Abstract
Small-Cell Lung Cancer (SCLC) accounts for 15 % of all lung cancer cases. Due to comorbidities and its aggressive behaviour, patients are highly symptomatic even at a non-metastatic stage. Standardizing the care pathway and introducing a simultaneous care approach could address the unmet needs of this disease. However, there is no uniformity in the involvement of palliative care physicians and in the simultaneous use of palliative care and cancer therapies. In this context, 13 experts from Lazio region undertook a process to deepen their understanding of the regional provision of simultaneous care to patients with SCLC by structuring and carrying out a survey. The survey covered 35–52 % of SCLC cases in the region, confirming heterogeneous results in both management and therapeutic approach, particularly in the area of simultaneous care. The working group met to define practical recommendations with the aim of standardizing the management of this disease.
Keywords: Small-cell lung cancer, Simultaneous care, Multidisciplinary team, Diagnostic and therapeutic care pathways
Highlights
-
•
The survey covered approximately 35–52 % of SCLC cases in the Lazio region.
-
•
A multidisciplinary team for lung cancer wasn't formalized in 25 % of hospitals.
-
•
A comprehensive assessment of patient needs was not conducted in 40 % of hospitals.
-
•
Monitoring systems for KPIs were absent in 75 % of hospitals.
-
•
38 % of healthcare professionals had not received training in simultaneous care.
1. Introduction
In 2020, approximately 41,000 new cases of lung cancer were estimated in Italy, of which Small-Cell Lung Cancer (SCLC) accounted for 10–15 % [1]. SCLC is a neuroendocrine tumour characterized by a high proliferative index and a rapid tendency to metastasize. As a result, 70–80 % of patients are diagnosed at an advanced stage (AS) of the disease, according to the Veterans Administration Lung Study Group (VALG) staging system [2,3]. The prognosis of SCLC is generally poor, with a median overall survival (OS) of less than 12 months [4].
Due to comorbidities and the aggressive nature of the disease, patients are often highly symptomatic even when diagnosed at a limited stage (LS); because of these characteristics, SCLC is one of the most pathologically and care-intensive neoplastic diseases, and therefore requires, in most cases, a multidisciplinary management approach.
Given the complex clinical picture, it is also essential to benefit from a care pathway leading to early diagnosis and an effective time management of all stages of the disease. Simultaneous care can address the unmet needs of the pathology by combining cancer treatment with radiotherapy, palliative care, psychological support, nutritional and integrative support, physical rehabilitation, holistic approaches, and smoking cessation programs. The main aim of simultaneous care is to improve the quality of life (QoL) of patients and families facing incurable diseases, through prevention, early identification, and relief of suffering and pain, physical, psychosocial and spiritual problems.
To date, in Italy, simultaneous care represents the paradigm for providing the best care for patients with advanced and/or metastatic cancer. Such paradigm is also recommended by the Working Group of the Italian Association of Medical Oncology (AIOM) and the Italian Society of Palliative Care (SICP) [5]. Standardization of this paradigm can create efficiencies, thus generating the extra time needed for personalized care. Several scientific papers have shown that such standardization improves outcomes for patients with advanced cancer by providing comprehensive care that addresses both oncological treatment and symptom management, but also supports advanced care planning and reduces unnecessary hospitalizations. Standardization could also bring measurable benefits as shown by several meta-analyses. These studies shown improvements in quality of life, symptom burden, and even survival rates in cancer patients. The findings from several trials also highlighted reduced stress for caregivers, improved mental health, and greater patient satisfaction with care [[6], [7], [8]].
However, in the Lazio region, approaches vary regarding the involvement of palliative care physicians and the simultaneous use of cancer therapies and palliative care (defined as early). In this scenario, a group of 13 experts from the Lazio region set the goal of helping to define of new and more effective care pathways for SCLC patients, aiming to improve their outcomes and QoL.
2. Material and methods
Starting from January 2023, a multidisciplinary working group of 13 experts from the Lazio region, including eight oncologists, two radiation oncologists, two palliative care physicians, and a clinical psychologist, held a series of periodic meetings. During these meetings they assessed the extent of simultaneous care provided to SCLC patients in the Lazio region and formalized a document containing clinical, organizational, soft skills development, and key performance indicators (KPIs).
The 13 experts came from different clinical, organizational, and social backgrounds within the Lazio region. This in-depth process was made possible by the analysis of the main available literature evidences and the subsequent formalization of a survey.
A review of the scientific literature was carried out to identify key studies on PubMed. Manual searches using keyword strings were performed for each main topic. Using keywords such as “small-cell lung cancer” and “simultaneous care”, the multidisciplinary working group targeted national and international articles from the last 21 years (2002–2023). To identify publications containing data and information relevant to the subject of the study, each expert critically reviewed the 25 articles retained from the initial screening and, finally, compiled a list of bibliographic references for inclusion. 9 articles were included in the final review.
Following the scientific literature review, the multidisciplinary working group drafted and compiled a survey (Supplementary Appendix) that allowed mapping areas of interest: general context, simultaneous care supply, organizational requirements, multidisciplinary team, patient assessment, pharmacological and non-pharmacological approaches, KPIs monitoring, training of healthcare professionals, and communication with patients. In order to standardize data collection, the multidisciplinary working group adopted the following definition of simultaneous care: “simultaneous care integrates active therapy (chemotherapy, immunotherapy and radiation therapy) with palliative care, psychological support, nutritional and integrative support, physical rehabilitation, exercise programs, holistic approaches. Assessment of the need for referral to these programs should be made at the time of the SCLC diagnosis and repeated periodically targeted early palliative care [9])."
The analysis included approximately 213 patients diagnosed with SCLC in the Lazio region, cared by the multidisciplinary team involved in this work during 2022 in the different healthcare facilities included.
3. Results
The analysis of the data reflected the professional management practices of approximately 213 patients with SCLC in the Lazio region, with variability ranging from 6 to 52 patients cared for in the last year (2022) in the different healthcare facilities included.
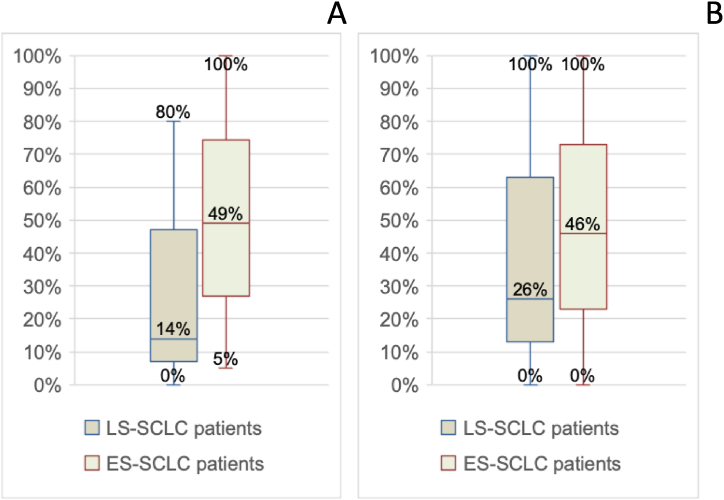
The percentages of patients enrolled in a palliative care program or a simultaneous care program varied widely, ranging from 0 % to 100 % in both cases. Fig. 1A and B showminimum, maximum, and median values.
Fig. 1.
A: Percentage variability of patients enrolled in a palliative care program (minimum, median, maximum, and interquartile range values). B: Percentage variability of patients enrolled in a simultaneous care program (minimum, median, maximum, and interquartile range values).
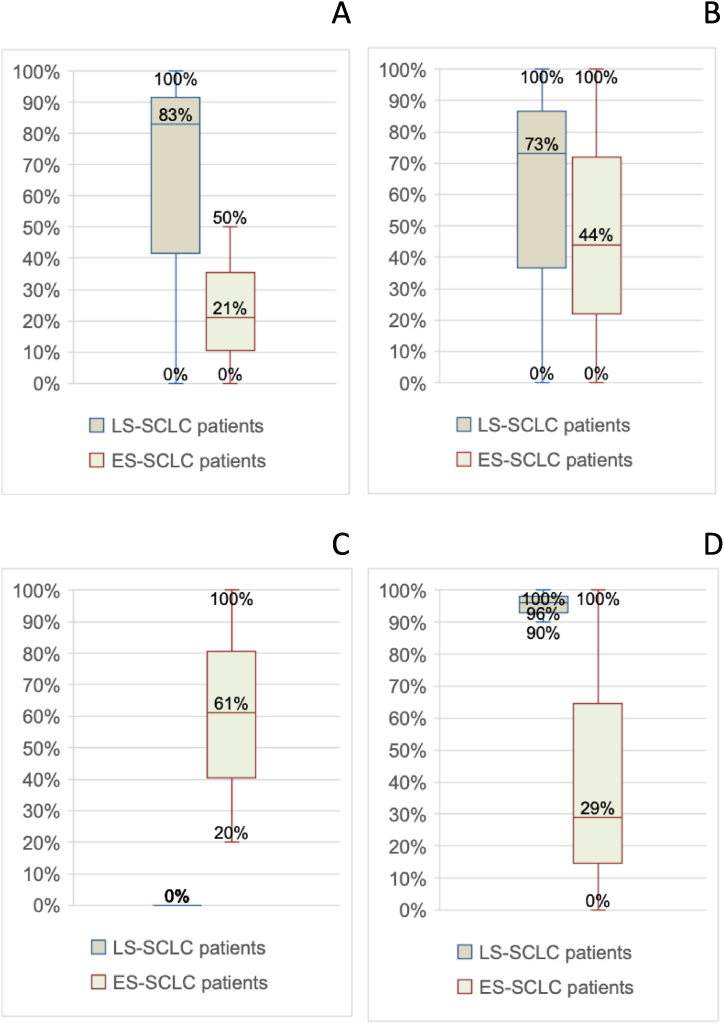
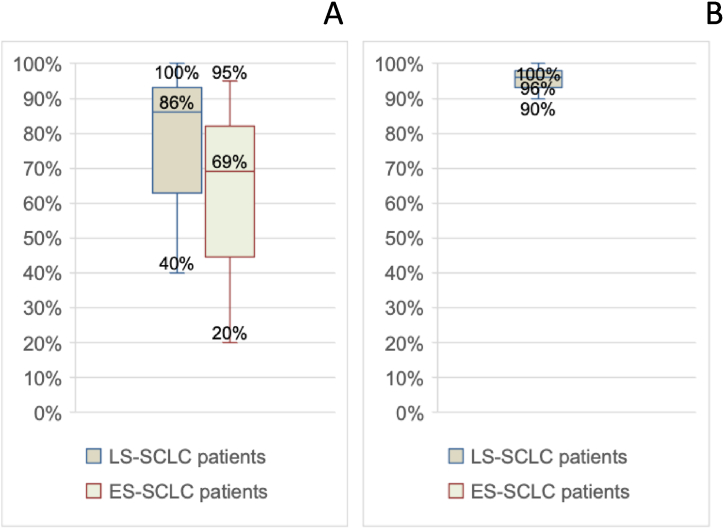
In terms of medical treatment approach, there was also a considerable percentage distribution, with 40 %–100 % of LS-SCLC patients receiving chemotherapy and 20 %–95 % of ES-SCLC patients receiving chemotherapy. Radiation therapy for thoracic disease in LS-SCLC patients ranged from 90 % to 100 % (Fig. 2A–B).
Fig. 3.
A. Percentage variability of patients undergoing palliative radiation therapy of thoracic disease (minimum, median, maximum values and interquartile range). B:Percentage variability of patients undergoing PCI (minimum, median, maximum, and interquartile range). C: Variability in percentage of patients undergoing radiation therapy for bone metastases (minimum, median, maximum, and interquartile range values). D: Percentage variability of patients undergoing mediastinal consolidation (minimum, median, maximum, and interquartile range values).
Fig. 2.
A: Percentage variability of patients undergoing curative chemotherapy (minimum, median, maximum, and interquartile range values). B: Percentage variability of patients undergoing radiation therapy for thoracic disease (minimum, median, maximum, and interquartile range values)./(minimum, median, maximum values and interquartile range). In all the other radiation therapy approaches included (palliative treatment of thoracic disease, prophylactic cranial irradiation (PCI), radiation therapy for bone metastases, mediastinal consolidation), widely distributed rates were observed (Fig. 3A–D).
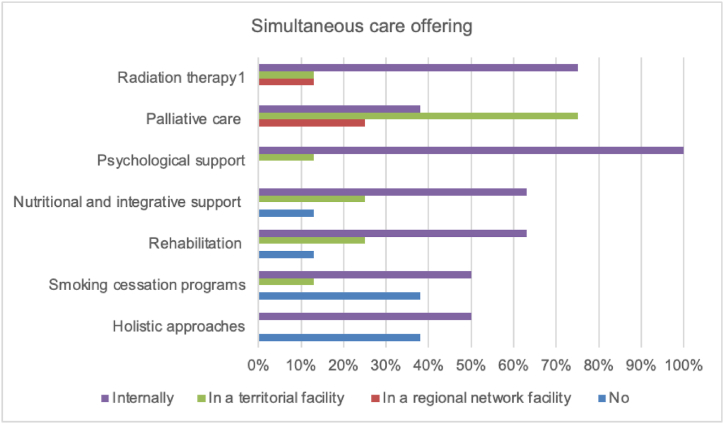
Simultaneous care offering (Fig. 4) showed that 100 % of facilities provided the execution of radiation therapy, palliative care, psychological support, and nutritional support either in-house or through connections with facilities in the territory and/or in the regional network. , On the other hand, smoking cessation programs and holistic approaches (music therapy, hot-cold therapy, acupuncture, massage) were not guaranteed in 38 % of cases, and rehabilitation and exercise programs were not guaranteed in 13 % of cases.
Fig. 4.
Simultaneous care, in addition to chemotherapy and immunotherapy, provided in-house or in conjunction with territorial and/or regional network facilities.
Analysis of organizational requirements (Table 1) showed that 25 % of the facilities included had no formalized PDTAs (Diagnostic and Therapeutic Care Pathways) dedicated to lung cancer, and only 33 % of formalized PDTAs included a specific pathway for simultaneous care. Moreover, the criteria for involving certain healthcare professionals, such as the clinical nutrition specialist, clinical psychologist, and palliative care physician, were not always clear, with 38 %, 25 %, and 12 % of cases unclarified respectively. In contrast, the criteria for including patients in palliative care were always clear (100 %). Connections with end-of-life facilities were clear and well-defined in all cases (100 %), while formalized procedures to ensure continuity of care were absent in 50 % of facilities.
Table 1.
results from data analysis– organizational requirements.
| Organizational requirements | Yes | No |
|---|---|---|
| Is there a formalized PDTA for lung cancer in your healthcare facility? | 75 % | 25 % |
| If so, does the PDTA include a dedicated pathway for simultaneous care? | 33 % | 66 % |
| Are the criteria for inclusion of patients in simultaneous care clearly defined in your healthcare facility? | 88 % | 12 % |
| Is it clear when the involvement of a clinical psychologist is required in your healthcare facility? | 75 % | 25 % |
| Is it clear when the involvement of a specialist in clinical nutrition is required in your healthcare facility? | 62 % | 38 % |
| Are the criteria for including patients in radiation therapy clearly defined in your healthcare facility? | 100 % | 0 % |
| Is it clear when the involvement of a palliative care physician is required in your healthcare facility? | 88 % | 12 % |
| Are the criteria for including patients in palliative care clearly defined in your healthcare facility? | 100 % | 0 % |
| Are the timelines for activating palliative care clearly defined in your healthcare facility? | 75 % | 25 % |
| Does your healthcare facility have connections with end-of-life facilities (e.g., hospices)? | 100 % | 0 % |
| If so, are the connections with end-of-life facilities well defined in your healthcare facility? | 87 % | 13 % |
| Is there a formalized procedure to ensure continuity of care until the end of life in your healthcare facility? | 50 % | 50 % |
With regard to the multidisciplinary team (Table 2), the findings showed that 25 % of facilities had no multidisciplinary teams dedicated to lung cancer, and that the palliative care physician was not involved in all new diagnoses in 80 % of cases. Some of the professionals dedicated to simultaneous care were missing, notably the clinical nutrition specialist (25 %), the interventional radiologist (25 %), and the radiation oncologist (13 %), were missing, but the clinical psychologist was always present (100 %) (see Table 3).
Table 2.
results from data analysis– multidisciplinary team.
| Multidisciplinary team | Yes | No |
|---|---|---|
| Does your healthcare facility have a multidisciplinary team dedicated to lung cancer? | 75 % | 25 % |
| If present in-house, is the palliative care physician involved in all new diagnoses in your healthcare facility? | 20 % | 80 % |
| Does your healthcare facility have a healthcare professional capable of placing an endobronchial or vascular stent? | 50 % | 50 % |
| Is there a clinical nutrition specialist in your healthcare facility? | 75 % | 25 % |
| Is there a radiation oncologist in your healthcare facility? | 87 % | 13 % |
| Is there a palliative care physician in your healthcare facility? | 88 % | 12 % |
| Is there an interventional radiologist in your healthcare facility? | 75 % | 25 % |
| Is there a clinical psychologist in your healthcare facility? | 100 % | 0 % |
Table 3.
results data analysis – patient assessment.
| Patient assessment | Yes | No |
|---|---|---|
| In your healthcare facility, does a healthcare professional conduct an assessment to evaluate the patient's needs regarding simultaneous care? | 62 % | 38 % |
| Does the professional assess the patient's performance status? | 100 % | 0 % |
| Does the professional assess the patient's nutritional status? | 88 % | 12 % |
| Does the professional assess the patient's pain? | 100 % | 0 % |
| Does the professional inquire about the presence of a caregiver? | 100 % | 0 % |
| Is the assessment repeated frequently during the patient's care process? | 75 % | 25 % |
In 38 % of facilities, a full assessment of the patient's concurrent care needs was not carried out However, some elements were frequently assessed, including Performance Status (PS) (100 %), pain evaluation (100 %), information on the presence of a caregiver (100 %), and nutritional status evaluation (88 %). In 25 % of the healthcare facilities, the assessment was not repeated during the patient's care process.
Regarding the non-pharmacological approach (Table 4), it was found that all common symptoms in SCLC patients, such as dyspnoea, pain, nausea, depression, anxiety, fatigue, and decreased appetite, were widely addressed in 100 % of the facilities involved, while drowsiness was addressed in 88 %. Concerning spiritual management, a dedicated professional was present in 75 % of cases.
Table 4.
results from data analysis – non-pharmacological approach.
| Non-pharmacological approach | Si | No |
|---|---|---|
| Is there a professional who can manage the spirituality of the patient in your healthcare facility? | 75 % | 25 % |
| Is dyspnoea, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
| Is pain, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
| Is nausea, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
| Is depression, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
| Is anxiety, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
| Is fatigue, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
| Is drowsiness, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 88 % | 12 % |
| Is decreased appetite, one of the most common symptoms of SCLC, taken care of in your healthcare facility? | 100 % | 0 % |
Consistently, the evaluation of the pharmacological approach (Table 5) showed that the use of essential drugs for managing common symptoms in SCLC patients was highly satisfactory: 100 % of facilities reported using opioids, benzodiazepines, adjuvant drugs for pain management, antiemetics, antiedemic drugs, diuretics, steroids, anticoagulants, and antibiotics, while the use of anti-constipation drugs was reported in 75 %.
Table 5.
results from data analysis – pharmacological approach.
| Pharmacological approach | Yes | No |
|---|---|---|
| In the treatment of SCLC patients, are opioids used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are benzodiazepines used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are adjuvant drugs for pain management used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are antiemetic drugs used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are antiedemic drugs used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are diuretics used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are steroids used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are anticoagulant drugs used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are antibiotics used in your healthcare facility? | 100 % | 0 % |
| In the treatment of SCLC patients, are anti-constipation drugs used in your healthcare facility? | 75 % | 25 % |
The analysis of the presence of specific KPIs (Table 6) highlighted a lack of monitoring activity: KPIs related to quality of life were present in only half of the healthcare facilities (50 %), KPIs related to the patient's care pathway were present in only 25 % of cases, as well as KPIs for monitoring the provision of simultaneous care. However, 75 % of professionals recognized the usefulness of implementing several specific KPIs.
Table 6.
results from data analysis – KPIs monitoring.
| KPIs monitoring | Yes | No |
|---|---|---|
| Is the patient's QoL monitored throughout the entire care process in your healthcare facility? | 50 % | 50 % |
| Are KPIs related to the SCLC patient's care pathway identified in your healthcare facility, and is there a monitoring system for these indicators? | 25 % | 75 % |
| If not, do you think it'd be useful to identify specific KPIs for SCLC in your healthcare facility? | 75 % | 25 % |
| Are the KPIs related to the provision of simultaneous care identified in your healthcare facility? | 25 % | 75 % |
Finally, 90 % of professionals were trained on the possibility for patients to express end-of-life declarations, 62 % were trained on simultaneous care, and 50 % were trained on effective and appropriate methods of communication with patients. Nevertheless, 87 % of facilities expressed their intention to participate in dedicated training courses. In 25 % of healthcare facilities, there were no clear and easily accessible information flyers on the patient's care pathway (Table 7).
Table 7.
results from data analysis – training of healthcare professionals and communication with patients.
| Training of healthcare professionals and communication with patients | Yes | No |
|---|---|---|
| Are healthcare professionals in your healthcare facility trained on simultaneous care? | 62 % | 38 % |
| Are healthcare professionals in your healthcare facility trained on the possibility for patients to express end-of-life declarations? | 88 % | 12 % |
| Are healthcare professionals in your healthcare facility trained on communication techniques with patients? | 50 % | 50 % |
| If not, do you think it'd be useful to provide training courses in your healthcare facility? | 100 % | 0 % |
| Would you be willing to participate in training courses? | 87 % | 13 % |
| Does the patient in your healthcare facility receive clear and easily understandable informational flyers regarding their care pathway? | 75 % | 25 % |
4. Discussion
The analysis of the data provided an overview of the management of approximately 35–52 % of SCLC cases in the Lazio region. In fact, in 2020, Italy estimated an incidence of around 41,000 cases of lung cancer, of which SCLC accounted for 10–15 % [1]. Considering that the Lazio region accounts for about one-tenth of the Italian population (6 million), the estimated incidence would be around 4100 new cases of lung cancer and 410–615 new diagnoses of SCLC (median survival <12 months). These data, in relation to the 213 patients included in the survey, indicate the aforementioned percentage values (35–52 %).
Table 8 presents the main unmet needs related to the management of these patients highlighted by the working group. Thus, the group formulated a series of clinical, organizational, soft skills development, and KPIs monitoring recommendations.
Table 8.
Main unmet needs related to the management of patients with SCLC in the Lazio region, highlighted by analysis of the data from completed surveys.
| Unmet clinical needs |
| Lack of uniformity in conducting assessments throughout the patient's care pathway and during clinical re-evaluation of patients undergoing treatment |
| Variability in the involvement of palliative care physicians and referral to palliative care |
| Lack of uniformity in the involvement of clinical nutrition specialists and clinical psychologists |
| Inconsistent criteria for determining the patient's ineligibility for active chemo-radiation therapy treatment |
| Risk of misusing corticosteroids in patients on active therapy |
| Inconsistent criteria for determining eligibility for different radiation therapy approaches |
| Limited referral to cigarette cessation programs |
| Unmet organizational needs |
| Absence of a structured network between the hospital and the territory to ensure access to all necessary professionals (radiation oncologists, interventional radiologists, clinical nutrition specialists, etc.) |
| Lack of a formalized PDTA for lung cancer |
| Absence of formalized multidisciplinary teams for the management of lung cancer |
| Inconsistent involvement of palliative care physicians within multidisciplinary teams |
| Inadequate referral to end-of-life services |
| Unmet needs in KPIs monitoring |
| Lack of a specific set of KPIs for monitoring the SCLC patient pathway |
| Absence of a specific set of KPIs for monitoring the delivery of simultaneous care |
| Absence of a specific set of KPIs for monitoring the activity of multidisciplinary teams |
| Unmet needs for soft skills |
| Low participation in training courses on patient communication methods |
| Low participation in training courses on simultaneous care |
| Underutilization of clear and easily understandable informational flyers |
4.1. Clinical recommendations
The first access of a patient represents the moment when the responsible professional for the initial examination (clinical oncologist, pulmonologist, radiation oncologist, or thoracic surgeon) initiates the care pathway. The patients that access this pathway is either self-referred, admitted to the emergency department or another ward, or referred by a general practitioner (GP) or another specialist. During the first examination, the working group recommends inquiring about the presence of a caregiver and evaluating the patient's PS using the ECOG Scale [10] or the Karnofsky Performance Status Scale [11]. Within a maximum of three weeks, if the suspicion of SCLC is confirmed, the diagnostic pathway and subsequent staging completion should be finalized. A confirmed SCLC diagnosis leads to a multidisciplinary discussion in which each clinician that relevant to define the preferred therapeutic approach should give his contribution. This includes the palliative care physician. Indeed, as demonstrated in numerous studies [[12], [13], [14], [15]], an early management by the multidisciplinary team, in conjunction to the combination of oncology therapies and palliative care from the time of diagnosis, leads to improvements in the patient's QoL and, in some cases, may extend the overall survival.
The implementation of the discussed therapeutic decision takes place after a comprehensive evaluation conducted by the clinical oncologist. In this context, the recommendation is to perform a comprehensive patient assessment, that needs to be repeated at each clinical re-evaluation. This occurs every 3–4 weeks for patients on infusion therapy or at the beginning and at the end of the patient's hospitalization. In particular, Table 9 summarizes the recommended clinical tools and their application.
Table 9.
recommended clinical tools and their application.
| Clinical tool | Application |
|---|---|
| Performance Status using the ECOG Scale or the Karnofsky Performance Status Scale | Measurement of a subject's general state of psychophysical well-being |
| Edmonton Symptom Assessment System (ESAS) [16] | Multidimensional assessment of patient symptoms such as pain, tiredness, nausea, depression, anxiety, drowsiness, lack of appetite, malaise and difficulty breathing |
| Visual Analogue Scale (VAS) [17] | Estimation of pain intensity |
| Fatigue Assessment Scale (FAS) [18] | Persistent fatigue rating scale |
| Malnutrition Universal Screening Tool (MUST) questionnaire [19] | Evaluation of patient's nutritional status |
| The European Organization for Research and Treatment of Cancer's Quality of Life Questionnaire (EORTC QLQ-C30) [20] | If needed. In the presence of a palliative care physician, to further assess needs from a palliative care perspective |
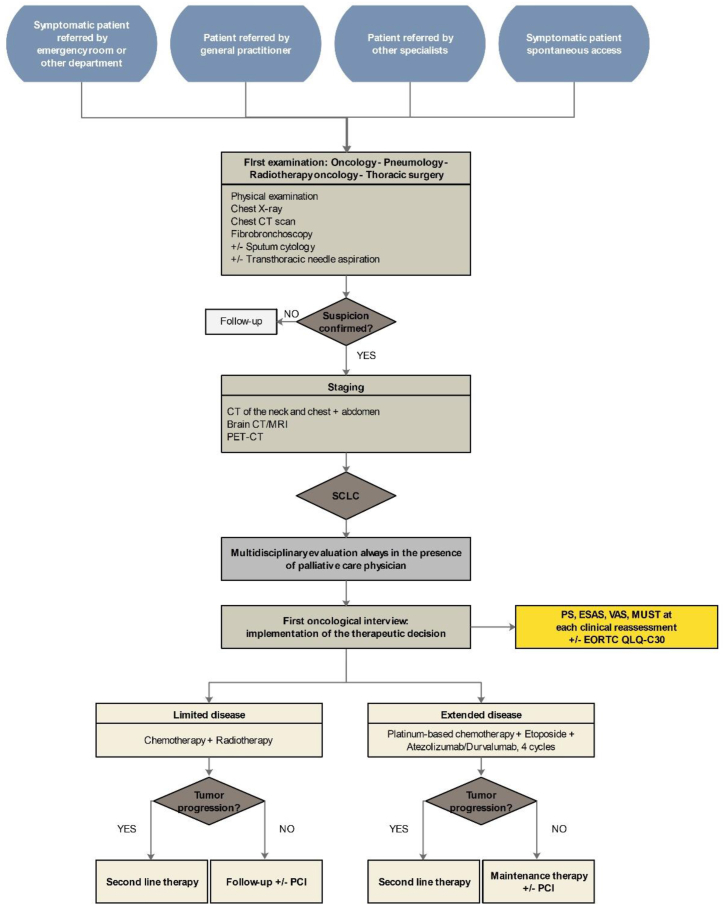
The flow chart in Fig. 5 provides a summary of the patient management pathway, from the first access to the initiation of therapy.
Fig. 5.
Flow chart of SCLC patients care pathway from the first access to treatment setup.
According to the working group, the palliative care physician should be available for collaboration with the clinical oncologist at any time. The oncologist, decides whether to activate the palliative care pathway, in accordance to the comprehensive evaluation of patients and their prognosis. It is essential for the two professionals to establish dynamic and flexible interaction, guided by the oncology specialist, with the support of the palliative care physician. The latter can provide their services in three different care settings, depending on the needs of patients and their families, to ensure continuous care within a network organization, as defined by the Prime Ministerial Decree of January 12, 2017, which establishes and updates essential levels of care (LEA) in palliative care.
The Palliative Care Clinic (in-house or territorial) (Article 15) [21] is dedicated to patients with good functional and motor autonomy, but affected by progressive and irreversible diseases. For such patients care is aimed at managing disease symptoms and providing possible psychological support to them and their families. Home Palliative Care (UCP-Dom, Article 23) [21] aims to support terminal patients and their families by providing comprehensive care (medical, social, psychological, and spiritual) at the patient's residence. Home palliative care are divided in two categories: one provided by the GP and nurses in the territory, and the other one provided by specialized territorial teams who directly collaborate with the in-hospital palliative care physician and the entire multidisciplinary team. Finally, the Hospice, or Residential Palliative Care Center (Article 31) [21], represents a temporary place of assistance and accommodation for patients with an advanced and terminal prognosis, to guide them towards the end of life with appropriate medical, nursing, psychological, and spiritual support, to reduce suffering and to provide support to the family.
The involvement of appropriate professionals during each phase of the disease is essential for the simultaneous care approach. Therefore, the working group has established criteria regarding the involvement of the clinical nutrition specialist and the clinical psychologist. More in details, a referral to the nutritionist to perform a nutritional assessment should always occur upon patient request, but also in case of a MUST score ≥1. The referral for a psychological support, on the other hand, should always take place upon patient request, family request, or care team recommendation, whether for curative or palliative treatment purposes.
The working group have recommended to determine ineligibility for active chemotherapy, radiation therapy treatment or immune-chemotherapy, by evaluating the presence of a poor PS (ECOG = 2 if comorbidity or ECOG ≥3), the life expectancy according to the Palliative Prognostic Score (PaP) [22], and the presence of comorbidities that contraindicate active treatment, through the estimation of the Charlson Comorbidity Index (CCI) [23].
Table 10 summarizes the main recommendations related to the use of radiation therapy [24,25] provided by the working group (see Table 11).
Table 10.
Key recommendations regarding the use of radiation therapy.
| Radiation therapy approach | Key recommendations |
|---|---|
| Radiation therapy for thoracic disease |
|
| Thoracic consolidation |
|
| PCI |
|
| Treatment of encephalic metastases |
|
| Treatment of bone metastases |
|
| Palliative treatment of thoracic disease |
|
| Stereotactic radiation therapy |
|
Table 11.
proposal of volume KPIs.
| Volume KPIs | ||
|---|---|---|
| KPI | Target percentage | Phase of PDTA |
| Number of new diagnoses per year | Not applicable | Diagnosis |
| Number of LS-SCLC patients at diagnosis | Not applicable | Diagnosis |
| Number of ES-SCLC patients at diagnosis | Not applicable | Diagnosis |
| Number of patients referred to palliative care services | Not applicable | Medical therapeutic approach |
| Number of surgical procedures per year | Not applicable | Surgical approach |
| Number of first-line chemotherapy treatments delivered per year | Not applicable | Medical therapeutic approach |
| Number of second-line chemotherapy treatments delivered per year | Not applicable | Medical therapeutic approach |
| Number of curative radiation therapy treatments delivered per year | Not applicable | Radiation therapy approach |
| Number of palliative radiation therapy treatments delivered per year | Not applicable | Radiation therapy approach |
In the context of adjuvant pharmacological approaches used during active therapy, inconsistencies in the use of corticosteroids are often observed. Therefore, the recommendations suggest using corticosteroids only in the presence and/or onset of moderate to severe respiratory symptoms (ESAS >4). This use is intended for premedication in chemotherapy, management of immunotherapy and/or concurrent treatment toxicity, and control of edema in patients with brain metastases. Generally, corticosteroid use should be limited in both duration and dosage, based on the resolution of acute situations.
The final clinical recommendation concerns referral to cigarette cessation programs. Promoting smoking cessation is crucial, particularly for patients who have already failed previous attempts, are undergoing treatments with curative intent, or are experiencing acute and/or worsening respiratory symptoms. Additionally, the working group consistently recommends referring family members and caregivers to smoking cessation programs.
4.2. Organizational recommendations
Although in 2015, in the Lazio region, hub centers for lung cancer were formalized through DCA U00419/2015, the involvement of local healthcare services and the establishment of a network for the non-surgical management of patients still appear limited. In response to additional unmet needs identified from the survey analysis, the working group recommends the implementation of an Integrated PDTA between Hospital Reference Centers (CRO), Local Health Authorities (ASL), and GPs ("Hospital-Territory PDTA”) for lung cancer. The purpose is to formalize a collaborative network, in compliance with the Organizational Guidelines for the regional oncology network under the Agreement State-Regions n.59/CRS of April 17, 2019. This should be implemented independently of the prior formalization of PDTAs by individual hospital facilities.
Within the shared network, or cancer network, it is essential to first identify the services offered by the involved healthcare facilities to identify the competence of each center (e.g., in case of radiation therapy, interventional radiology, or clinical nutrition) and to establish the criteria for transitioning between different structures within the network. This includes the establishment of Oncological Access and Continuity of Care Points (PACO). The working group also recommend the ideal structure of the 'hospital-territory PDTA' for lung cancer. This structure should include: services provided by the network facilities, methods and criteria for timely access of the patient (Oncology Access and Continuity Point), formalization of the Multidisciplinary Team, standardized diagnosis (in terms of initial assessment and molecular pathology) and staging criteria, definition of clinical and organizational criteria for timely referral to on-call professionals' consultation and transition to other network facilities, methods and criteria to access the simultaneous care (early palliative care), therapeutic approach (surgical, medical and radiation therapy) and follow up.
Alongside the development of PDTAs, the formalization of dedicated multidisciplinary teams (MDT) for lung cancer could serve as a key tool to address the challenges in the Lazio region. Specifically, the MDT should consist of a core team comprising an oncologist, palliative care physician, surgeon, pulmonologist, pathologist, radiologist, radiation oncologist, physiatrist, nutritionist, clinical psychologist, care manager, data manager, and any other relevant professionals available on call. The role of the interprofessional care manager is crucial, whose function should also include ensuring the proper functioning of the care pathway from an organizational perspective by coordinating activities and serving as the point of contact with the MDT. To this end, it is essential to predefine the clinical, organizational, and soft skill competencies required for the case manager (job profile).
To implement the above organizational recommendations, the working group has proposed a series of operational steps involving the engagement of Health Directorates, General Directorates, Institutional Stakeholders, Specialists from CROs and territorial facilities, and GPs responsible for the initiative. Additionally, the plan envisions incorporating current authorizing resolutions and conducting a clinical-organizational assessment to explore the context in more depth. This will be followed by determining the necessary timelines for implementing the action plan and, finally, drafting PDTAs in accordance with the resolutions and context-specific requirements, supplemented by a panel of KPIs to monitor activities. The ultimate goal is to simplify professional patient management practices and enhance the quality of care and life for the patients.
4.3. KPIs
The following proposed KPIs (Table 12, Table 13, Table 14) are the result of the clinical experience
Table 12.
proposal of process KPIs.
| Process KPIs | ||
|---|---|---|
| KPI | Target percentage | Phase of PDTA |
| Waiting time ≤7 days between the execution of the first specialist medical examination and its request | ≥80 % | Diagnosis |
| Waiting time ≤21 days to complete the diagnostic-staging process from the first access of the patient | ≥70 % | Staging |
| Time for reporting cytological/histological examination results ≤15 days | ≥90 % | Diagnosis and staging |
| Waiting time ≤15 days between diagnosis and oncology treatment | ≥90 % | Medical therapeutic approach |
| Waiting time ≤7 between the activation of a palliative care program and its request | ≥90 % | Simultaneous care |
| Percentage of patients evaluated for QoL using validated tools | ≥90 % | Diagnosis |
| Percentage of patients receiving informative flyers | ≥80 % | Follow up |
Table 13.
Proposal of simultaneous care KPIs.
| Simultaneous care KPIs | ||
|---|---|---|
| KPI | Target percentage | Phase of PDTA |
| Percentage of patients in a palliative care program at time of death | ≥95 % | Simultaneous care |
| Percentage of patients receiving curative chemotherapy within the last 30 days of life | <30 % | Simultaneous care |
| Percentage of patients receiving curative radiation therapy within the last 30 days of life | <10 % | Simultaneous care |
| Percentage of patients who benefited from a palliative care service within the last 30 days of life | ≥95 % | Simultaneous care |
| Percentage of patients who died in hospital | <10 % | Simultaneous care |
| Percentage of patients who died in hospice or at home | ≥90 % | Simultaneous care |
| Percentage of patients referred to a simultaneous care program before starting the oncology treatments | ≥75 % | Simultaneous care |
| Percentage of patients with documented evaluation of dyspnoea | 100 % | Simultaneous care |
Table 14.
Proposal of MDT KPIs.
| MDT KPIs | ||
|---|---|---|
| KPI | Target percentage | Phase of PDTA |
| Number of cases discussed in MDT per eligible patients per year | ≥80 % | MDT |
| Percentage of missed MDT meetings compared to the scheduled ones | <10 % | MDT |
| Waiting time <7 days from the referral to MDT discussion to its execution | ≥90 % | MDT |
| Waiting time <15 days between MDT discussion and the beginning of curative oncology treatments | ≥60 % | Medical therapeutic approach |
| Waiting ≤30 days between MDT discussion and surgical intervention | ≥90 % | Surgical approach |
of the working group, supported by previous formalized PDTAs of individual hospital facilities.
4.4. Developing of soft skills
Soft skills, like understanding towards others, empathy, active listening, and effective communication, represent a transversal competencies necessary to enhance the quality of healthcare professionals work. The working group have proposed the implementation of structured training courses in order to improve development of soft skills for healthcare professionals involved in the integrated and multidisciplinary care of SCLC patients. The proposal aims to train on effective communication techniques towards patients and other professionals, address psychological and social issues, and explore emotional implications experienced by patients and their families. Additionally, the training aims to identify the best coping strategies, acquire relational competencies, and recognize and prevent burnout.
Moreover, the working group proposes the development of frontal training programs focused on the following main topics: supportive therapies and main palliative treatment approaches, management of opioids and antibiotics in end-of-life care, dyspnoea management, treatment modification in accordance with the disease phase and the setting of care, main criteria for referral to supportive care programs, medico-legal aspects and patient rights.
In conclusion, the working group recognizes the importance of providing support to cancer patients in understanding the main aspects of their disease management process.
The definition of informative flyers that summarize the care pathway of patients with a diagnosis of SCLC could guide them through the different phases of their disease, from the diagnosis to the end-of-life care.
The working group suggests the involvement of the main patient associations to structure the best informative flyers from a patient's point of view.
The working group also recommend the contents that should be present within the informative flyers. These are: basic information about SCLC in term of its frequency, and possible causes, its diagnosis, classification and staging, the most common bio-psychosocial-spiritual symptoms, the treatment options including possible side effects, information on ongoing clinical trials, the structure of the multidisciplinary team involved in the management of the disease, offered services in the field of simultaneous care and/or complementary interventions (e.g., referral to smoking cessation programs), and finally patient rights and support groups or patient associations.
5. Conclusions
The project provided a representative analysis of the regional context regarding the provision of simultaneous care in patients with SCLC, highlighting significant discrepancies both in clinical and organizational approaches. To implement the formulated recommendations, the involvement of major scientific societies, such as AIOM, SICP, and AIRO (Italian Association of Radiation Oncology), as well as patient associations and key institutional stakeholders, is recommended.
CRediT authorship contribution statement
Sabrina Mariotti: Writing – review & editing, Validation, Methodology, Conceptualization. Gabriele Minuti: Writing – review & editing, Validation, Methodology, Conceptualization. Lorenza Landi: Writing – review & editing, Validation, Supervision, Investigation, Conceptualization. Emilio Bria: Writing – review & editing, Validation, Supervision, Investigation, Conceptualization. Giorgia Carlucci: Writing – original draft, Investigation, Funding acquisition, Formal analysis, Data curation. Mariantonietta Di Salvatore: Writing – review & editing, Validation, Conceptualization. Raffaele Giusti: Writing – review & editing, Validation, Conceptualization. Aurelia Iurato: Writing – review & editing, Validation, Conceptualization. Sara Ramella: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization. Maria Adelaide Ricciotti: Writing – review & editing, Supervision, Conceptualization. Gian Paolo Spinelli: Writing – review & editing, Validation, Conceptualization. Marco Tineri: Writing – review & editing, Validation, Conceptualization. Francesco Scarcella: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization. Mario Rosario D'andrea: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies
Mariotti S: MD, PhD, Oncologist at University Hospital Tor Vergata, involved in the last 15 years mainly in the field of lung cancers and gynecological malignancies. Co-Investigator in many phase III and IV clinical trials
Minuti G: MD involved in the last 10 years in clinical studies from phase I to phase III and in translational research on Lung Cancer
Landi L: Director of “Clinical Trials Unit: Phase 1 and Precision Medicine” at National Cancer Institute, IRCCS, Regina Elena in Rome, Principal Investigator in several Clinical Trials focus on Lung Cancer and Thoracic Malignancies
Bria E: Medical Oncologist, Associate Professor of Medical Oncology, Università Cattolica del Sacro Cuore (UCSC), Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy. His scientific track includes methodology/design of newer early-phases studies, definition/validation of prognostic models, analysis of bio-molecular predictors, methodology of guidelines (Lung/Breast/BSC), analysis of investigational molecular pathways, meta-analysis/systematic reviews and surrogate end-points development/validation
Carlucci G: Pharmacist with consolidated experience in clinical data management for observational research studies; healthcare consultant with solid expertise in the oncological field
Di Salvatore M: Medical Oncologist (MD, phD) at Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy specialized in treamtent of Lung Cancer. Passionate about fundamental research but her fulfilment is at the patient's bedside
Giusti R: Medical Oncologist at Sant’ Andrea Hospital of Rome. Clinical and scientific research activities dedicated to promotion and personalization of supportive care in cancer and improving patient quality of life
Iurato A: MD, Radiation Oncologist at Fondazione Policlinico Universitario Campus Bio-Medico, Rome, her field of interest is clinical application of novel radiotherapy techniques like Adaptive Radiotherapy, SABR (Stereotactic Ablative Radiotherapy), VMAT (Volumetric Arc Therapy) and IGRT (ImageGuided Radiation Therapy). She is involved in clinical and translational research regarding lung cancer treatments. She has experience as radiation oncologist in lung cancer segmentation, combination of radiation and chemotherapy, target agents and immunotherapy. She is author of original articles in national and international peer-reviewed journals
Ramella S: Professor of Radiation Oncology at Campus Bio-Medico University, Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy with 17 years of experience and over 2240 citations. She has international teaching experiences with ESRO and ESTRO, she is the coordinator of the Lung Cancer subgroup ESTRO Guidelines and was track chair for WCLC 2022. Her main interest lies in lung cancer treatment with regard to combination of radiation and chemotherapy, target agents and immunotherapy. In the era of personalized medicine, her research has also focused on exploiting radiomics data in Non–Small Cell Lung Cancer (NSCLC). Her latest research is directed to develop a Radiomics-based Decision Support System (RDSSs) to predict outcomes during chemoradiation. Her efforts have been recognized by the University Strategic Project 2019 Award for the project “A Collaborative Multi-Sources Radio Pathomics Approach for Personalized Oncology in NSCLC”
Ricciotti MA: Palliative oncologist with over 10 years of experience in the field of territorial and intra-hospital palliative care, adjunct professor at the School of Specialization in Medicine and Palliative Care at the Catholic University of the Sacred Heart of Rome
Spinelli GP: Medical Oncologist (MD,PhD) at Territorial Oncology of ASL Latina – University “Sapienza”-Polo Pontino, specialized in treatment of lung cancer and other solid tumors
Tineri M: Psychologist with over 10 years of experience in Hospice and Hospital care, with a focus on caregivers and bereavement support, continuity of care, palliative care consultancy and palliative care research. Lecturer in the Master of Psycho-Oncology at the IRE, Istituto Nazionale Tumori Regina Elena
Scarcella F: Palliative care physician with over 20 years of experience in Hospice and Hospital care, with focus on outpatient activities, simultaneous care, continuity of care, consultancy in palliative care and research in palliative care; Professor for several Palliative Care and Pain Therapy courses at the Catholic University of the Sacred Heart of Rome
D'Andrea MR: medical oncologist, Director of the Oncology and Palliative care network of the ASL Roma 4
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39324.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.AIOM (Italian Association of Medical Oncology) Guidelines . 2021. Lung Cancer. [Google Scholar]
- 2.Minuti G., et al. Management of small-cell lung cancer patient in the regions of Lazio, Umbria and Sardinia. Recent Prog Med. 2021;112(10):639–646. doi: 10.1701/3679.36653. [DOI] [PubMed] [Google Scholar]
- 3.Micke P., et al. Staging small-cell lung cancer: Veterans administration lung study group versus international association for the study of lung cancer--what limits limited disease? Lung Cancer. 2002;37(3):271–276. doi: 10.1016/s0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 4.Jackman D.M., Johnson B.E. Small-cell lung cancer. Lancet. 2005;366(9494):1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 5.AIOM-SICP Cure Palliative Precoci e Simultanee. 2015. https://www.sicp.it/wp-content/uploads/2018/12/27_2015_documento_AIOM-SICP_WEB.pdf
- 6.Brunello A., Galiano A., Schiavon S., Nardi M., Feltrin A., Pambuku A., De Toni C., Dal Col A., Lamberti E., Pittarello C., et al. Simultaneous care in oncology: a 7-year experience at esmo designated centre at veneto Institute of oncology, Italy. Cancers. 2022;14:2568. doi: 10.3390/cancers14102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temel J.S., Greer J.A., Muzikansky A., Gallagher E.R., Admane S., Jackson V.A., Dahlin C.M., Blinderman C.D., Jacobsen J., Pirl W.F., Billings J.A., Lynch T.J. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010 Aug 19;363(8):733–742. doi: 10.1056/NEJMoa1000678. PMID: 20818875. [DOI] [PubMed] [Google Scholar]
- 8.Bakitas M., Lyons K.D., Hegel M.T., Balan S., Brokaw F.C., Seville J., Hull J.G., Li Z., Tosteson T.D., Byock I.R., Ahles T.A. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009 Aug 19;302(7):741–749. doi: 10.1001/jama.2009.1198. PMID: 19690306; PMCID: PMC3657724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui D. IASLC 2023 World Conference on Lung Cancer. 2023. Components of successful early integration: screening tools and who to refer. [Google Scholar]
- 10.Oken M.M., et al. Toxicity and response criteria of the eastern cooperative oncology group. Am. J. Clin. Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 11.Karnofsky D.A., Burchenal J.H. In: Evaluation of Chemotherapeutic Agents. MacLeod C.M., editor. Columbia University Press; New York: 1949. The clinical evaluation of chemotherapeutic agents in cancer; pp. 196–197. [Google Scholar]
- 12.Temel J.S., et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 13.Temel J.S., et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J. Clin. Oncol. 2016;35(8):834–841. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakitas M.A., et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakitas M.A., et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015;33(13):1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruera E., et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J. Palliat. Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 17.Hayes M.H.S., Patterson D.G. Experimental development of the graphic rating method. Psychol. Bull. 1921;18:98–99. [Google Scholar]
- 18.Michielsen H.J., et al. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J. Psychosom. Res. 2003;54(4):345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 19.Pei-Chun C., et al. The Malnutrition Universal Screening Tool (MUST) and a nutrition education program for high risk cancer patients: strategies to improve dietary intake in cancer patients. Biomedicine. 2015;5(3):17. doi: 10.7603/s40681-015-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaronson N.K., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 21.Decreto del Presidente del Consiglio dei Ministri 12 gennaio 2017: definizione e aggiornamento dei livelli essenziali di assistenza, di cui all’articolo 1 comma 7 del decreto legislativo 30 dicembre 1992, n.502 (17A02015) Gazzetta Ufficiale Serie Generale n.65 del 18.03.2017. Suppl. Ordinario n.15. 2017 [Google Scholar]
- 22.Pirovano M., et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17(4):231–239. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 23.Charlson M.E., et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Simone C.B., et al. Radiation therapy for small cell lung cancer: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2020;10(3):158–173. doi: 10.1016/j.prro.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daly M.E., et al. Radiation therapy for small-cell lung cancer: ASCO guideline endorsement of an ASTRO guideline. J. Clin. Oncol. 2021;39(8):931–939. doi: 10.1200/JCO.20.03364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.