1. Introduction
Meibomian Gland Dysfunction (MGD) is considered as a major etiological factor in the spectrum of dry eye diseases (DED), presenting as a chronic and multifaceted ocular pathology [1]. This condition is characterized by terminal duct obstruction and aberrant alterations in meibomian gland secretions [2]. The secretions, collectively termed meibum, play a pivotal role in maintaining the integrity of the tear film's lipid layer. Disruptions in the quality or quantity of meibum are foundational in the pathogenesis of evaporative DED.
MGD often accompanies ocular surface inflammation, encompassing conditions such as meibomitis and meibomitis-related keratoconjunctivitis (MRKC) [3,4]. This relationship of meibomian gland dysfunction and ocular surface inflammation has been the subject of scientific scrutiny. Among the various hypotheses proposed, the MRKC concept, as elucidated by Suzuki et al., offers a comprehensive perspective in understanding this confluence. MRKC is categorized into two distinct subtypes: the phlyctenular-type and the non-phlyctenular-type. The phlyctenular-type MRKC is characterized by a confluence of genetic predispositions, particularly the HLA-A26 and -DR8 alleles, and a heightened susceptibility to bacterial infections from species such as Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus and Propionibacterium acnes [[5], [6], [7], [8]]. This subtype is hypothesized to be driven by delayed-type hypersensitivity reactions, with Demodex infestation also implicated as a significant contributing factor [9]. In comparison, the non-phlyctenular-type MRKC predominantly involves an inflammatory cascade triggered by alterations in the tear film's lipid layer, leading to progressive ocular surface damage. This subtype underscores the intricate relationship between lipid layer integrity and ocular surface health [10].
The incorporation of Intense Pulsed Light (IPL) therapy in 2002, initially designed for dermatological use, marked a development in the therapeutic approach to MGD [11]. The utility of IPL in MGD management has been substantiated through its multifarious mechanisms of action, which include the destruction of superficial blood vessels, modulation of inflammatory processes, normalization of meibum consistency, and reduction of microbiome colonization [[12], [13], [14], [15], [16], [17], [18]]. Despite the established efficacy of IPL therapy in the management of MGD, a substantial gap persists in comprehending its optimal application. This is especially pertinent in the strategic integration of IPL therapy with other established therapeutic modalities such as anti-inflammation medications [11,[19], [20], [21], [22], [23]]. The clinical practice of combining topical anti-inflammatory agents, especially steroids which are commonly used by clinicians in the clinical setting when ocular surface inflammation is present, with IPL therapy for the treatment of MGD requires a thorough investigation of their potential synergistic or adverse effects. This study is designed to critically assess the efficacy of IPL therapy both as a stand-alone treatment and in combination with topical steroids, focusing on cases of MGD that are further complicated by ocular surface inflammation.
2. Methods
This retrospective comparative study was conducted at Eyejun Ophthalmic Clinic. The study was reviewed and approved by the Institutional Review Board of the Public Institutional Bioethics Committee (IRB approval number: P01-202401-01-024), adhering to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants.
2.1. Subjects
The study included consecutive patients diagnosed with MGD accompanied by ocular surface inflammation, treated with IPL therapy for the first time between April and July 2023. We excluded patients with corneal infiltration due to potential vision-threatening complications and the necessity for continuous steroid eye drop use. The inclusion criteria for IPL treatment were patients with MGD Stage 3 or higher, as defined by the International Workshop on Meibomian Gland Dysfunction in 2011 [2]. Subjects were divided into two groups: those receiving IPL treatment alone and those receiving IPL treatment in conjunction with steroid eye drops. Exclusion criteria included patients with prior dry eye treatments excluding lubricants, acute infections which was not related to MRKC, use of glaucoma eye drops, recent ophthalmic procedures within the past 6 months, and those receiving systemic immunotherapy for systemic diseases.
2.2. Treatment protocols
Patients underwent four sessions of IPL treatment at three-week intervals using the M22 machine (Lumenis Ltd., Yokneam, Israel). The treatment involved a 590-nm filter and a 6 mm cylindrical light guide, with fluence levels set between 12 and 19 J/cm2 based on Fitzpatrick skin types, following protocols established in previous studies. Both upper and lower eyelids were treated in each session, with the procedure repeated twice. Post-treatment, meibomian gland expression was performed immediately using eyelid compression forceps. Patients were advised to practice eyelid hygiene at home twice daily, following a 10-min hot massage. In the combination group, fluorometholone 0.1 % was administered twice daily.
2.3. Outcome measurements
The primary outcomes of this investigation were the changes in Meibomian Gland Expressibility (MGE) and Meibum Quality (MQ) between the two participant groups. This study used the more severe results from either the upper or lower eyelids, following the grading criteria established by the International Workshop on Meibomian Gland Dysfunction Guidelines [2]. Supplementary evaluations included the Ocular Surface Disease Index (OSDI) for quantifying symptoms, lid margin deformity, and tear meniscus height [24]. The Cornea and Contact Lens Research Unit (CCLRU) grading scales were utilized for assessing bulbar/palpebral conjunctival redness and palpebral conjunctival roughness using white light reflex as a diagnostic tool of ocular surface inflammation [25]. The CCLRU grading scale used images from anterior segment photography according to the guideline, and the average value of three readings of the same image was used. The inter-/intra-class coefficients (ICC) of bulbar/palpebral conjunctival redness and palpebral conjunctival roughness were obtained by two-way mixed model, and were 0.816, 0.723, and 0.671, respectively. Furthermore, the SICCA score was implemented to evaluate corneal and conjunctival erosions [26].
2.4. Statistical analyses
The assessment of data normality was conducted employing the Kolmogorov–Smirnov test, and all p-values of the continuous variables or ranked variables over 3 levels were greater than 0.05. Baseline characteristics of the study cohorts were compared utilizing an independent t-test for numerical data and chi-square analysis for categorical data. To evaluate the differences in MGE and MQ between groups, a repeated measures ANOVA was implemented. Subsequent post-hoc analyses were performed applying Tukey's Honest Significant Difference (HSD) test. A p-value threshold of less than 0.05 was established for determining statistical significance.
3. Results
In our study, we compared the baseline demographics and clinical characteristics of patients who received IPL alone (n = 238) and those received IPL combined with topical steroid (n = 260). Our analysis revealed no significant differences between the two groups in terms of age, sex, predisposing factors, clinical parameters including OSDI scores, meibomian gland indices, tear meniscus height, and SICCA scores (Table 1).
Table 1.
Baseline demographics and characteristics of IPL alone group and IPL with topical steroid group.
| IPL alone (n = 238) | IPL + steroid (n = 260) | p-value | |
|---|---|---|---|
| Age (years) | 50.29 ± 16.04 | 50.43 ± 14.63 | 0.919 |
| Sex (n, % of female) | 184 (77.3) | 186 (71.5) | 0.151 |
| Predisposing factors (n, %) | |||
| Allergy | 120 (40.4) | 136 (42.3) | 0.720 |
| Rosacea | 71 (29.8) | 62 (23.8) | 0.132 |
| Sjogren syndrome | 12 (5.0) | 14 (5.4) | 0.864 |
| Acne medication | 10 (4.2) | 11 (4.2) | 0.987 |
| Hormonal treatment | 6 (2.5) | 5 (1.9) | 0.650 |
| OSDI (scores) | 28.53 ± 7.16 | 27.62 ± 8.13 | 0.518 |
| Meibomian gland indices | |||
| Meibomian gland expressibility (grades) | 2.90 ± 1.06 | 2.79 ± 1.12 | 0.262 |
| Meibum quality (grades) | 2.99 ± 1.18 | 2.83 ± 1.32 | 0.221 |
| Lid margin deformity (n, %) | |||
| Vascular enlargement | 142 (59.7) | 169 (65.0) | 0.219 |
| Notching | 36 (15.1) | 40 (15.3) | 0.936 |
| Plugging | 47 (19.7) | 52 (20.0) | 0.944 |
| Mucocutaneous junction shift | 60 (25.2) | 77 (29.6) | 0.271 |
| Tear meniscus height (nm) | 207.21 ± 31.96 | 205.60 ± 32.07 | 0.575 |
| SICCA (scores) | 1.24 ± 0.41 | 1.30 ± 0.38 | 0.091 |
| CCLRU grading scale (grades) | |||
| Bulbar redness | 2.71 ± 1.23 | 2.73 ± 1.15 | 0.851 |
| Palpebral conjunctival redness | 2.85 ± 1.63 | 2.84 ± 1.73 | 0.947 |
| Palpebral conjunctival roughness | 2.23 ± 1.01 | 2.35 ± 0.89 | 0.159 |
∗ Statistically significance.
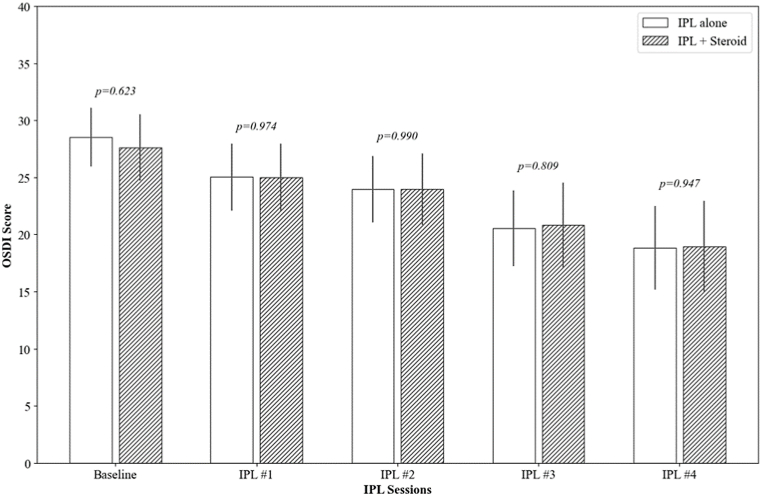
At baseline, the OSDI scores were comparable between the IPL alone group (28.53 ± 7.16) and the IPL combined with steroid group (27.62 ± 8.13), with no significant difference (p = 0.518). Over the course of the treatment, both groups showed a significant reduction in OSDI scores. After the first IPL session, the reduction in OSDI scores was statistically significant in both groups (−3.50 ± 4.36, p = 0.010 and −2.64 ± 5.62, p = 0.031, respectively). This trend of improvement continued progressively through the subsequent sessions, with both groups showing a significant decrease in OSDI scores by the fourth session (−9.71 ± 12.31, p < 0.001 and −8.66 ± 10.32, p < 0.001, respectively). There were no significant differences in the OSDI score reductions between the two groups at any of the follow-up points (p = 0.352) (Fig. 1).
Fig. 1.
The changes in the Ocular Surface Disease Index (OSDI) over IPL sessions. The p-value was calculated by repeated measures ANOVA with Tuckey's HSD post hoc test at each follow-up point to compare the IPL alone group and the IPL combined with topical steroid group.
∗ Statistically significance.
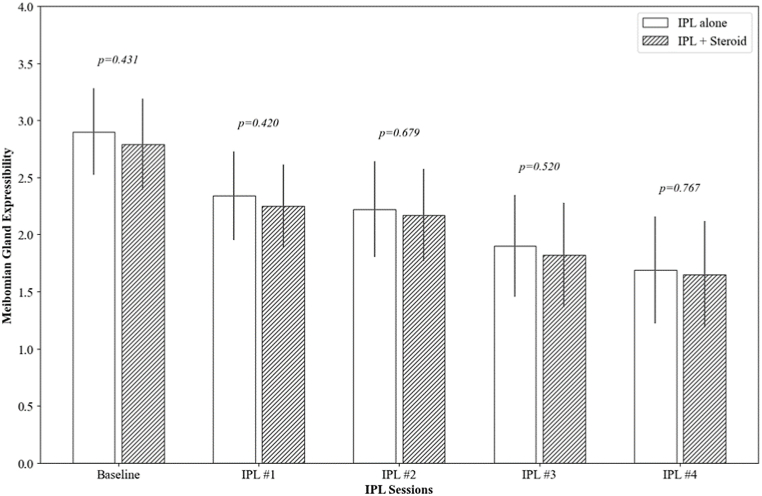
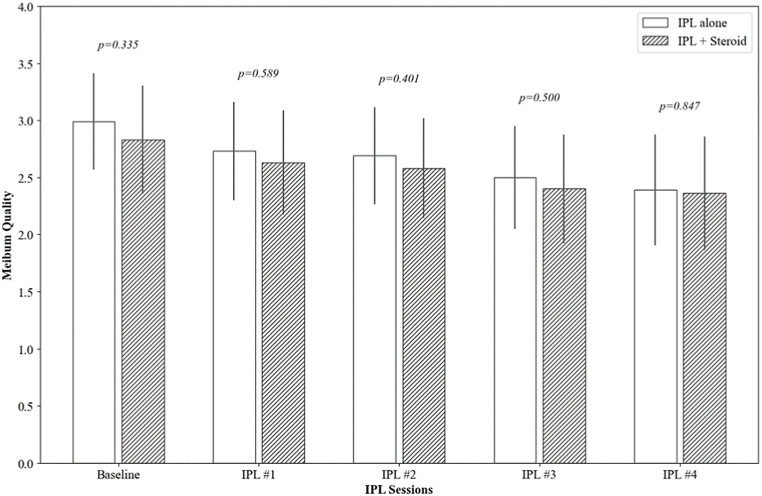
Both groups showed significant improvement in MGE from their respective baselines (2.90 ± 1.06 and 2.79 ± 1.12, respectively) to the fourth session (1.69 ± 1.31 and 1.65 ± 1.30, respectively), with each follow-up session showing statistically significant improvement (p < 0.001). Similarly, MQ scores also improved in both groups, from 2.99 ± 1.18 to 2.39 ± 1.36 in the IPL alone group and from 2.83 ± 1.32 to 2.36 ± 1.39 in the IPL combined with steroid group, with significant improvements noted in the later sessions (p < 0.001 at the fourth session for both groups). Notably, there were no significant differences between the two treatment groups in the degree of improvement in either MGE or MQ at any of the time points (p = 0.256 and 0.184, respectively) (Fig. 2).
Fig. 2.
The changes in meibomian gland expressibility (MGE) and meibum quality (MQ) over IPL sessions. The p-value was calculated by repeated measures ANOVA with Tuckey's HSD post hoc test at each follow-up point to compare the IPL alone group and the IPL combined with topical steroid group.
∗ Statistically significance.
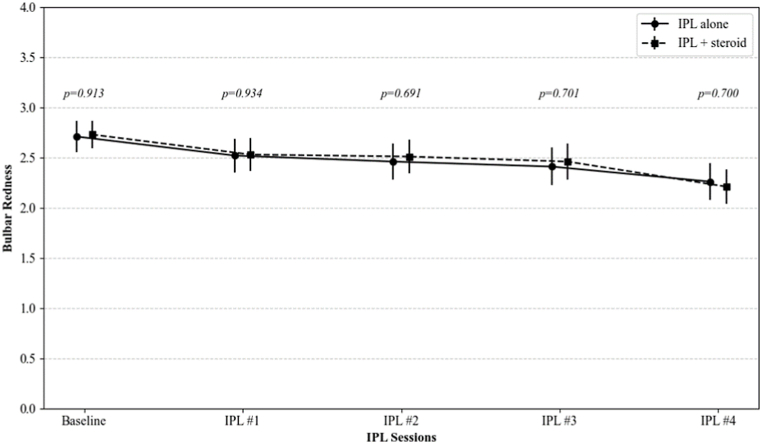
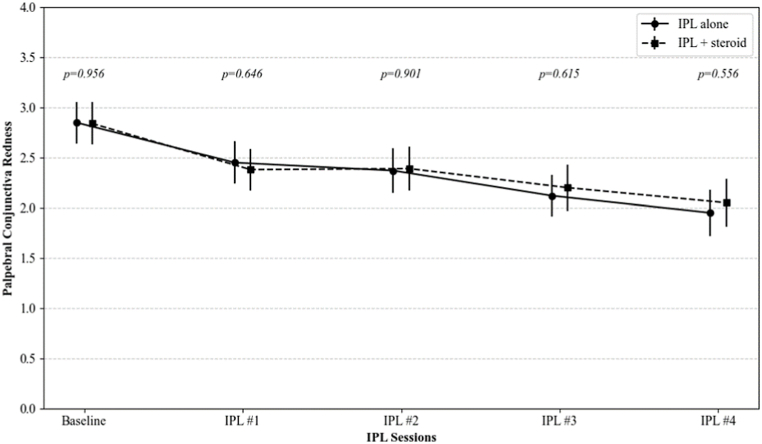
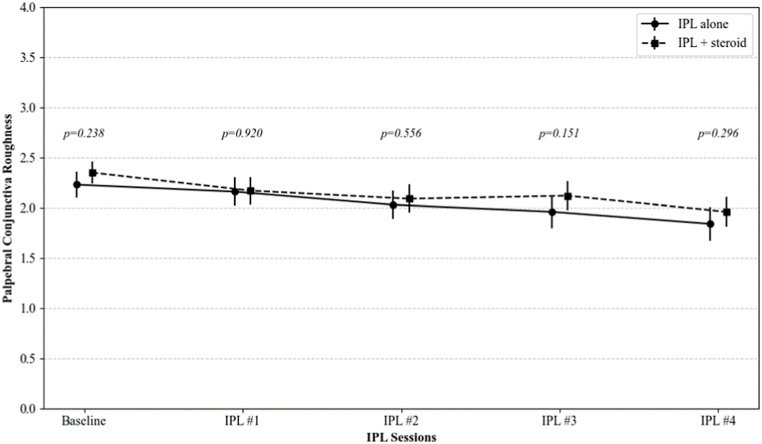
Bulbar and palpebral conjunctival redness, and palpebral conjunctival roughness were significantly decreased after IPL treatments (from 2.71, 2.85, and 2.23 to 2.26, 1.99, and 1.83 in IPL group, and from 2.73, 2.84, and 2.35 to 2.22, 2.05, and 1.98 in IPL combined with topical steroid group, respectively; all p < 0.001 at the fourth session for both groups). There were no significant differences in the changes of these CCLRU indicators between the two groups at any of the follow-up points (p = 0.700, 0.556, and 0.296, respectively) (Fig. 3).
Fig. 3.
The changes in the CCLRU grading scale on bulbar redness, palpebral conjunctival redness, and palpebral conjunctival roughness over IPL sessions. The p-value was calculated by repeated measures ANOVA with Tuckey's HSD post hoc test at each follow-up point to compare the IPL alone group and the IPL combined with topical steroid group.
∗ Statistically significance.
Regarding subjective discomfort and objective complications, 9 patients (3.8 %) in the IPL alone group and 11 patients (4.2 %) in the IPL combined with steroid group reported subjective concerns of skin pigmentation. In addition, 3 patients from each group reported skin erythema and associated edema. A total of 2.1 % (n = 5) in the IPL alone group and 2.3 % (n = 6) in the combined group, experienced transient eye discomfort and redness following the IPL treatment, which resolved spontaneously within one week. In the group receiving IPL combined with steroid, there were 18 patients (6.9 %) had an increase in intraocular pressure greater than 5 mmHg at the end of the treatment sessions. This condition improved with discontinuation of steroid eye drops and the short-term use of antiglaucoma eye drops.
4. Discussion
The aim of this research was to describe the differences in symptomatology and clinical manifestations between monotherapy with IPL and its combination with corticosteroids. Our findings reveal no significant discrepancy in the efficacy of these treatments in terms of both patient-reported symptoms and clinical parameters of MGD. Notably, complications associated with corticosteroids were exclusively observed in the combination therapy group.
Our results for patient symptom and meibomian gland functionality align with previous study [27,28]. Additionally, prior research has explored the effects of IPL on conjunctival redness [29]. Although a trend toward reduced conjunctival redness was observed after three IPL sessions spaced two weeks apart, the reduction did not reach statistical significance. In the present study, all three clinical markers of conjunctival inflammation demonstrated statistically significant changes. However, the extent of improvement was modest, with only a 0.5-grade reduction over four sessions conducted at three-week intervals. These findings raise questions about the clinical relevance of using these indicators to assess the therapeutic effect of each IPL session.
IPL therapy offers a dual modality in inflammation modulation: directly through the obliteration of superficial telangiectasias and cytokine modulation, and indirectly through improvements in the quantity and quality of meibum [13,18,30]. Despite the established anti-inflammatory properties of IPL, it is frequently combined with anti-inflammatory agents, and research on the adjunctive therapies remains limited [11,[19], [20], [21]]. To the best of our knowledge, this study is the first to demonstrate that long-term adjunctive use of topical steroids does not improve the therapeutic efficacy of IPL.
This study has several limitations. First, as a retrospective design, it is prone to potential biases and limits causal inference. Second, ocular surface inflammation was evaluated using the CCLRU grading scales, which, despite showing substantial agreement (ICC 0.6–0.8), are subjective. More objective methods, such as tear cytokine analysis, are needed. Third, this study focused on long-term adjunctive steroid use, without evaluating short-term use within the first week, which warrants caution in interpretation as early steroid effects may have been overlooked.
In conclusion, IPL alone did not show a significant difference in symptomatic relief or clinical improvement in MGD compared to its concurrent use with conjugated steroid therapy during IPL sessions. Therefore, the continual use of corticosteroid eye drops for additional anti-inflammatory effect in conjunction with IPL does not appear advisable, especially considering the associated complications.
CRediT authorship contribution statement
Hyunmin Ahn: Writing – original draft, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jae Lim Chung: Validation, Resources, Funding acquisition. Young Jun Kim: Resources, Funding acquisition. Ikhyun Jun: Validation. Tae-im Kim: Validation. Kyoung Yul Seo: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and financial disclosure
a. Funding/support: This research was supported by Korea Mouse Phenotyping Project (RS-2024-00400118) from the Ministry of Science and ICT through the National Research Foundation.
b. Financial Disclosures: The authors have no financial disclosures related to this manuscript.
References
- 1.Craig J.P., Nichols K.K., Akpek E.K., et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Nichols K.K., Foulks G.N., Bron A.J., et al. The international workshop on meibomian gland dysfunction: executive summary. Invest. Ophthalmol. Vis. Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki T. Inflamed obstructive meibomian gland dysfunction causes ocular surface inflammation. Invest. Ophthalmol. Vis. Sci. 2018;59(14):DES94–DES101. doi: 10.1167/iovs.17-23345. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T. Meibomitis-related keratoconjunctivitis: implications and clinical significance of meibomian gland inflammation. Cornea. 2012;31:S41–S44. doi: 10.1097/ICO.0b013e31826a04dd. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty J.M., McCULLEY J.P. Comparative bacteriology of chronic blepharitis. Br. J. Ophthalmol. 1984;68(8):524–528. doi: 10.1136/bjo.68.8.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mates A. Characterization and properties of purified Staphylococcus aureus lipase. Microbios. 1974;9(33):61–74. [PubMed] [Google Scholar]
- 7.Singer T.R., Isenberg S.J., Apt L. Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br. J. Ophthalmol. 1988;72(6):448–451. doi: 10.1136/bjo.72.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T., Sano Y., Sasaki O., Kinoshita S. Ocular surface inflammation induced by Propionibacterium acnes. Cornea. 2002;21(8):812–817. doi: 10.1097/00003226-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Forton F., Germaux M.-A., Brasseur T., et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J. Am. Acad. Dermatol. 2005;52(1):74–87. doi: 10.1016/j.jaad.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Bron A.J., de Paiva C.S., Chauhan S.K., et al. Tfos dews ii pathophysiology report. Ocul. Surf. 2017;15(3):438–510. doi: 10.1016/j.jtos.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Toyos R., McGill W., Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomedicine and laser surgery. 2015;33(1):41–46. doi: 10.1089/pho.2014.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papageorgiou P., Clayton W., Norwood S., Chopra S., Rustin M. Treatment of rosacea with intense pulsed light: significant improvement and long‐lasting results. Br. J. Dermatol. 2008;159(3):628–632. doi: 10.1111/j.1365-2133.2008.08702.x. [DOI] [PubMed] [Google Scholar]
- 13.Bäumler W., Vural E., Landthaler M., Muzzi F., Shafirstein G. The effects of intense pulsed light (IPL) on blood vessels investigated by mathematical modeling. Laser Surg. Med.: The Official Journal of the American Society for Laser Medicine and Surgery. 2007;39(2):132–139. doi: 10.1002/lsm.20408. [DOI] [PubMed] [Google Scholar]
- 14.Borchman D., Foulks G.N., Yappert M.C., et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest. Ophthalmol. Vis. Sci. 2011;52(6):3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul. Surf. 2019;17(2):360–364. doi: 10.1016/j.jtos.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto V.G., Sadick N.S., Lloreta J., Nicholson J., Shea C.R. Effects of intense pulsed light on sun‐damaged human skin, routine, and ultrastructural analysis. Laser Surg. Med.: The Official Journal of the American Society for Laser Medicine and Surgery. 2002;30(2):82–85. doi: 10.1002/lsm.10042. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Song N., Gong L. Therapeutic effect of intense pulsed light on ocular demodicosis. Curr. Eye Res. 2019;44(3):250–256. doi: 10.1080/02713683.2018.1536217. [DOI] [PubMed] [Google Scholar]
- 18.Liu R., Rong B., Tu P., et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am. J. Ophthalmol. 2017;183:81–90. doi: 10.1016/j.ajo.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Giannaccare G., Taroni L., Senni C., Scorcia V. Intense pulsed light therapy in the treatment of meibomian gland dysfunction: current perspectives. Clin. Optom. 2019:113–126. doi: 10.2147/OPTO.S217639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arita R., Mizoguchi T., Fukuoka S., Morishige N. Multicenter study of intense pulsed light therapy for patients with refractory meibomian gland dysfunction. Cornea. 2018;37(12):1566–1571. doi: 10.1097/ICO.0000000000001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vora G.K., Gupta P.K. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr. Opin. Ophthalmol. 2015;26(4):314–318. doi: 10.1097/ICU.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 22.Ballesteros-Sanchez A., Gargallo-Martinez B., Sánchez-González M.C., Sanchez-Gonzalez J.-M. Intense pulse light combined with low-level light therapy in dry eye disease: a systematic review. Eye Contact Lens. 2023;49(1):8–13. doi: 10.1097/ICL.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 23.Ballesteros-Sánchez A., Sánchez-González J.-M., Gutiérrez-Ortega R., Gargallo-Martínez B. Diamond Bur Microblepharoexfoliation combined with intense pulse light and meibomian gland expression for evaporative dry eye: a short-term controlled clinical trial. Ophthalmology and Therapy. 2024;13(5):1223–1237. doi: 10.1007/s40123-024-00919-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffman R.M., Christianson M.D., Jacobsen G., Hirsch J.D., Reis B.L. Reliability and validity of the ocular surface disease index. Arch. Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 25.Efron N., Morgan P.B., Katsara S.S. Validation of grading scales for contact lens complications. Ophthalmic Physiol. Opt. 2001;21(1):17–29. [PubMed] [Google Scholar]
- 26.Whitcher J.P., Shiboski C.H., Shiboski S.C., et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren's Syndrome International Registry. Am. J. Ophthalmol. 2010;149(3):405–415. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi M., Han S.J., Ji Y.W., et al. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci. Rep. 2019;9(1):7648. doi: 10.1038/s41598-019-44000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tashbayev B., Yazdani M., Arita R., Fineide F., Utheim T.P. Intense pulsed light treatment in meibomian gland dysfunction: a concise review. Ocul. Surf. 2020;18(4):583–594. doi: 10.1016/j.jtos.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Hergueta M.C., Alió del Barrio J.L., Canto-Cerdan M., Amesty M.A. Efficacy and safety of intense pulsed light direct eyelid application. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-17986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor M., Porter R., Gonzalez M. Intense pulsed light may improve inflammatory acne through TNF-α down-regulation. J. Cosmet. Laser Ther. 2014;16(2):96–103. doi: 10.3109/14764172.2013.864198. [DOI] [PubMed] [Google Scholar]