Abstract
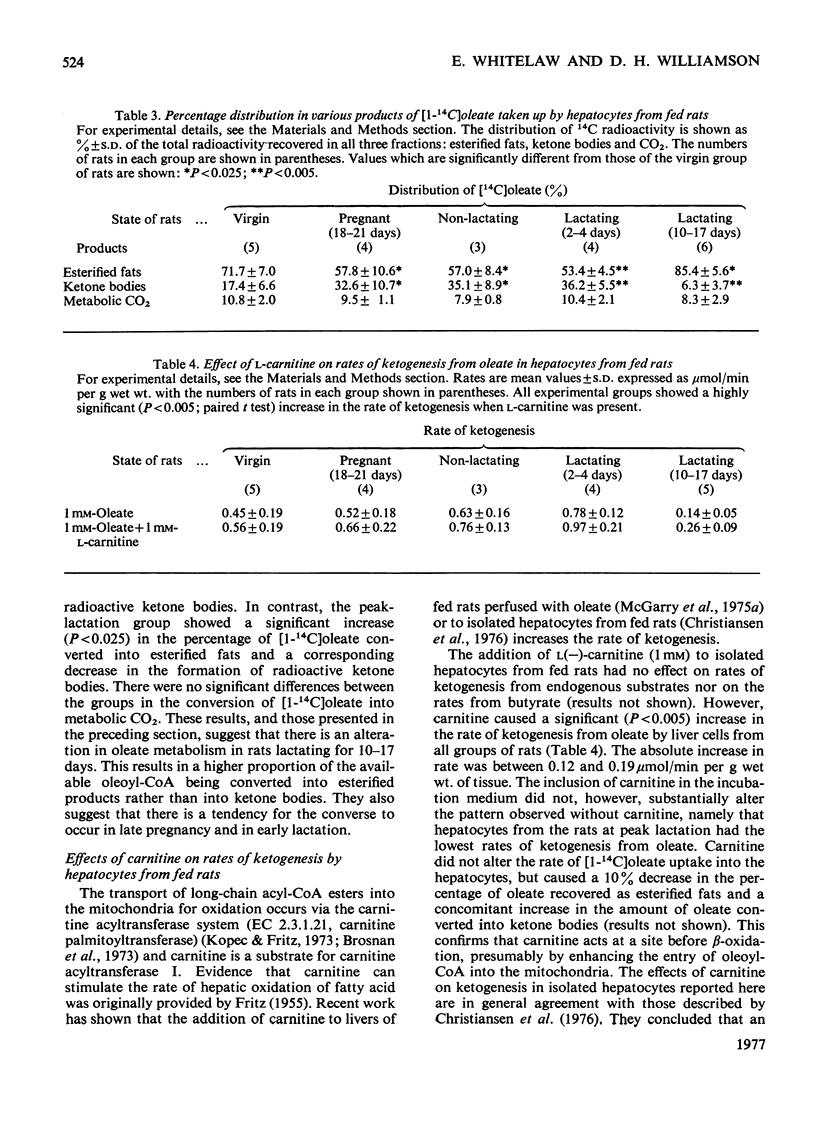
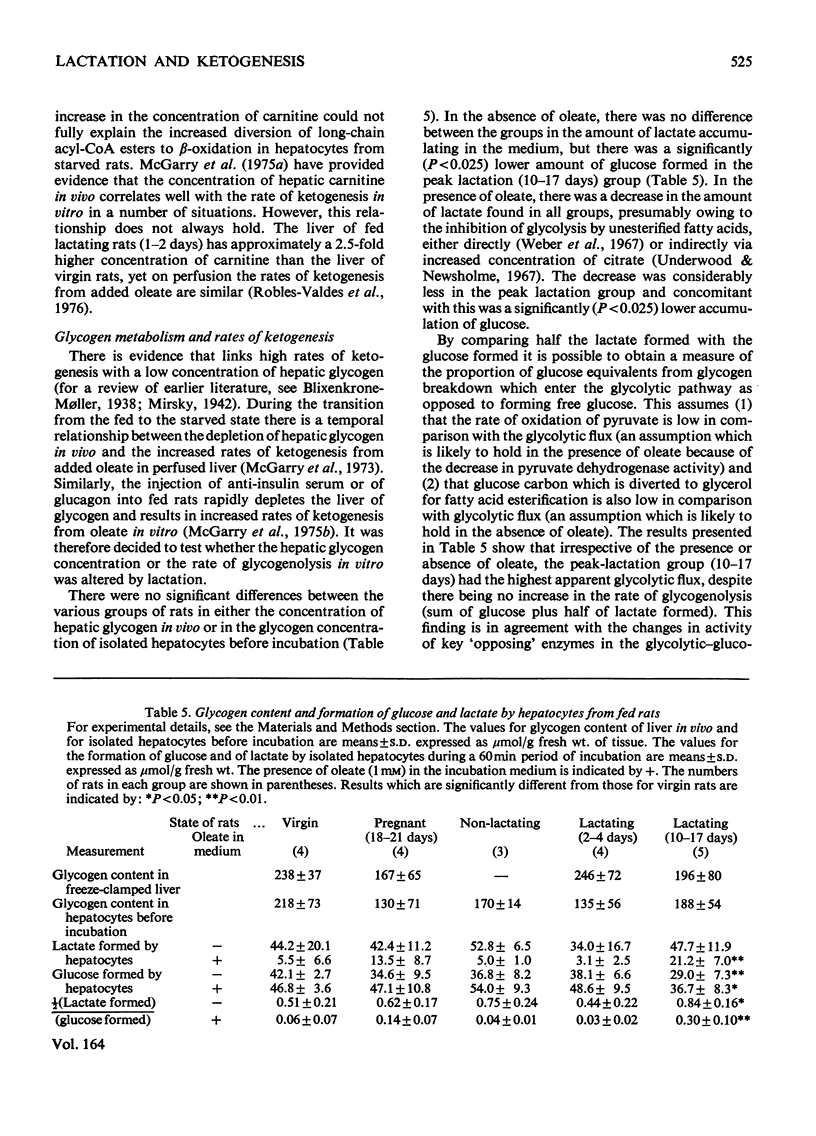
1. Rates of ketogenesis from endogenous butyrate or oleate were measured in isolated hepatocytes prepared from fed rats during different reproductive states [virgin, pregnant, early-lactating (2-4 days) and peak-lactating (10-17 days)]. In the peak-lactation group there was a decrease (25%) in the rate of ketogenesis from butyrate, but there were no differences in the rates between the other groups. Wth oleate, the rate of ketogenesis was increased in the pregnant and in the early-lactation groups compared with the virgin group, whereas the rate was 50% lower in the peak-lactation group. 2. Experiments with [1-(14)C]oleate indicated that these differences in rates of ketogenesis were not due to alterations in the rate of oleate utilization, but to changes in the amount of oleoyl-CoA converted into ketone bodies. 3. Although the addition of carnitine increased the rates of ketogenesis from oleate in all groups of rats, it did not abolish the differences between the groups. 4. Measurements of the accumulation of glucose and lactate showed that hepatocytes from rats at peak lactation had a higher rate of glycolytic flux than did hepatocytes from the other groups. After starvation, the rate of ketogenesis from oleate was still lower in the peak-lactation group compared with the control group. This suggests that the alteration in ketogenic capacity in the former group is not merely due to a higher glycolytic flux. 5. It is concluded that livers from rats at peak lactation have a lower capacity to produce ketone bodies from long-chain fatty acids which is due to an alteration in the partitioning of long-chain acyl-CoA esters between the pathways of triacylglycerol synthesis and beta-oxidation. The physiological relevance of this finding is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Amenomori Y., Chen C. L., Meites J. Serum prolactin levels in rats during different reproductive states. Endocrinology. 1970 Mar;86(3):506–510. doi: 10.1210/endo-86-3-506. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Christiansen R., Borrebaek B., Bremer J. The effect of (-)carnitine on the metabolism of palmitate in liver cells isolated from fasted and refed rats. FEBS Lett. 1976 Mar 1;62(3):313–317. doi: 10.1016/0014-5793(76)80083-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- FRITZ I. The effect of muscle extracts on the oxidation of palmitic acid by liver slices and homogenates. Acta Physiol Scand. 1955 Oct 12;34(4):367–385. doi: 10.1111/j.1748-1716.1955.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Hamosh M., Clary T. R., Chernick S. S., Scow R. O. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970 Sep 8;210(3):473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H. Measurements of substrate uptake by mammary gland of the rat. Biochem J. 1972 Oct;129(5):1171–1173. doi: 10.1042/bj1291171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Comparison of properties of carnitine palmitoyltransferase I with those of carnitine palmitoyltransferase II, and preparation of antibodies to carnitine palmitoyltransferases. J Biol Chem. 1973 Jun 10;248(11):4069–4074. [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of ketogenesis and clinical aspects of the ketotic state. Metabolism. 1972 May;21(5):471–489. doi: 10.1016/0026-0495(72)90059-5. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in hepatic ketogenesis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4385–4388. doi: 10.1073/pnas.72.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The significance of changes in tissue clearing-factor lipase activity in relation to the lipaemia of pregnancy. Biochem J. 1968 Feb;106(3):677–682. doi: 10.1042/bj1060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Williamson D. H. Lactating mammary gland of the rat: a potential major site of ketone-body utilization. Biochem J. 1972 Jun;128(2):459–460. doi: 10.1042/bj1280459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Prolactin receptors in rat liver: possible induction by prolactin. Science. 1975 Apr 4;188(4183):57–59. doi: 10.1126/science.163493. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Comparison of glucose metabolism in the lactating mammary gland of the rat in vivo and in vitro. Effects of starvation, prolactin or insulin deficiency. Biochem J. 1977 Apr 15;164(1):153–159. doi: 10.1042/bj1640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Valdes C., McGarry J. D., Foster D. W. Maternal-fetal carnitine relationship and neonatal ketosis in the rat. J Biol Chem. 1976 Oct 10;251(19):6007–6012. [PubMed] [Google Scholar]
- Simpson A. A., Simpson M. H., Sinha Y. N., Schmidt G. H. Changes in concentration of prolactin and adrenal corticosteroids in rat plasma during pregnancy and lactation. J Endocrinol. 1973 Sep;58(3):675–676. doi: 10.1677/joe.0.0580675. [DOI] [PubMed] [Google Scholar]
- Smith R. W. The effects of pregnancy and lactation on the activities in rat liver of some enzymes associated with glucose metabolism. Biochim Biophys Acta. 1975 Nov 10;411(1):22–29. doi: 10.1016/0304-4165(75)90281-0. [DOI] [PubMed] [Google Scholar]
- Smith R. W. The effects of pregnancy, lactation and involution on the metabolism of glucose and acetate by rat liver tissue. J Dairy Res. 1973 Oct;40(3):339–351. doi: 10.1017/s0022029900014710. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Walsh A. Effect of lactation on lipolysis in rat adipose tissue. Lipids. 1976 May;11(5):418–420. doi: 10.1007/BF02532850. [DOI] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Lea M. A., Convery H. J., Stamm N. B. Regulation of gluconeogenesis and glycolysis: studies of mechanisms controlling enzyme activity. Adv Enzyme Regul. 1967;5:257–300. doi: 10.1016/0065-2571(67)90020-9. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., McKeown S. R., Ilic V. Interactions of glucose, acetoacetate and insulin in mammary-gland slices of lactating rats. Biochem J. 1975 Aug;150(2):145–152. doi: 10.1042/bj1500145. [DOI] [PMC free article] [PubMed] [Google Scholar]


