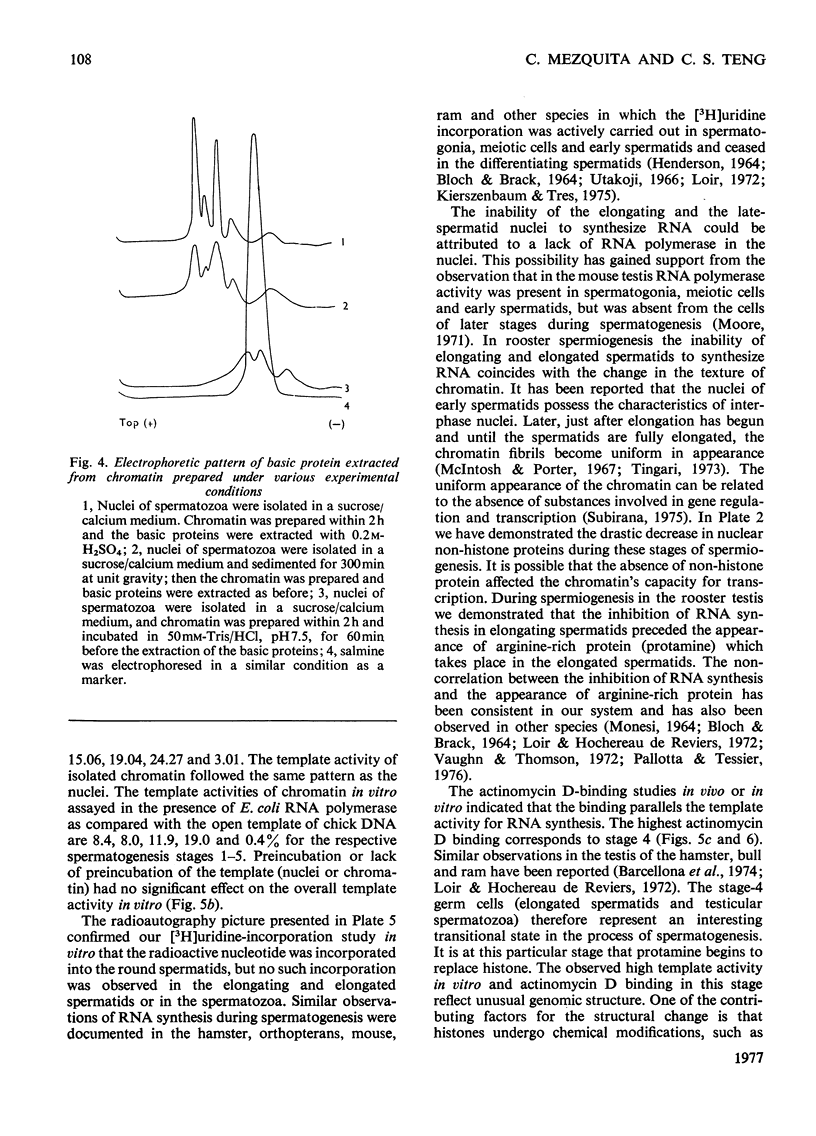
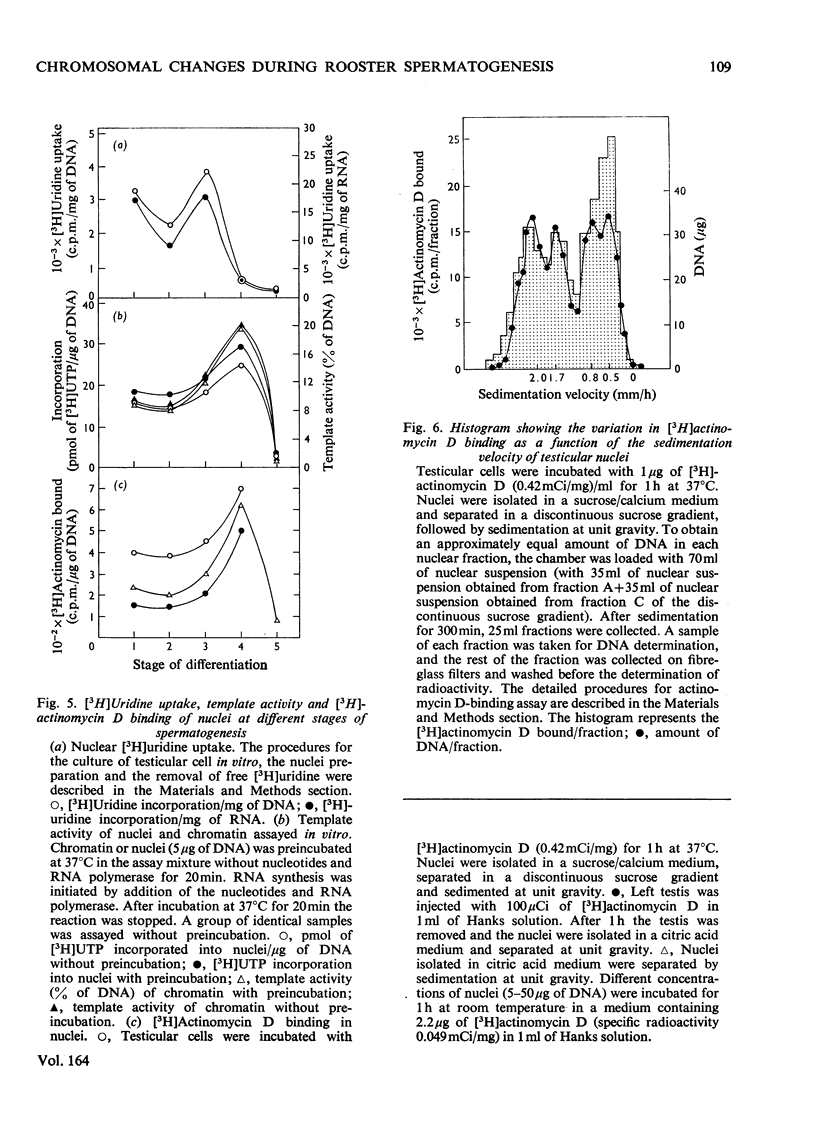
Abstract
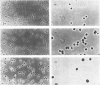
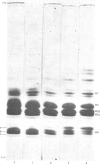
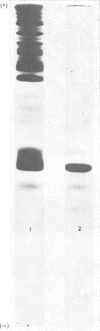
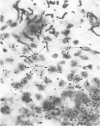
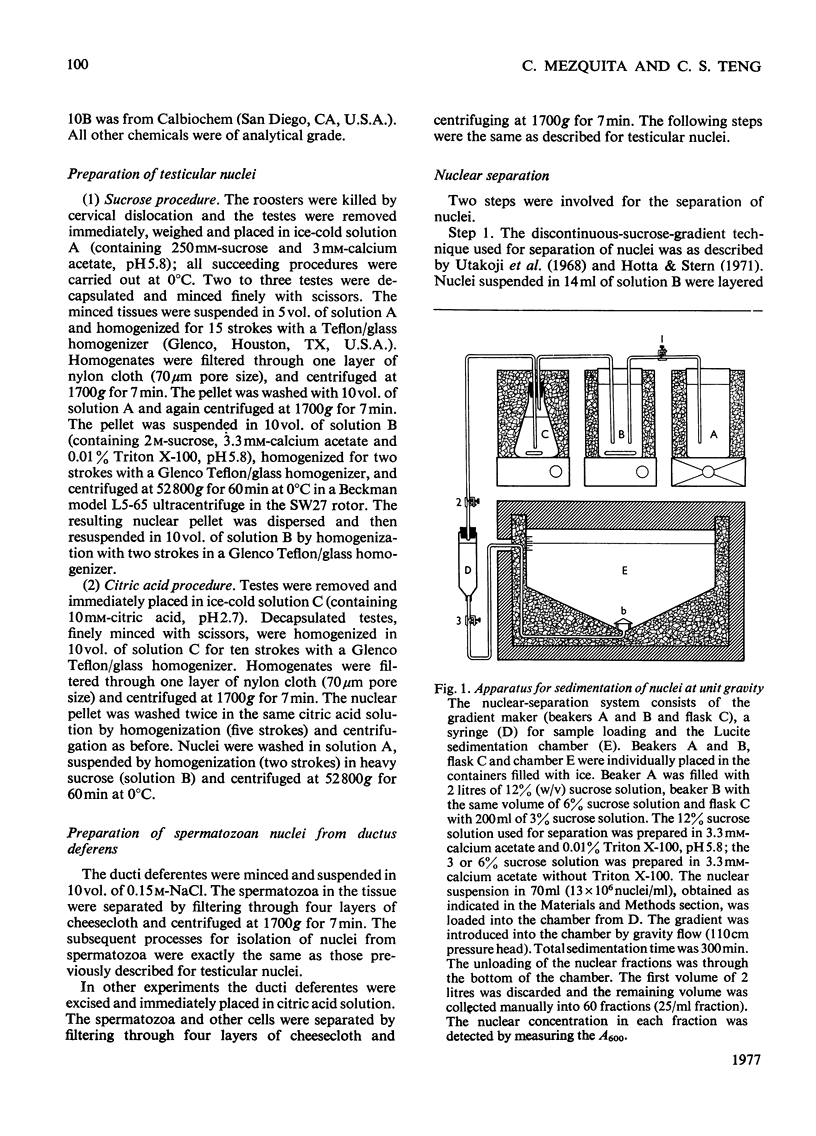
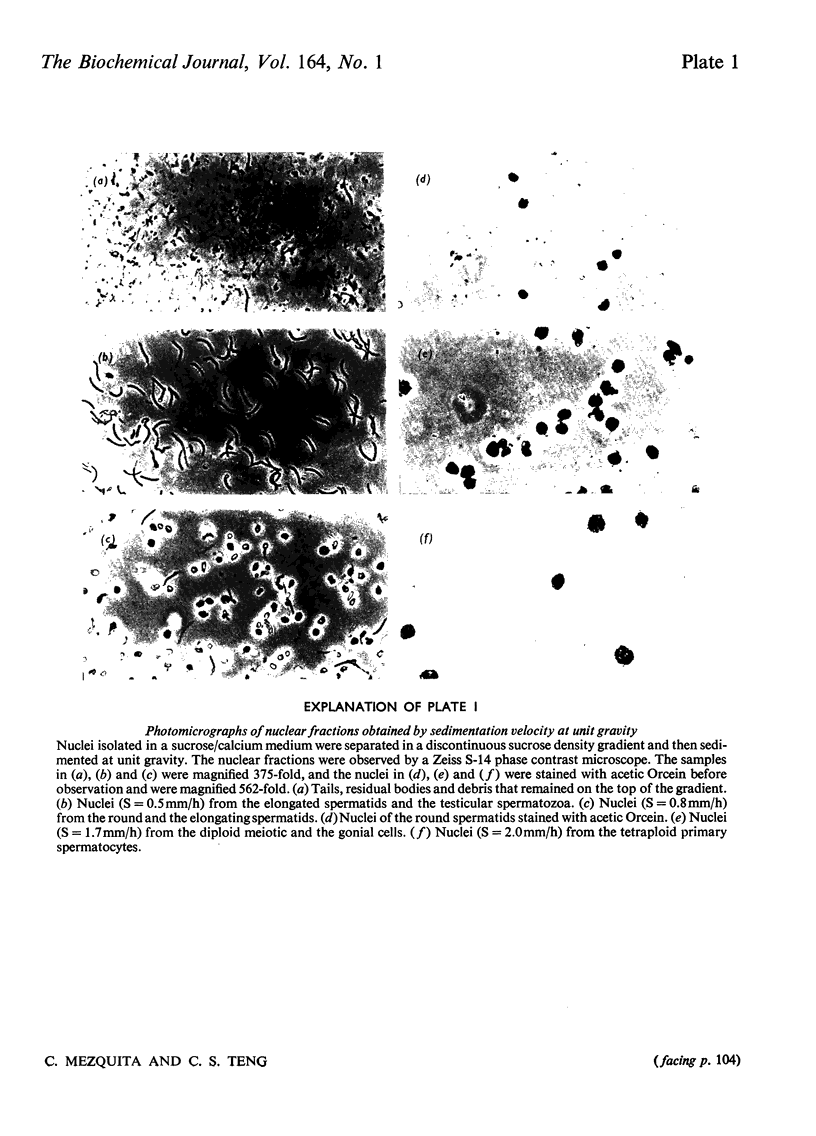
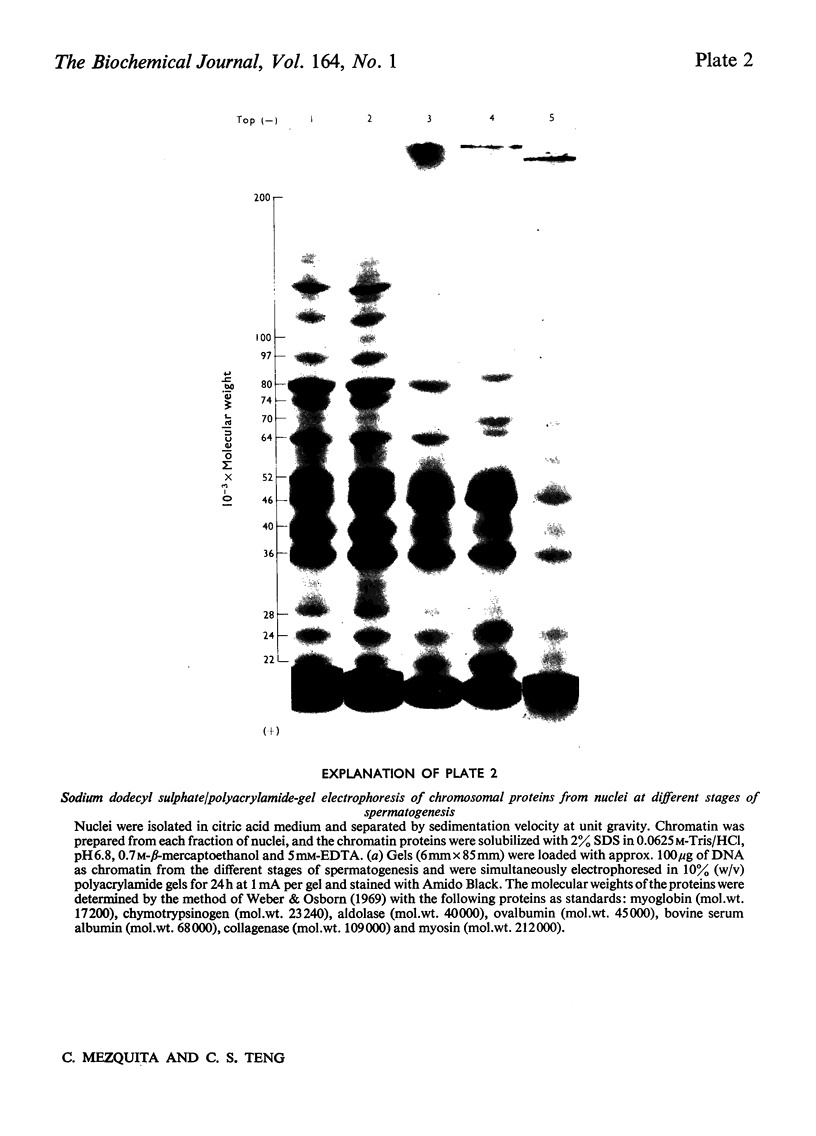
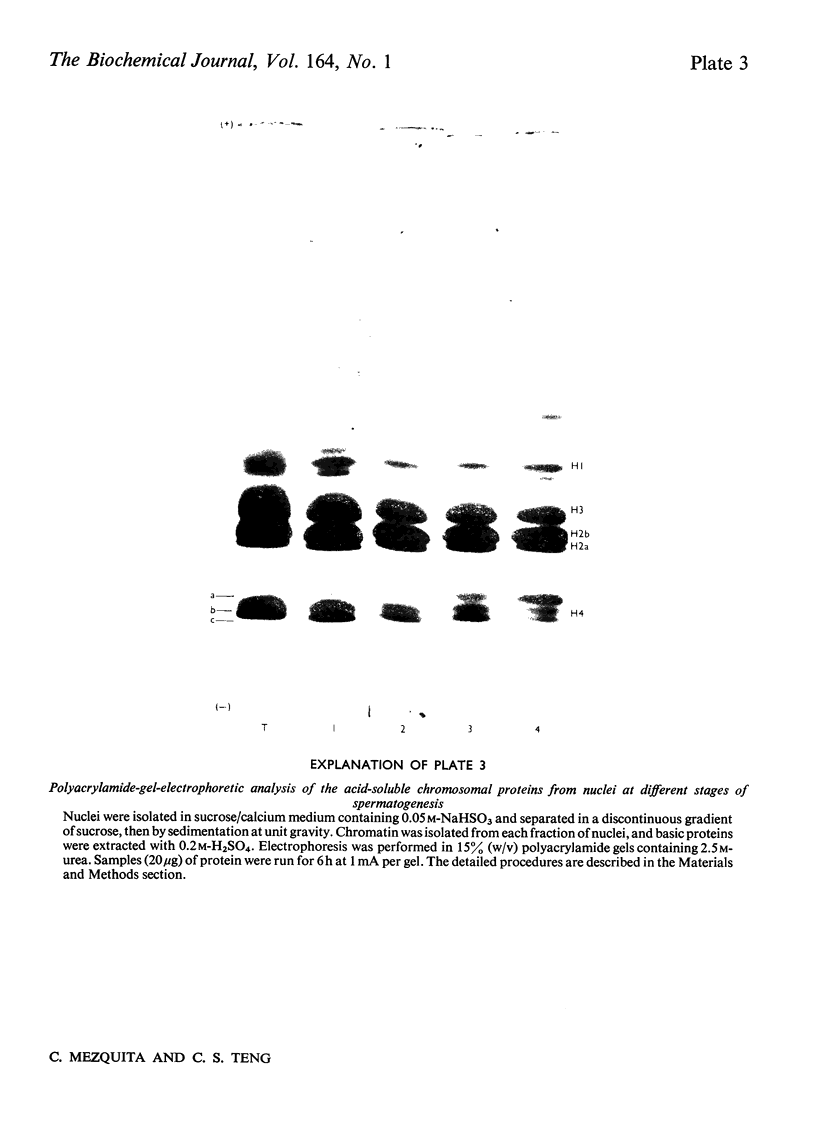
We developed a technique to separate nuclei of rooster testis by centrifugation through a discontinuous sucrose density gradient and by sedimentation at unit gravity. Four different major fractions obtained from testicular nuclei and one from the vas deferens were characterized according to their velocity of sedimentation, morphology and DNA content. The ratios (w/w) of basic proteins, non-histone proteins and RNA to DNA decreased during spermiogenesis both in nuclei and chromatin. Changes in the electrophoretic patterns of histones and non-histone proteins were detected especially in the elongated spermatids. The lack of uptake of [3H]uridine in elongating and elongated spermatids and in spermatozoa was demonstrated by radioautography and by the detection of labelled RNA extracted from different fractions of nuclei. Template activity for RNA synthesis and the binding of actinomycin D by testicular nuclei reached a peak in the elongated spermatid stage, when the histones are replaced by the protamine.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcellona W. J., Brackeen R. B., Brinkley B. R. Differential binding of tritiated actinomycin to the nuclei of mammalian spermatogenic cells in vivo. J Reprod Fertil. 1974 Jul;39(1):41–48. doi: 10.1530/jrf.0.0390041. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Dunn J. J. DNA-dependent RNA polymerase from phage T4 infected E. coli: an enzyme missing a factor required for transcription of T4 DNA. Biochem Biophys Res Commun. 1969 Jan 27;34(2):230–237. doi: 10.1016/0006-291x(69)90636-6. [DOI] [PubMed] [Google Scholar]
- Bellvé A. R., Anderson E., Hanley Bowdoin L. Synthesis and amino acid composition of basic proteins in mammalian sperm nuclei. Dev Biol. 1975 Dec;47(2):349–365. doi: 10.1016/0012-1606(75)90289-4. [DOI] [PubMed] [Google Scholar]
- Bernon D. E., Buckland R. B. The activity of some peptide hydrolase enzymes in fresh and stored poultry semen from full sib groups of males and their relationship to fertility. Poult Sci. 1975 Sep;54(5):1492–1498. doi: 10.3382/ps.0541492. [DOI] [PubMed] [Google Scholar]
- Brachet J., Hulin N. Binding of tritiated actinomycin and cell differentiation. Nature. 1969 May 3;222(5192):481–482. doi: 10.1038/222481a0. [DOI] [PubMed] [Google Scholar]
- Branson R. E., Grimes S. R., Jr, Yonuschot G., Irvin J. L. The histones of rat testis. Arch Biochem Biophys. 1975 Jun;168(2):403–412. doi: 10.1016/0003-9861(75)90269-6. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Hartree E. F. Comparison of neutral proteinase activities in cock and ram spermatozoa and observations on a proacrosin in cock spermatozoa. J Reprod Fertil. 1976 Jan;46(1):155–164. doi: 10.1530/jrf.0.0460155. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Trout testis cells. 3. Acetylation of histones in different cell types from developing trout testis. J Biol Chem. 1972 Sep 10;247(17):5506–5510. [PubMed] [Google Scholar]
- DALY M. M., MIRSKY A. E., RIS H. The amino acid composition and some properties of histones. J Gen Physiol. 1951 Mar 20;34(4):439–450. doi: 10.1085/jgp.34.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGMAN C. W., SPORN M. B. STUDIES ON CHROMATIN. I. ISOLATION AND CHARACTERIZATION OF NUCLEAR COMPLEXES OF DEOXYRIBONUCLEIC ACID, RIBONUCLEIC ACID, AND PROTEIN FROM EMBRYONIC AND ADULT TISSUES OF THE CHICKEN. J Biol Chem. 1964 Oct;239:3483–3492. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bolund L., Ringertz N. R. Actinomycin binding of normal and phytohaemagglutinin stimulated lymphocytes. Exp Cell Res. 1969 Apr;55(1):120–122. doi: 10.1016/0014-4827(69)90465-0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Gledhill B. L., Ringertz N. R. Changes in deoxyribonucleo-protein during spermiogenesis in the bull. 3 H-Actinomycin D binding capacity. Exp Cell Res. 1969 Dec;58(2):435–438. doi: 10.1016/0014-4827(69)90527-8. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Chae C-B, Irvin J. L. Acetylation of histones of rat testis. Arch Biochem Biophys. 1975 Jun;168(2):425–435. doi: 10.1016/0003-9861(75)90271-4. [DOI] [PubMed] [Google Scholar]
- Guraya S. S. Histochemistry of avian spermatogenesis. Acta Morphol Acad Sci Hung. 1970;18(2):139–146. [PubMed] [Google Scholar]
- Ho J. J., Meizel S. Electrophoretic detection of multiple forms of trypsin-like activity in spermatozoa of the domestic fowl. J Reprod Fertil. 1970 Oct;23(1):177–179. doi: 10.1530/jrf.0.0230177. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. Meiotic protein in spermatocytes of mammals. Nat New Biol. 1971 Nov 17;234(46):83–86. doi: 10.1038/newbio234083a0. [DOI] [PubMed] [Google Scholar]
- Hymer W. C., Evans W. H., Kraicer J., Mastro A., Davis J., Griswold E. Enrichment of cell types from the rat adenohypophysis by sedimentation at unit gravity. Endocrinology. 1973 Jan;92(1):275–287. doi: 10.1210/endo-92-1-275. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadohama N., Turkington R. W. Changes in acidic chromatin proteins during the hormone-dependent development of rat testis and epididymis. J Biol Chem. 1974 Oct 10;249(19):6225–6233. [PubMed] [Google Scholar]
- Kaye J. S., McMaster-Kaye R. Histones of spermatogenous cells in the house cricket. Chromosoma. 1974;46(4):397–419. doi: 10.1007/BF00331629. [DOI] [PubMed] [Google Scholar]
- Kaye J. S., McMaster-Kaye R. The fine structure and chemical composition of nuclei during spermiogenesis in the house cricket. I. Initial stages of differentiation and the loss of nonhistone protein. J Cell Biol. 1966 Oct;31(1):159–179. doi: 10.1083/jcb.31.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975 May;65(2):258–270. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaroo K. K., Jahnke G., Irvin J. L. Changes in basic chromosomal proteins during spermatogenesis in the mature rat. Arch Biochem Biophys. 1975 Jun;168(2):413–424. doi: 10.1016/0003-9861(75)90270-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loir M., Hochereau-de Reviers M. T. Deoxyribonucleoprotein changes in ram and bull spermatids. J Reprod Fertil. 1972 Oct;31(1):127–130. doi: 10.1530/jrf.0.0310127. [DOI] [PubMed] [Google Scholar]
- Loir M. Métabolisme de l'acide ribonucléique et des protéines dans les spermatocytes et les spermatides du bélier (Ovis aries). II. Variation de l'incorporation et devenir de la 3H-lysine, de la 3H-arginine et de la 35S-cystine. Ann Biol Anim Biochim Biophys. 1972;12(3):411–429. doi: 10.1051/rnd:19720306. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MIRSKY A. E., RIS H. The desoxyribonucleic acid content of animal cells and its evolutionary significance. J Gen Physiol. 1951 Mar 20;34(4):451–462. doi: 10.1085/jgp.34.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONESI V. RIBONUCLEIC ACID SYNTHESIS DURING MITOSIS AND MEIOSIS IN THE MOUSE TESTIS. J Cell Biol. 1964 Sep;22:521–532. doi: 10.1083/jcb.22.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick W. J., Maclean N., Baynes Y. A. RNA synthesis in chicken erythrocytes. Nat New Biol. 1972 Aug 2;238(83):137–139. doi: 10.1038/newbio238137a0. [DOI] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Marushige K., Marushige Y., Wong T. K. Complete displacement of somatic histones during transformation of spermatid chromatin: a model experiment. Biochemistry. 1976 May 18;15(10):2047–2053. doi: 10.1021/bi00655a004. [DOI] [PubMed] [Google Scholar]
- Marushige K., Ozaki H. Properties of isolated chromatin from sea urchin embryo. Dev Biol. 1967 Nov;16(5):474–488. doi: 10.1016/0012-1606(67)90060-7. [DOI] [PubMed] [Google Scholar]
- Marushige Y., Marushige K. Enzymatic unpacking of bull sperm chromatin. Biochim Biophys Acta. 1975 Sep 22;403(1):180–191. doi: 10.1016/0005-2744(75)90020-0. [DOI] [PubMed] [Google Scholar]
- McCOY T. A., MAXWELL M., KRUSE P. F., Jr Amino acid requirements of the Novikoff hepatoma in vitro. Proc Soc Exp Biol Med. 1959 Jan;100(1):115–118. doi: 10.3181/00379727-100-24542. [DOI] [PubMed] [Google Scholar]
- McIndoe W. M., Lake P. E. The distribution of some hydrolytic enzymes in the semen of the domestic fowl, Gallus domesticus. J Reprod Fertil. 1974 Oct;40(2):359–365. doi: 10.1530/jrf.0.0400359. [DOI] [PubMed] [Google Scholar]
- McIntosh J. R., Porter K. R. Microtubules in the spermatids of the domestic fowl. J Cell Biol. 1967 Oct;35(1):153–173. doi: 10.1083/jcb.35.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Moore G. P. DNA-dependent RNA synthesis in fixed cells during spermatogenesis in mouse. Exp Cell Res. 1971 Oct;68(2):462–465. doi: 10.1016/0014-4827(71)90176-5. [DOI] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Nakano M., Tobita T., Ando T. Studies on a protamine (galline) from fowl sperm. I. Fractionation and some characterization. Int J Pept Protein Res. 1973;5(3):149–159. doi: 10.1111/j.1399-3011.1973.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Von Hippel P. H. On the structure and stability of DNA-protamine and DNA-polypeptide complexes. J Mol Biol. 1968 Apr 14;33(1):265–281. doi: 10.1016/0022-2836(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Pallotta D., Tessier A. Amino acid composition of sperm histones in the house cricket Acheta domesticus. Can J Biochem. 1976 Jan;54(1):56–61. doi: 10.1139/o76-009. [DOI] [PubMed] [Google Scholar]
- Panyim S., Bilek D., Chalkley R. An electrophoretic comparison of vertebrate histones. J Biol Chem. 1971 Jul 10;246(13):4206–4215. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Paoletti R. A., Huang R. C. Characterization of sea urchin sperm chromatin and its basic proteins. Biochemistry. 1969 Apr;8(4):1615–1625. doi: 10.1021/bi00832a043. [DOI] [PubMed] [Google Scholar]
- Platz R. D., Grimes S. R., Meistrich M. L., Hnilica L. S. Changes in nuclear proteins of rat testis cells separated by velocity sedimentation. J Biol Chem. 1975 Aug 10;250(15):5791–5800. [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Littau V. C., Allfrey V. G. Changes in histone acetyl content and in nuclear non-histone protein composition of avian erythroid cells at different stages of maturation. J Biol Chem. 1974 Nov 25;249(22):7358–7368. [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Template properties of DNA-polypeptide complexes. J Mol Biol. 1970 Jun 14;50(2):333–344. doi: 10.1016/0022-2836(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Shires A., Carpenter M. P., Chalkley R. New histones found in mature mammalian testes. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2714–2718. doi: 10.1073/pnas.72.7.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subirana J. A. Studies on the thermal denaturation of nucleohistones. J Mol Biol. 1973 Mar 5;74(3):363–386. doi: 10.1016/0022-2836(73)90378-1. [DOI] [PubMed] [Google Scholar]
- Teng C. S. Nuclear acidic protein of the developing Oncopeltus embryos. Biochim Biophys Acta. 1974 Nov 6;366(4):385–395. doi: 10.1016/0005-2787(74)90036-7. [DOI] [PubMed] [Google Scholar]
- Teng N. H., Piatigorsky J., Ingram V. M. Histones of chick embryonic lens nuclei. Dev Biol. 1974 Nov;41(1):72–76. doi: 10.1016/0012-1606(74)90283-8. [DOI] [PubMed] [Google Scholar]
- Tingari M. D. Observations on the fine structure of spermatozoa in the testis and excurrent ducts of the male fowl, Gallus domesticus. J Reprod Fertil. 1973 Aug;34(2):255–265. doi: 10.1530/jrf.0.0340255. [DOI] [PubMed] [Google Scholar]
- Utakoji T. Chronology of nucleic acid synthesis in meiosis of the male Chinese hamster. Exp Cell Res. 1966 Jun;42(3):585–596. doi: 10.1016/0014-4827(66)90271-0. [DOI] [PubMed] [Google Scholar]
- VENDRELY C., KNOBLOCH A., VENDRELY R. Contribution à l'étude biochimique comparée de diverses désoxyribonucléoprotéines d'origine animale. Biochim Biophys Acta. 1956 Mar;19(3):472–479. doi: 10.1016/0006-3002(56)90470-x. [DOI] [PubMed] [Google Scholar]
- Vaughn J. C., Thomson L. A. A kinetic study of DNA and basic protein metabolism during spermatogenesis in the sand crab, Emerita analoga. J Cell Biol. 1972 Feb;52(2):322–337. doi: 10.1083/jcb.52.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangh L., Ruiz-Carrillo A., Allfrey V. G. Separation and analysis of histone subfractions differing in their degree of acetylation: some correlations with genetic activity in development. Arch Biochem Biophys. 1972 May;150(1):44–56. doi: 10.1016/0003-9861(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yanagimachi R., Teichman R. J. Cytochemical demonstration of acrosomal proteinase in mammalian and avian spermatozoa by a silver proteinate method. Biol Reprod. 1972 Feb;6(1):87–97. doi: 10.1093/biolreprod/6.1.87. [DOI] [PubMed] [Google Scholar]
- Zirkin B. R. The protein composition of nuclei during spermiogenesis in the leopard frog, Rana pipiens. Chromosoma. 1970;31(2):231–240. doi: 10.1007/BF00285150. [DOI] [PubMed] [Google Scholar]