the first quartile, the risk of depressive symptoms increased in the highest quartile of PM2.5 (OR: 1.838, 95% CI: 1.657–2.038), the third quartile of PM10 (OR: 1.746, 95% CI: 1.575–1.935), the highest quartile of SO2 (OR: 1.986, 95% CI: 1.789–2.204), and the highest quartile of CO (OR: 1.606, 95% CI: 1.657–2.038) in the completely adjusted model, with all P values being <0.05.
Table 2.
Associations between per 10 μg m−3 increase in CO, O3, NO2, SO2, PM2.5, and PM10 and depressive symptoms risk in single-pollutant models.
| Variables | Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| PM2.5 | 1.131 (1.110, 1.153) | <0.001 | 1.132 (1.110, 1.154) | <0.001 | 1.130 (1.108, 1.153) | <0.001 |
| PM10 | 1.067 (1.056, 1.078) | <0.001 | 1.067 (1.056, 1.078) | <0.001 | 1.066 (1.055, 1.078) | <0.001 |
| NO2 | 1.004 (0.971, 1.038) | 0.812 | 1.000 (0.967, 1.034) | 0.983 | 1.019 (0.985, 1.054) | 0.281 |
| SO2 | 1.292 (1.255, 1.330) | <0.001 | 1.293 (1.256, 1.332) | <0.001 | 1.276 (1.238, 1.315) | <0.001 |
| CO | 1.007 (1.006, 1.008) | <0.001 | 1.007 (1.006, 1.008) | <0.001 | 1.007 (1.006, 1.008) | <0.001 |
| O3 | 0.730 (0.707, 0.754) | <0.001 | 0.725 (0.701, 0.749) | <0.001 | 0.732 (0.708, 0.757) | <0.001 |
Note.
1. Model 1: The crude model.
2. Model 2: The base model, adjusted for age at baseline and sex.
3. Model 3: The main model, further adjusted for educational level, marital status, employment status, urbanicity, cigarette smoking, alcohol drinking, and social activity.
4. OR, odds ratio. CI, confidence interval.
Fig. 1.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between long-term air pollution exposure and incidence of depressive symptoms. a, PM2.5. b, PM10. c, NO2. d, SO2. e, CO. f, O3.
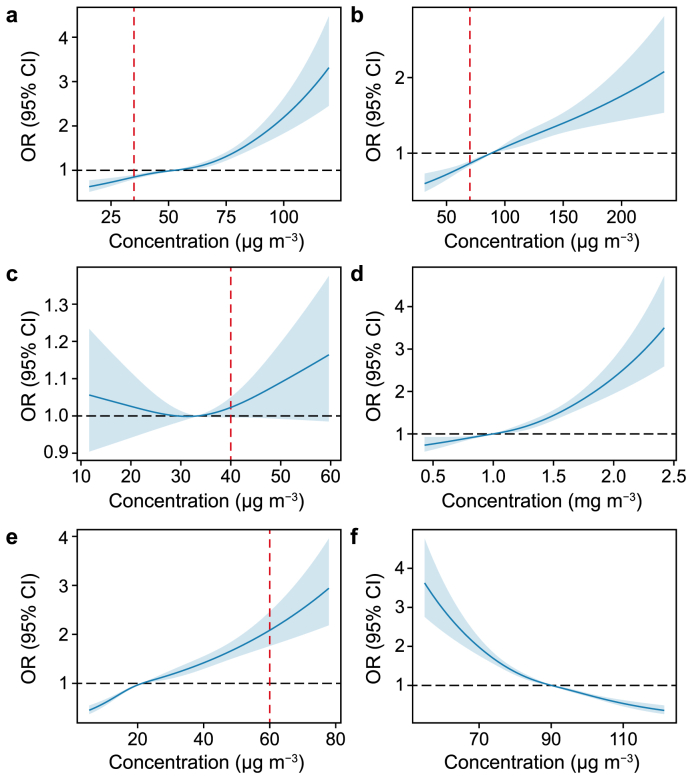
Fig. 2 depicts the exposure-response (E-R) relationships for all pollutants related to depressive symptoms. The data indicated that the relationships between PM10 and SO2 with depressive symptoms were nearly linear, with no evident thresholds (Fig. 2b, e). By contrast, for PM2.5 and CO, the curves remained relatively flat at lower concentrations but dramatically increased beyond 52 μg m−3 for PM2.5 and 1 mg m−3 for CO, displaying J-shaped patterns (Fig. 2a, d). The curve for NO2 presented an inflection point, with a gradual decline in risk below 33 μg m−3, followed by an inverted curve as the concentration continued to increase (Fig. 2c). Regarding O3, the curve demonstrated a reversed J-shape, showing a sharper incline below 90 μg m−3 but tended to plateau at higher concentrations (Fig. 2f).
Fig. 2.
The exposure-response relationships between air pollutants and depressive symptoms. a, PM2.5. b, PM10. c, NO2. d, CO. e, SO2. f, O3. The red vertical dotted line reflects the air quality thresholds set by Chinese national standards, with matching amounts set at 60 μg m−3 for SO2, 40 μg m−3 for NO2, 70 μg m−3 for PM10, and 35 μg m−3 for PM2.5. OR, odds ratio; CI, confidence interval.
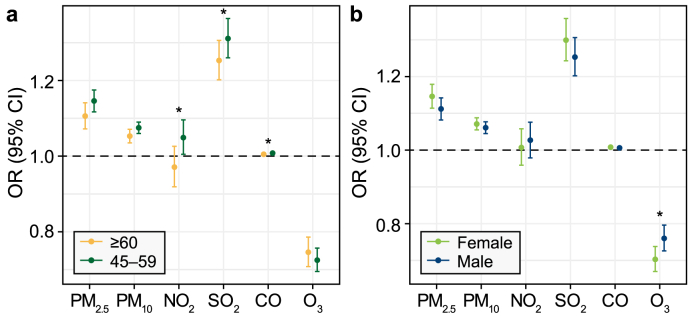
Furthermore, the findings from the subgroup analyses based on age and sex are presented in Fig. 3. In the analysis stratified by age, more pronounced associations between NO2, SO2, and CO with depressive symptoms were observed among individuals aged 45–59 years compared with those aged 60 years and older (Fig. 3a). For PM10 and PM2.5, no discernible disparities in the effects were found between the age groups (Fig. 3a). In terms of sex as a potential effect modifier, the females appeared to be more susceptible than the males to the effects of air pollutant exposure to SO2, CO, PM10, and PM2.5 (Fig. 3b). However, the distinctions in susceptibility within the sex groups did not prove statistically significant.
Fig. 3.
Associations of per 10 μg m−3 increase in CO, O3, NO2, SO2, PM2.5, and PM10 with depressive symptoms, stratified by subgroups: a, age; b, sex. ∗P < 0.05. OR, odds ratio; CI, confidence interval.
The results of the two-pollutant models indicated that the correlation of SO2 remained robust after controlling for other air pollutants in the model, while the correlations of CO, PM10, and PM2.5 became insignificant after controlling for SO2. For NO2, its correlation turned notable upon adjustment for other pollutants but exhibited a significant negative correlation when considering CO, SO2, PM10, and PM2.5. With O3 as the controlling factor, NO2 reversed to a significant positive correlation. In addition, the effect of PM10 was no longer statistically significant when PM2.5 was factored into the model (Table 3).
Table 3.
Associations (OR and 95% CI) between air pollutants (per 10 μg m−3 increase) and depressive symptoms risk in two-pollutant models.
| Variables | Adjusted for PM2.5 | Adjusted for PM10 | Adjusted for NO2 | Adjusted for SO2 | Adjusted for CO | Adjusted for O3 |
|---|---|---|---|---|---|---|
| PM2.5 | – | 1.118 (1.055, 1.186)∗ | 1.265 (1.230, 1.302)∗ | 1.025 (0.998, 1.053) | 1.079 (1.049, 1.110)∗ | 1.212 (1.187, 1.238)∗ |
| PM10 | 1.007 (0.975, 1.040) | – | 1.121 (1.104, 1.139)∗ | 1.009 (0.994, 1.023) | 1.037 (1.021, 1.052)∗ | 1.114 (1.101, 1.128)∗ |
| NO2 | 0.758 (0.721, 0.797)∗ | 0.793 (0.756, 0.832)∗ | – | 0.869 (0.835, 0.903)∗ | 0.872 (0.836, 0.908)∗ | 1.147 (1.106, 1.190)∗ |
| SO2 | 1.247 (1.197, 1.299)∗ | 1.259 (1.209, 1.311)∗ | 1.352 (1.306, 1.398)∗ | – | 1.274 (1.219, 1.331)∗ | 1.350 (1.308, 1.394)∗ |
| CO | 1.004 (1.002, 1.005)∗ | 1.004 (1.003, 1.006)∗ | 1.010 (1.008, 1.011)∗ | 1.000 (0.999, 1.002) | – | 1.009 (1.007, 1.010)∗ |
| O3 | 0.667 (0.644, 0.691)∗ | 0.659 (0.635, 0.683)∗ | 0.701 (0.677, 0.726)∗ | 0.695 (0.671, 0.719)∗ | 0.708 (0.684, 0.732)∗ | – |
Note.
1. The Model 3 covariates were included.
2. ∗P < 0.05.
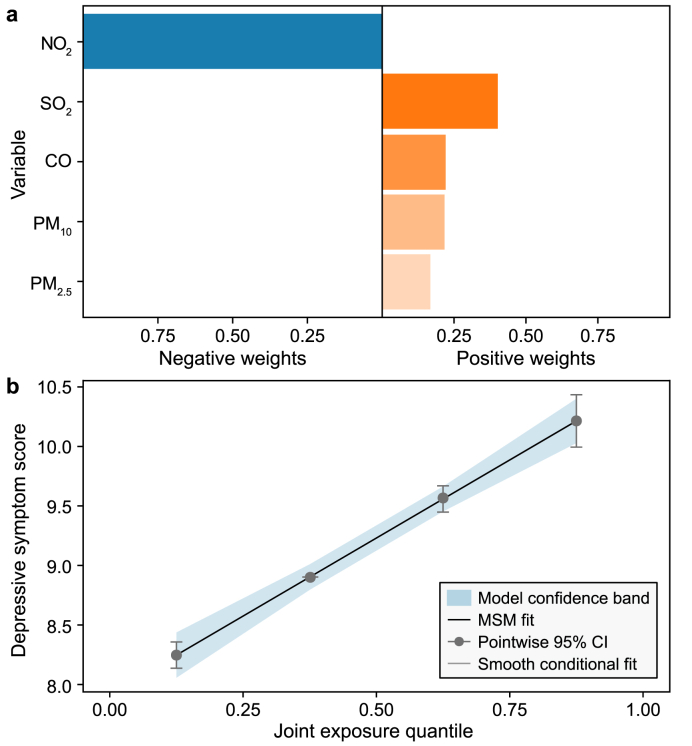
The coefficients in the multi-pollutant model indicated the combined impact on depressive symptoms when the levels of mixed ambient air pollutants increased by a quarter simultaneously (Table 4). In the initial analysis, when all six air pollutants were examined, the results indicated a nonsignificant correlation between air pollution and depressive symptoms (OR: 0.954, 95% CI: 0.908–1.003, P > 0.05). This nonsignificance could indicate that certain pollutants may counteract the effects of others when considered in combination. After controlling for O3 in the analysis, a significant relationship with depressive symptoms was observed for CO, NO2, SO2, PM10, and PM2.5, and the OR for each quantile increase in the combined air pollutant concentrations was 1.382 (95% CI: 1.324–1.442, P < 0.05). The combined exposure effect was substantially greater than the individual effects. In the qgcomp model, the results from simultaneously including both PM2.5 and PM10 were essentially in line with those obtained when PM2.5 or PM10 were included in the model. As depicted in Fig. 4, SO2 contributed most significantly to the positive effect on depressive symptoms, with a weight of 40.0%, followed by CO, PM2.5, and PM10. NO2 was the only contaminant that was assigned a negative weight.
Table 4.
ORs (95% CI) of incidence risk of depressive symptoms associated with air pollutants in multi-pollutant models.
| Mixture variables | OR (95% CI) | P |
|---|---|---|
| PM2.5 + PM10 + CO + SO2 + NO2 + O3 | 0.954 (0.908, 1.003) | 0.066 |
| PM10 + CO + SO2 + NO2 + O3 | 0.955 (0.909, 1.003) | 0.063 |
| PM2.5 + CO + SO2 + NO2 + O3 | 0.979 (0.933, 1.026) | 0.371 |
| PM2.5 + PM10 + CO + SO2 + NO2 | 1.382 (1.324, 1.442) | <0.001 |
| PM10 + CO + SO2 + NO2 | 1.383 (1.325, 1.443) | <0.001 |
| PM2.5 + CO + SO2 + NO2 | 1.378 (1.320, 1.439) | <0.001 |
Note.
1. OR, odds ratio. CI, confidence interval.
2. The Model 3 covariates were included.
Fig. 4.
Qgcomp model regression index weights (a) and joint effects with 95% CI (b) of air pollutants (CO, SO2, NO2, PM10, and PM2.5) on depressive symptoms. The Model 3 covariates were included. MSM, marginal structural model; CI, confidence interval. The MSM fit line (black line) completely overlaps with the smooth conditional fit line (grey line), thus covering the latter.
Mediation analyses examined potential mediators for the four air contaminants that correlated positively with depressive symptoms (Table 5). Among the mediating variables, ADL exhibited a mediating effect that ranged from 5.70% for SO2 to 9.22% for PM10. The IADL had a more pronounced mediating impact, explaining 9.46% for SO2 to 11.63% for PM10. Sleep duration failed to demonstrate a statistically significant mediating effect in this situation. By contrast, cognitive function played a strong mediating role in the process through which air pollution led to depressive symptoms; the proportion of mediation effect ranged from 23.52% for PM10 to 28.59% for PM2.5.
Table 5.
Potential mediators of the association between air pollutants and depressive symptoms.
| Mediators | Direct effect |
Indirect effect |
Total effect |
Proportion Mediated |
||||
|---|---|---|---|---|---|---|---|---|
| Coefficients (95% CI) | P | Coefficients (95% CI) | P | Coefficients (95% CI) | P | % (95% CI) | P | |
| Cognitive function | ||||||||
| PM2.5 | 0.1758 (0.1210, 0.2306) | <0.001 | 0.0704 (0.0576, 0.0832) | <0.001 | 0.2462 (0.1908, 0.3016) | <0.001 | 28.59 (21.99, 38.00) | <0.001 |
| PM10 | 0.0988 (0.0689, 0.1287) | <0.001 | 0.0304 (0.0236, 0.0372) | <0.001 | 0.1292 (0.0983, 0.1601) | <0.001 | 23.52 (17.27, 32.00) | <0.001 |
| CO | 0.0110 (0.0077, 0.0145) | <0.001 | 0.0035 (0.0028, 0.0042) | <0.001 | 0.0145 (0.0111, 0.0179) | <0.001 | 23.95 (17.55, 33.00) | <0.001 |
| SO2 | 0.3380 (0.2510, 0.4250) | <0.001 | 0.1340 (0.1140, 0.1540) | <0.001 | 0.4710 (0.3900, 0.5520) | <0.001 | 28.40 (22.70, 36.00) | <0.001 |
| Sleep | ||||||||
| PM2.5 | 0.2439 (0.1948, 0.2931) | <0.001 | 0.0022 (−0.0058, 0.0102) | 0.58 | 0.2461 (0.1969, 0.2953) | <0.001 | 0.89 (−2.33, 4.11) | 0.58 |
| PM10 | 0.1324 (0.1048, 0.1599) | <0.001 | −0.0022 (−0.0064, 0.0001) | 0.34 | 0.1302 (0.1018, 0.1587) | <0.001 | −1.65 (−5.36, 1.00) | 0.34 |
| CO | 0.0156 (0.0127, 0.0186) | <0.001 | −0.0004 (−0.0008, 0.0001) | 0.1 | 0.0153 (0.0123, 0.1819) | <0.001 | −2.55 (−5.67, 0.58) | 0.1 |
| SO2 | 0.5100 (0.4362, 0.5838) | <0.001 | −0.0102 (−0.0223, 0.0019) | 0.1 | 0.4998 (0.4285, 0.5711) | <0.001 | −2.05 (−4.60, 0.50) | 0.1 |
| ADL | ||||||||
| PM2.5 | 0.0328 (0.0260, 0.0396) | <0.001 | 0.0026 (0.0016, 0.0036) | <0.001 | 0.0354 (0.0287, 0.0421) | <0.001 | 7.39 (4.43, 12.00) | <0.001 |
| PM10 | 0.0161 (0.0126, 0.0196) | <0.001 | 0.0016 (0.0011, 0.0022) | <0.001 | 0.0177 (0.0143, 0.0211) | <0.001 | 9.22 (5.96, 12.48) | <0.001 |
| CO | 0.0017 (0.0013, 0.0021 | <0.001 | 0.0001 (0.0000, 0.0002) | <0.001 | 0.0018 (0.0014, 0.0022) | <0.001 | 6.01 (2.60, 9.42) | <0.001 |
| SO2 | 0.0619 (0.0516, 0.0715) | <0.001 | 0.0037 (0.0022, 0.0052) | <0.001 | 0.0656 (0.0560, 0.7523) | <0.001 | 5.70 (3.41, 7.99) | <0.001 |
| IADL | ||||||||
| PM2.5 | 0.0277 (0.0226, 0.0328) | <0.001 | 0.0033 (0.0024, 0.0043) | <0.001 | 0.0310 (0.0258, 0.0362) | <0.001 | 10.64 (7.43, 13.85) | <0.001 |
| PM10 | 0.0141 (0.0112, 0.0169) | <0.001 | 0.0019 (0.0014, 0.0023) | <0.001 | 0.0159 (0.0130, 0.0188) | <0.001 | 11.63 (8.43, 14.82) | <0.001 |
| CO | 0.0016 (0.0013, 0.0019) | <0.001 | 0.0002 (0.0001, 0.0003) | <0.001 | 0.0018 (0.0015, 0.0021) | <0.001 | 11.25 (8.19, 14.32) | <0.001 |
| SO2 | 0.0571 (0.0496, 0.0646) | <0.001 | 0.0060 (0.0045, 0.0742) | <0.001 | 0.0631 (0.0553, 0.0708) | <0.001 | 9.46 (7.12, 11.80) | <0.001 |
Note.
1. ADL, activities of daily living. IADL, instrumental activities of daily living.
2. PM2.5, PM10, CO, and SO2 were included as continuous data in the mediation analyses.
3. The Model 3 covariates were included.
The sensitivity analyses conducted using a linear mix-effects model revealed that the effects of CO, SO2, PM10, and PM2.5 on the depressive symptoms scores persisted, but the effect of PM10 was not significant (Supplementary Material Table S4). When repeated measures of the depressive symptoms scores were analyzed, the results of the qgcomp model were almost identical to those in the main analysis, with no significant negative correlation when all six air pollutants were monitored but with a significant positive correlation after adjusting for O3 (Supplementary Material Table S5). Recognizing that physical health conditions might exacerbate mental health issues and potentially lead to depressive symptoms; additional analyses were performed. The correlations between air pollutants and depressive symptoms were not substantially altered, even after adjusting for medical history (Supplementary Material Table S6). When cases of depressive symptoms occurring within the first two years of follow-up were excluded to mitigate the impact of reverse causation, the patterns of associations remained similar, although the strength of the associations was somewhat weaker than those in the main models (Supplementary Material Table S7).
4. Discussion
This study comprehensively evaluates the associations between air pollutants and depressive symptoms in China. It established independent relationships of exposures to CO, SO2, PM10, and PM2.5 with the incidence of depressive symptoms. When linked to depressive symptoms, the E-R curves for the individual air pollutants were either linear or J-shaped, suggesting the absence of safe threshold levels. SO2 demonstrated a persistently strong correlation even when other pollutants were considered, whereas when PM2.5 concentrations were factored into the model, the effect of PM10 was no longer statistically discernible. The analysis using the qgcomp model demonstrated that co-exposure to CO, SO2, NO2, PM10, and PM2.5 was positively correlated with the occurrence of depressive symptoms. SO2 emerged as the primary contributor to the positive effect, while NO2 was the sole contributor to the negative correlation. In addition, cognitive function, ADL, and IADL partially mediated the depressive symptoms caused by air pollutants.
This study supports the positive link between depressive symptoms and all examined contaminants except for O3. The positive correlation with NO2 was not significant, which aligns with the findings of previous research. Numerous studies have confirmed the positive correlation of PM2.5 with depression [17,46,47]. For instance, a study encompassing 75 cities in China revealed that short-term exposures to ambient PM2.5, PM10, NO2, SO2, and CO substantially increased the risk of hospitalization for depressive disorder [10]. Similarly, time-series research conducted in Canada demonstrated substantial connections between depression and exposure to CO, SO2, NO2, and PM10 [48]. In addition, a Korean cross-sectional study indicated that chronic exposures to CO, NO2, and PM10 (excluding SO2) were linked to depressive disorder [49]. In concordance with our results, a nested case-control study in Korea showed no link between NO2 and depression throughout the follow-up periods [50]. Conversely, a meta-analysis of 39 articles revealed a direct correlation between NO2 and PM2.5 exposures and increased risk of depressive disorder, with relative risks (RR) of 1.037 (95% CI: 1.011–1.064) and 1.074 (95% CI: 1.021–1.120) per 10 μg m−3 increase, respectively [8]. Furthermore, a significant negative correlation with O3 exposure was observed in this study. This result was also detected in an investigation in Ningbo, China [18], which reported a significant negative link with O3 on the sixth lag day. These results were not entirely consistent, which might be due to variations in the study populations, design, weather conditions, and air pollution levels.
Analyzing E-R curves is essential for health impact evaluation and the development of improved strategies. This study revealed a continuous increase in the E-R curves for CO, PM10, SO2, and PM2.5 concentrations in relation to depressive symptoms. A study in Shijiazhuang, China, observed a linear correlation between air pollution levels and hospitalization rates due to mental health issues [51]. Another study that covered 26 Chinese cities reached a similar conclusion [12]. When compared with the Chinese national standards of air pollutants (as indicated by the red vertical dotted line in Fig. 2), our findings indicate that exposure levels below the air quality standards, particularly 60 μg m−3 for SO2 and 4 mg m−3 for CO, can still present a risk for the development of depressive symptoms. This underscored the necessity of continued efforts to reduce these pollutants. Although particulate matter may not be considered harmful to mental health at the standard concentrations in China, the mean exposure concentrations (63.55 μg m−3 for PM2.5 and 92.66 μg m−3 for PM10) among the population exceeded these standards (35 μg m−3 for PM2.5 and 70 μg m−3 for PM10), suggesting a potential danger. Therefore, the implementation of stricter standards and a reduction in exposure to various contaminants are necessary to help mitigate the burden of depression.
Despite the unclear biological mechanism that links air pollution to depression, several possible pathways have been proposed. Previous studies have indicated that SO2 could potentially cause brain injury by interacting with lipids, nucleic acids, along with proteins through free radical activity [52]. Exposure to SO2 has also been linked to alterations in the plasticity of synapses in the hippocampus [53]. In addition, particulate matters such as PM2.5 are hypothesized to activate the hypothalamic-pituitary-adrenal axis, compromise the integrity of the blood-brain barrier, and boost the generation of reactive oxygen species, thereby stimulating the release of proinflammatory mediators [54,55]. Essentially, air pollutants could affect the central nervous system through oxidative stress and inflammatory responses, potentially via systemic circulation, the trigeminal nerve, or olfactory receptor neurons [56]. Further investigation is necessary to elucidate the precise processes that link air pollution exposure to mental health outcomes.
Identifying a sensitive subpopulation is crucial for the development of effective public health strategies. Previous research findings have indicated that females may exhibit higher susceptibility to air pollutant exposure than males [12,57], which is similar to our results for PM10, CO, SO2, and PM2.5. However, the sex disparities observed between the groups did not reach statistical significance. Factors such as work-related co-exposures and hormonal status could contribute to this sex disparity [58]. In terms of age, the participants aged 45–59 years appeared to be more vulnerable, which aligned with the finding of a previous study that anxiety outpatients typically fall within the age range of 37–59 years [59]. The age-specific discrepancy may be attributed to greater social pressure. Conversely, two studies on hospitalization for depression have reported stronger associations in the group aged 65 years or older [10,12], and studies of other diseases have suggested that older individuals are consistently more vulnerable to the effects of air pollution [60,61]. In brief, the reasons for these discrepancies are likely to be multifaceted. Further exploration of factors such as genetic susceptibility, socioeconomic status, and previous health records in future studies may shed further light on this complex issue.
In the multiple pollutant analysis, SO2 was identified as a stable and primary contributor to the adverse effects of multiple air pollutants on depressive symptoms. Following SO2, PM2.5, PM10, and CO also showed significant contributions, whereas the adverse impacts associated with NO2 were mostly mitigated and overshadowed by those of the other contaminants. The two-exposure model confirmed that the effect of SO2 on depressive symptoms was not affected by other pollutants and might have affected the effects of other pollutants. Comparable findings were found in studies in Hefei, China [62,63], where SO2 retained a strong effect even after adjustment for other air pollutants, which suggests its pivotal role in the mixture of air pollutants. In addition, Zhou et al. noted that the impacts of PM10 and NO2 were diminished after adjustment for SO2, rendering the association with NO2 statistically insignificant [59]. The dual-pollutant model also found that PM10 had a limited independent contribution to depressive symptoms when PM2.5 exposure levels were considered. Similarly, previous research indicated that finer particulate matter (PM2.5) is more detrimental to human health, and even the health risks associated with PM10 may primarily stem from its PM2.5 component [64,65]. Moreover, a case-crossover study found that PM2.5 had a more pronounced negative effect on hospital admissions linked to anxiety than PM10, which suggests a greater influence on anxiety [66]. Nevertheless, the published evidence regarding the relative contributions of each air pollutant and its combined effects on depression is limited. A cohort study in the United Kingdom that involved 389,185 participants examined the joint impacts of air pollutants and depressive disorders using air pollution scores derived from a principal component analysis [67]. The results revealed that prolonged exposure to various air pollutants was associated with an increased risk of mental disorders. Overall, the specific contribution of each air contaminant to long-term health impacts remains uncertain owing to limited evidence. This study suggests that SO2 may exhibit the most substantial long-term association with depressive symptoms among the various air pollutants, emphasizing the importance of reducing SO2 concentrations to a relatively low threshold in China's future air quality management strategies.
The mediation analyses in this study revealed that cognitive function, ADL, and IADL may underlie the relationship between air pollution and depressive symptoms, with cognitive function exerting a particularly robust mediating effect. Although epidemiological studies that investigated the mediating role of cognitive capacity in the impact of air pollution on mental health are limited, our findings confirm this view. A cross-sectional study showed that a composite cognitive score was a partial mediating factor in the relationship between exposure to PM2.5 and depressive symptoms [68]. Another longitudinal study that focused on older women arrived at a similar conclusion [69]. There is a paucity of evidence regarding the potential mediating roles of ADL and IADL in the link between air pollution and depression. However, an American study suggested that time spent on physical activity might partly explain the link between PM2.5 levels and depression [70]. In addition, a Chinese study identified both cognitive impairment and ADL limitation as risk factors of depression [71], which may lend some support to the notion that ADL and IADL are mediating functions, although further research is necessary to confirm this. Therefore, further investigation is required to fully understand the mediation effects of these factors on the relationship between air pollution and depression. Such insights are pivotal for clarifying the pathways through which air pollution may influence mental health and for crafting more precise and effective intervention strategies.
Compared with previous studies, this study represents a large-scale investigation into the combined impacts of multiple air pollutants on depressive symptoms among middle-aged and elderly individuals in China. It provides a comprehensive analysis by incorporating all six major air pollutants (CO, O3, SO2, NO2, PM10, and PM2.5) in relation to depressive symptoms while also considering various individual-level factors that could influence these associations. Furthermore, a multi-pollutant model was used to address the potential synergistic or additive effects and collinearity among air pollutants, thus characterizing the combined impacts of air pollution on depressive symptoms and discerning the specific contribution of each pollutant. Moreover, the potential mediating factors were assessed to provide evidence for the mechanism research.
This study has several limitations that should be mentioned. First, the reliance on self-reported information from the participant questionnaires might have potentially introduced information bias. For example, the CESD-10 scale, used as a screening tool, was based on subjective participant reports rather than clinical diagnoses, which could misrepresent the actual conditions. Second, the exposure levels to the air pollutants were assessed solely based on the participants' residential cities. Given the vast scale of Chinese cities, this approach may not account for regional variations within a single city and could potentially result in exposure misclassification. This limitation could affect the accuracy of our findings. Therefore, future studies should consider using exposure data with higher spatial resolution to more accurately assess the health effects of air pollution and capture spatial heterogeneity within cities. Third, the study did not consider other sources of indoor air pollutants, such as solid fuel used in households and smoking, which could lead to underestimating household air pollution levels. Finally, the findings might have limited generalizability, as the study population was restricted to middle-aged and elderly individuals, who are known to have a higher prevalence of depression.
5. Conclusion
In conclusion, this study identified a positive connection between long-term exposures to PM2.5, PM10, CO, and SO2 and the incidence of depressive symptoms. The findings suggest that cognitive function, ADL, and IADL may partially mediate this association. Among the air pollutants examined, SO2 was the primary and stable contributor to the increased risk of depressive symptoms, both in individual and combined exposures. The research presents increasing proof regarding the detrimental effects of airborne contaminants on mental health and thus advocates for specific interventions such as stricter regulations, along with improved air quality management, to bolster public mental health.
CRediT author contribution
Yuqing Hao: Writing - Original Draft, Methodology, Data Curation, Formal Analysis, Investigation. Longzhu Xu: Validation, Writing - Review & Editing. Meiyu Peng: Validation, Writing - Review & Editing. Zhugen Yang: Writing - Review & Editing. Weiqi Wang: Supervision, Methodology. Fanyu Meng: Writing - Review & Editing, Supervision.
Ethical approval
Approval for the original CHARLS was obtained from the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015), and all participants signed informed consent at the time of participation.
Data availability statement
The datasets that support this article are publicly available from the project of the China Health and Retirement Longitudinal Study (CHARLS) and China High Air Pollutants (CHAP) datasets.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge funding from Project LH2021E097, supported by the Natural Science Foundation of Heilongjiang Province, Project QMPT-2007, supported by Harbin Medical University, with the support of the China Scholarship Council.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2024.100515.
Contributor Information
Weiqi Wang, Email: 201901044@hrbmu.edu.cn.
Fanyu Meng, Email: mengfanyu1984@hrbmu.edu.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Ambient (outdoor) air pollution [https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health].
- 2.Hayes R.B., Lim C., Zhang Y., Cromar K., Shao Y., Reynolds H.R., Silverman D.T., Jones R.R., Park Y., Jerrett M., et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 2020;49(1):25–35. doi: 10.1093/ije/dyz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z., Cui L., Cui X., Li X., Yu K., Yue K., Dai Z., Zhou J., Jia G., Zhang J. The association between high ambient air pollution exposure and respiratory health of young children: a cross sectional study in Jinan, China. Sci. Total Environ. 2019;656:740–749. doi: 10.1016/j.scitotenv.2018.11.368. [DOI] [PubMed] [Google Scholar]
- 4.Afsar B., Elsurer Afsar R., Kanbay A., Covic A., Ortiz A., Kanbay M. Air pollution and kidney disease: review of current evidence. Clin. Kidney J. 2019;12(1):19–32. doi: 10.1093/ckj/sfy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Depressive disorder (depression) [https://www.who.int/news-room/fact-sheets/detail/depression].
- 6.Greenberg P.E., Fournier A.A., Sisitsky T., Simes M., Berman R., Koenigsberg S.H., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2010 and 2018) PharmacoEcon. 2021;39(6):653–665. doi: 10.1007/s40273-021-01019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braithwaite I., Zhang S., Kirkbride J.B., Osborn D.P.J., Hayes J.F. Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: a systematic review and meta-analysis. Environ. Health Perspect. 2019;127(12) doi: 10.1289/ehp4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borroni E., Pesatori A.C., Bollati V., Buoli M., Carugno M. Air pollution exposure and depression: a comprehensive updated systematic review and meta-analysis. Environ. Pollut. 2022;292(Pt A) doi: 10.1016/j.envpol.2021.118245. [DOI] [PubMed] [Google Scholar]
- 9.Newbury J.B., Arseneault L., Beevers S., Kitwiroon N., Roberts S., Pariante C.M., Kelly F.J., Fisher H.L. Association of air pollution exposure with psychotic experiences during adolescence. JAMA Psychiatr. 2019;76(6):614–623. doi: 10.1001/jamapsychiatry.2019.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu X., Guo T., Si Y., Wang J., Zhang W., Deng F., Chen L., Wei C., Lin S., Guo X., et al. Association between ambient air pollution and daily hospital admissions for depression in 75 Chinese cities. Am. J. Psychiatr. 2020;177(8):735–743. doi: 10.1176/appi.ajp.2020.19070748. [DOI] [PubMed] [Google Scholar]
- 11.Szyszkowicz M., Kousha T., Kingsbury M., Colman I. Air pollution and emergency department visits for depression: a multicity case-crossover study. Environ. Health Insights. 2016;10:155–161. doi: 10.4137/ehi.S40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F., Liu H., Li H., Liu J., Guo X., Yuan J., Hu Y., Wang J., Lu L. Ambient concentrations of particulate matter and hospitalization for depression in 26 Chinese cities: a case-crossover study. Environ. Int. 2018;114:115–122. doi: 10.1016/j.envint.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Kioumourtzoglou M.A., Power M.C., Hart J.E., Okereke O.I., Coull B.A., Laden F., Weisskopf M.G. The association between air pollution and onset of depression among middle-aged and older women. Am. J. Epidemiol. 2017;185(9):801–809. doi: 10.1093/aje/kww163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu X., Shi L., Kubzansky L.D., Wei Y., Castro E., Li H., Weisskopf M.G., Schwartz J.D. Association of long-term exposure to air pollution with late-life depression in older adults in the US. JAMA Netw. Open. 2023;6(2) doi: 10.1001/jamanetworkopen.2022.53668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan S.J., Heinrich J., Bloom M.S., Zhao T.Y., Shi T.X., Feng W.R., Sun Y., Shen J.C., Yang Z.C., Yang B.Y., et al. Ambient air pollution and depression: a systematic review with meta-analysis up to 2019. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.134721. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Zhao D., Hong Y.S., Chang Y., Ryu S., Kang D., Monteiro J., Shin H.C., Guallar E., Cho J. Long-term particulate matter exposure and onset of depression in middle-aged men and women. Environ. Health Perspect. 2019;127(7) doi: 10.1289/ehp4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K.N., Lim Y.H., Bae H.J., Kim M., Jung K., Hong Y.C. Long-term fine particulate matter exposure and major depressive disorder in a community-based urban cohort. Environ. Health Perspect. 2016;124(10):1547–1553. doi: 10.1289/ehp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei F., Wu M., Qian S., Li D., Jin M., Wang J., Shui L., Lin H., Tang M., Chen K. Association between short-term exposure to ambient air pollution and hospital visits for depression in China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138207. [DOI] [PubMed] [Google Scholar]
- 19.Ehsanifar M., Tameh A.A., Farzadkia M., Kalantari R.R., Zavareh M.S., Nikzaad H., Jafari A.J. Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol. Environ. Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 20.Fonken L.K., Xu X., Weil Z.M., Chen G., Sun Q., Rajagopalan S., Nelson R.J. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatr. 2011;16(10):987–995. doi: 10.1038/mp.2011.76. 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Qian X., Xing J., Wang J., Sun Y., Wang Q., Li H. Particulate matter triggers depressive-like response associated with modulation of inflammatory cytokine homeostasis and brain-derived neurotrophic factor signaling pathway in mice. Toxicol. Sci. 2018;164(1):278–288. doi: 10.1093/toxsci/kfy086. [DOI] [PubMed] [Google Scholar]
- 22.Salvi A., Patki G., Liu H., Salim S. Psychological impact of vehicle exhaust exposure: insights from an animal model. Sci. Rep. 2017;7(1):8306. doi: 10.1038/s41598-017-08859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petkus A.J., Resnick S.M., Wang X., Beavers D.P., Espeland M.A., Gatz M., Gruenewald T., Millstein J., Chui H.C., Kaufman J.D., et al. Ambient air pollution exposure and increasing depressive symptoms in older women: the mediating role of the prefrontal cortex and insula. Sci. Total Environ. 2022;823 doi: 10.1016/j.scitotenv.2022.153642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H., Liang X., Wang L., Wei J., Guo B., Zeng C., Feng S., Wang S., Yang X., Pan Y., et al. Role of metabolic risk factors in the relationship between ambient fine particulate matter and depressive symptoms: evidence from a longitudinal population study. Ecotoxicol. Environ. Saf. 2024;270 doi: 10.1016/j.ecoenv.2023.115839. [DOI] [PubMed] [Google Scholar]
- 25.Qiu T., Fang Q., Zeng X., Zhang X., Fan X., Zang T., Cao Y., Tu Y., Li Y., Bai J., et al. Short-term exposures to PM(2.5), PM(2.5) chemical components, and antenatal depression: exploring the mediating roles of gut microbiota and fecal short-chain fatty acids. Ecotoxicol. Environ. Saf. 2024;277 doi: 10.1016/j.ecoenv.2024.116398. [DOI] [PubMed] [Google Scholar]
- 26.Yin J., John A., Cadar D. Bidirectional associations of depressive symptoms and cognitive function over time. JAMA Netw. Open. 2024;7(6) doi: 10.1001/jamanetworkopen.2024.16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altuğ H., Fuks K.B., Hüls A., Mayer A.K., Tham R., Krutmann J., Schikowski T. Air pollution is associated with depressive symptoms in elderly women with cognitive impairment. Environ. Int. 2020;136 doi: 10.1016/j.envint.2019.105448. [DOI] [PubMed] [Google Scholar]
- 28.Zhou T., Li R., Shi Y., Tian G., Yan Y. The associations between sleep duration, cognitive function, and depressive symptoms: an analysis of Chinese adolescents from China Family Panel Studies. J. Affect. Disord. 2022;319:252–259. doi: 10.1016/j.jad.2022.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Allen J.L., Klocke C., Morris-Schaffer K., Conrad K., Sobolewski M., Cory-Slechta D.A. Cognitive effects of air pollution exposures and potential mechanistic underpinnings. Curr. Environ. Health Rep. 2017;4(2):180–191. doi: 10.1007/s40572-017-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brockmeyer S., D'Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl. Neurosci. 2016;7(1):24–30. doi: 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H. Determining the effect of air quality on activities of daily living disability: using tracking survey data from 122 cities in China. BMC Publ. Health. 2022;22(1):835. doi: 10.1186/s12889-022-13240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Q., Liu X., Liu Z. The impact of air pollution on physical disability in a middle-aged and older Chinese population using regression discontinuity design. Health Place. 2023;79 doi: 10.1016/j.healthplace.2022.102958. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X., Wang Y., Luo Y., Ding R., Shi Z., He P. Bidirectional, longitudinal associations between depressive symptoms and IADL/ADL disability in older adults in China: a national cohort study. BMC Geriatr. 2024;24(1):659. doi: 10.1186/s12877-024-05248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS) Int. J. Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Chen X., Yan Z. Depression in the house: the effects of household air pollution from solid fuel use among the middle-aged and older population in China. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo F., Guo L., Thapa A., Yu B. Social isolation and depression onset among middle-aged and older adults in China: moderating effects of education and gender differences. J. Affect. Disord. 2021;283:71–76. doi: 10.1016/j.jad.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Wei J., Li Z., Xue W., Sun L., Fan T., Liu L., Su T., Cribb M. The ChinaHighPM(10) dataset: generation, validation, and spatiotemporal variations from 2015 to 2019 across China. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106290. [DOI] [PubMed] [Google Scholar]
- 38.Wei J., Li Z., Cribb M., Huang W., Xue W., Sun L., Guo J., Peng Y., Li J., Lyapustin A., et al. Improved 1 km resolution PM2.5 estimates across China using enhanced space–time extremely randomized trees. Atmos. Chem. Phys. 2020;20(6):3273–3289. doi: 10.5194/acp-20-3273-2020. [DOI] [Google Scholar]
- 39.Wei J., Li Z., Lyapustin A., Sun L., Peng Y., Xue W., Su T., Cribb M. Reconstructing 1-km-resolution high-quality PM2.5 data records from 2000 to 2018 in China: spatiotemporal variations and policy implications. Rem. Sens. Environ. 2021;252 doi: 10.1016/j.rse.2020.112136. [DOI] [Google Scholar]
- 40.Wei J., Li Z., Wang J., Li C., Gupta P., Cribb M. Ground-level gaseous pollutants (NO2, SO2, and CO) in China: daily seamless mapping and spatiotemporal variations. Atmos. Chem. Phys. 2023;23(2):1511–1532. doi: 10.5194/acp-23-1511-2023. [DOI] [Google Scholar]
- 41.Wei J., Liu S., Li Z., Liu C., Qin K., Liu X., Pinker R.T., Dickerson R.R., Lin J., Boersma K.F., et al. Ground-level NO(2) surveillance from space across China for high resolution using interpretable spatiotemporally weighted artificial intelligence. Environ. Sci. Technol. 2022;56(14):9988–9998. doi: 10.1021/acs.est.2c03834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei J., Li Z., Li K., Dickerson R.R., Pinker R.T., Wang J., Liu X., Sun L., Xue W., Cribb M. Full-coverage mapping and spatiotemporal variations of ground-level ozone (O3) pollution from 2013 to 2020 across China. Rem. Sens. Environ. 2022;270 doi: 10.1016/j.rse.2021.112775. [DOI] [Google Scholar]
- 43.Gao X., Jiang M., Huang N., Guo X., Huang T. Long-term air pollution, genetic susceptibility, and the risk of depression and anxiety: a prospective study in the UK biobank cohort. Environ. Health Perspect. 2023;131(1) doi: 10.1289/ehp10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K.C., Lo Y.C., Liao C.C., Jou Y.Y., Huang H.B. Associations between symptoms of depression and air pollutant exposure among older adults: results from the taiwan longitudinal study on aging (TLSA) Front. Publ. Health. 2021;9 doi: 10.3389/fpubh.2021.779192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keil A.P., Buckley J.P., O'Brien K.M., Ferguson K.K., Zhao S., White A.J. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 2020;128(4) doi: 10.1289/ehp5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin H., Guo Y., Kowal P., Airhihenbuwa C.O., Di Q., Zheng Y., Zhao X., Vaughn M.G., Howard S., Schootman M., et al. Exposure to air pollution and tobacco smoking and their combined effects on depression in six low- and middle-income countries. Br. J. Psychiatr. 2017;211(3):157–162. doi: 10.1192/bjp.bp.117.202325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pun V.C., Manjourides J., Suh H. Association of ambient air pollution with depressive and anxiety symptoms in older adults: results from the NSHAP study. Environ. Health Perspect. 2017;125(3):342–348. doi: 10.1289/ehp494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szyszkowicz M., Rowe B.H., Colman I. Air pollution and daily emergency department visits for depression. Int. J. Occup. Med. Environ. Health. 2009;22(4):355–362. doi: 10.2478/v10001-009-0031-6. [DOI] [PubMed] [Google Scholar]
- 49.Lee H., Myung W., Jeong B.H., Choi H., Jhun B.W., Kim H. Short- and long-term exposure to ambient air pollution and circulating biomarkers of inflammation in non-smokers: a hospital-based cohort study in South Korea. Environ. Int. 2018;119:264–273. doi: 10.1016/j.envint.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.Y., Bang M., Wee J.H., Min C., Yoo D.M., Han S.M., Kim S., Choi H.G. Short- and long-term exposure to air pollution and lack of sunlight are associated with an increased risk of depression: a nested case-control study using meteorological data and national sample cohort data. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143960. [DOI] [PubMed] [Google Scholar]
- 51.Song J., Zheng L., Lu M., Gui L., Xu D., Wu W., Liu Y. Acute effects of ambient particulate matter pollution on hospital admissions for mental and behavioral disorders: a time-series study in Shijiazhuang, China. Sci. Total Environ. 2018;636:205–211. doi: 10.1016/j.scitotenv.2018.04.187. [DOI] [PubMed] [Google Scholar]
- 52.Sang N., Hou L., Yun Y., Li G. SO(2) inhalation induces protein oxidation, DNA-protein crosslinks and apoptosis in rat hippocampus. Ecotoxicol. Environ. Saf. 2009;72(3):879–884. doi: 10.1016/j.ecoenv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Yao G., Yun Y., Sang N. Differential effects between one week and four weeks exposure to same mass of SO2 on synaptic plasticity in rat hippocampus. Environ. Toxicol. 2016;31(7):820–829. doi: 10.1002/tox.22093. [DOI] [PubMed] [Google Scholar]
- 54.Hahad O., Lelieveld J., Birklein F., Lieb K., Daiber A., Münzel T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020;21(12) doi: 10.3390/ijms21124306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Block M.L., Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang Z., Xu C., Cao Y., Kan H.D., Chen R.J., Yao C.Y., Liu X.L., Xiang Y., Wu N., Wu L., et al. The association between short-term ambient air pollution and daily outpatient visits for schizophrenia: a hospital-based study. Environ. Pollut. 2019;244:102–108. doi: 10.1016/j.envpol.2018.09.142. [DOI] [PubMed] [Google Scholar]
- 58.Clougherty J.E. A growing role for gender analysis in air pollution epidemiology. Environ. Health Perspect. 2010;118(2):167–176. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y.M., Fan Y.N., Yao C.Y., Xu C., Liu X.L., Li X., Xie W.J., Chen Z., Jia X.Y., Xia T.T., et al. Association between short-term ambient air pollution and outpatient visits of anxiety: a hospital-based study in northwestern China. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111071. [DOI] [PubMed] [Google Scholar]
- 60.Wang N., Mengersen K., Tong S., Kimlin M., Zhou M., Wang L., Yin P., Xu Z., Cheng J., Zhang Y., et al. Short-term association between ambient air pollution and lung cancer mortality. Environ. Res. 2019;179(Pt A) doi: 10.1016/j.envres.2019.108748. [DOI] [PubMed] [Google Scholar]
- 61.Tian Y., Liu H., Liang T., Xiang X., Li M., Juan J., Song J., Cao Y., Wang X., Chen L., et al. Fine particulate air pollution and adult hospital admissions in 200 Chinese cities: a time-series analysis. Int. J. Epidemiol. 2019;48(4):1142–1151. doi: 10.1093/ije/dyz106. [DOI] [PubMed] [Google Scholar]
- 62.Zhang C., Ding R., Xiao C., Xu Y., Cheng H., Zhu F., Lei R., Di D., Zhao Q., Cao J. Association between air pollution and cardiovascular mortality in Hefei, China: a time-series analysis. Environ. Pollut. 2017;229:790–797. doi: 10.1016/j.envpol.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 63.Zhu F., Ding R., Lei R., Cheng H., Liu J., Shen C., Zhang C., Xu Y., Xiao C., Li X., et al. The short-term effects of air pollution on respiratory diseases and lung cancer mortality in Hefei: a time-series analysis. Respir. Med. 2019;146:57–65. doi: 10.1016/j.rmed.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 64.Lin H., Tao J., Du Y., Liu T., Qian Z., Tian L., Di Q., Rutherford S., Guo L., Zeng W., et al. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ. Pollut. 2016;208(Pt B):758–766. doi: 10.1016/j.envpol.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 65.Liu J., Yin H., Tang X., Zhu T., Zhang Q., Liu Z., Tang X., Yi H. Transition in air pollution, disease burden and health cost in China: a comparative study of long-term and short-term exposure. Environ. Pollut. 2021;277 doi: 10.1016/j.envpol.2021.116770. [DOI] [PubMed] [Google Scholar]
- 66.Yue J.L., Liu H., Li H., Liu J.J., Hu Y.H., Wang J., Lu L., Wang F. Association between ambient particulate matter and hospitalization for anxiety in China: a multicity case-crossover study. Int. J. Hyg Environ. Health. 2020;223(1):171–178. doi: 10.1016/j.ijheh.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Yang T., Wang J., Huang J., Kelly F.J., Li G. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatr. 2023;80(4):305–313. doi: 10.1001/jamapsychiatry.2022.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren T., Yu X., Yang W. Do cognitive and non-cognitive abilities mediate the relationship between air pollution exposure and mental health? PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petkus A.J., Wang X., Beavers D.P., Chui H.C., Espeland M.A., Gatz M., Gruenewald T., Kaufman J.D., Manson J.E., Resnick S.M., et al. Outdoor air pollution exposure and inter-relation of global cognitive performance and emotional distress in older women. Environ. Pollut. 2021;271 doi: 10.1016/j.envpol.2020.116282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang R., Liu Y., Xue D., Yao Y., Liu P., Helbich M. Cross-sectional associations between long-term exposure to particulate matter and depression in China: the mediating effects of sunlight, physical activity, and neighborly reciprocity. J. Affect. Disord. 2019;249:8–14. doi: 10.1016/j.jad.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Zhang F., Yang W. Interaction between activities of daily living and cognitive function on risk of depression. Front. Publ. Health. 2024;12 doi: 10.3389/fpubh.2024.1309401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets that support this article are publicly available from the project of the China Health and Retirement Longitudinal Study (CHARLS) and China High Air Pollutants (CHAP) datasets.