Abstract
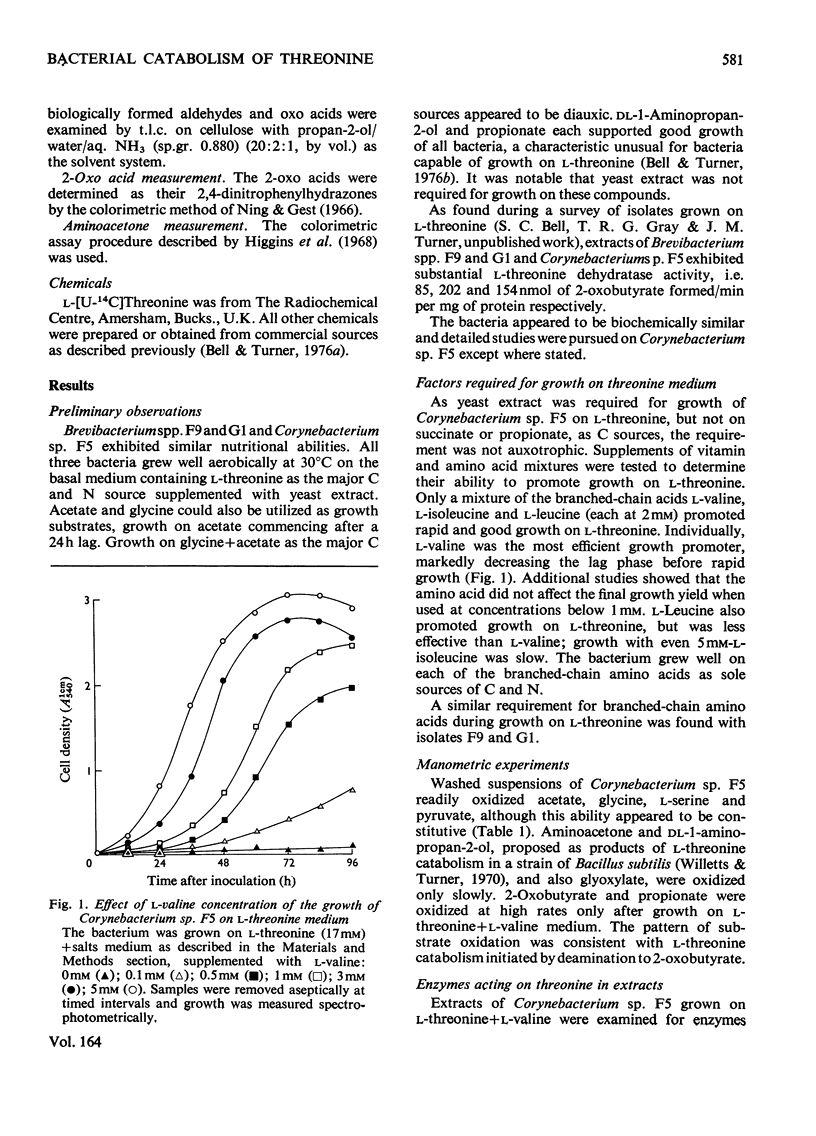
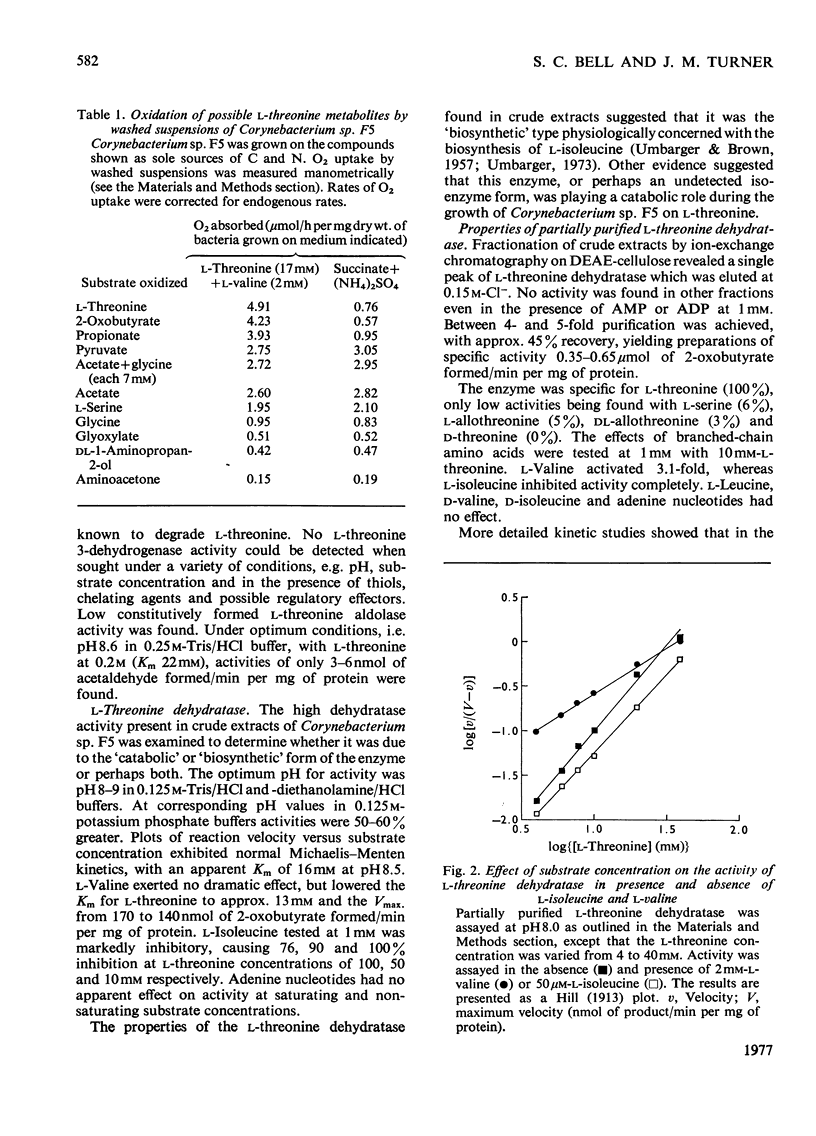
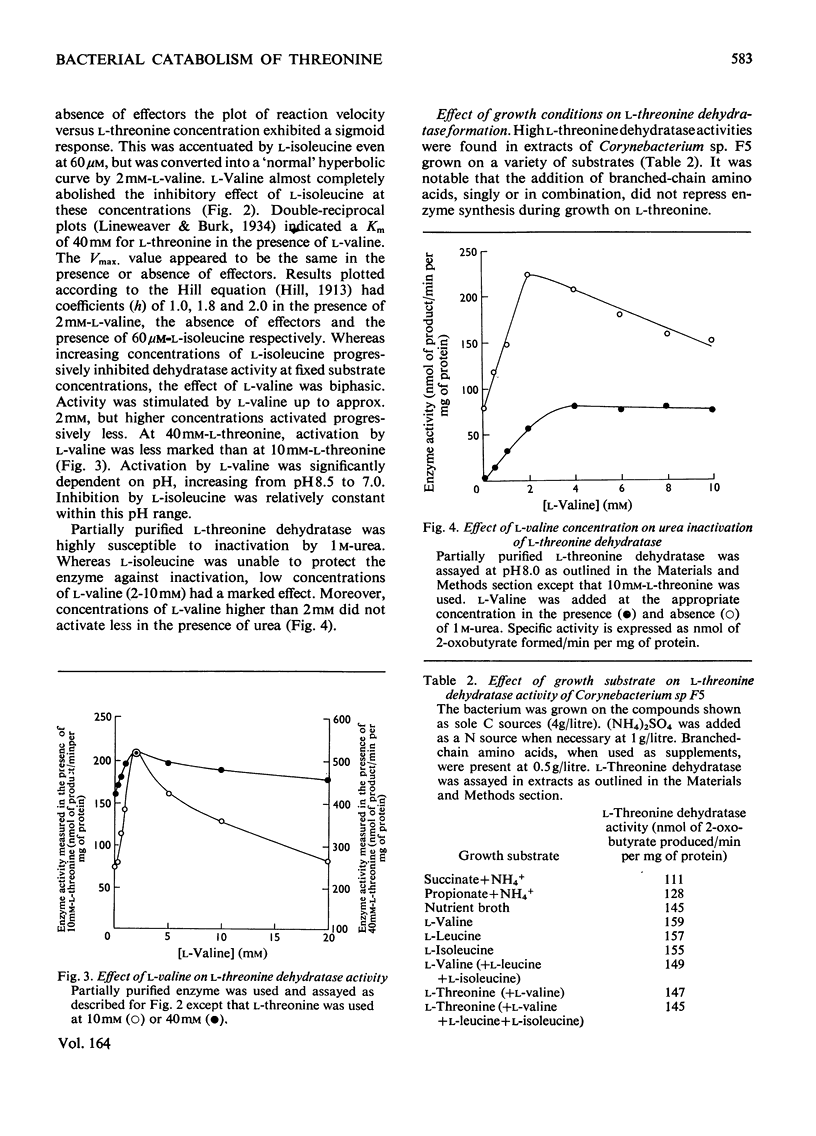
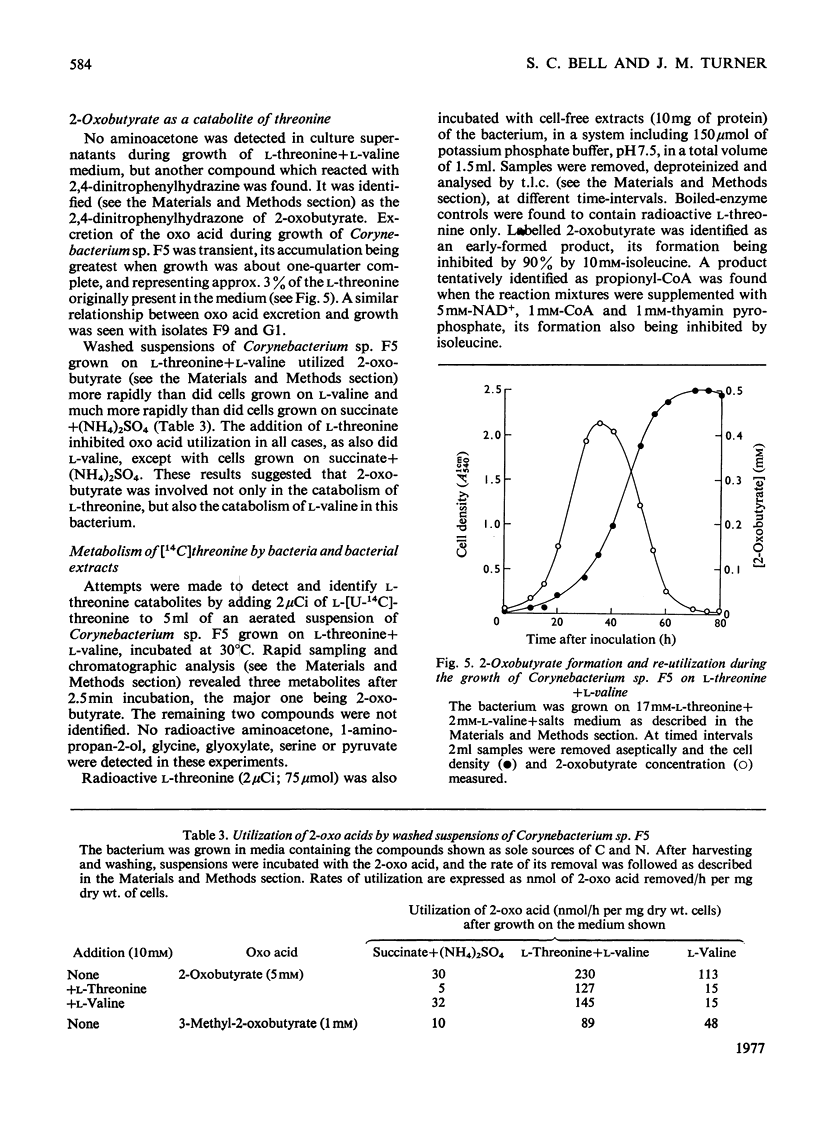
1. Three bacterial isolates capable of growth on l-threonine medium only when supplemented with branched-chain amino acids, and possessing high l-threonine dehydratase activity, were examined to elucidate the catabolic route for the amino acid. 2. Growth, manometric, radiotracer and enzymic experiments indicated that l-threonine was catabolized by initial deamination to 2-oxobutyrate and thence to propionate. No evidence was obtained for the involvement of l-threonine 3-dehydrogenase or l-threonine aldolase in threonine catabolism. 3. l-Threonine dehydratase of Corynebacterium sp. F5 (N.C.I.B. 11102) was partially purified and its kinetic properties were examined. The enzyme exhibited a sigmoid kinetic response to substrate concentration. The concentration of substrate giving half the maximum velocity, [S0.5], was 40mm and the Hill coefficient (h) was 2.0. l-Isoleucine inhibited enzyme activity markedly, causing 50% inhibition at 60μm, but did not affect the Hill constant. At the fixed l-threonine concentration of 10mm, the effect of l-valine was biphasic, progressive activation occurring at concentrations up to 2mm-l-valine, but was abolished by higher concentrations. Substrate-saturation plots for the l-valine-activated enzyme exhibited normal Michaelis–Menten kinetics with a Hill coefficient (h) of 1.0. The kinetic properties of the enzyme were thus similar to those of the `biosynthetic' isoenzyme from Rhodopseudomonas spheroides rather than those of the enteric bacteria. 4. The synthesis of l-threonine dehydratase was constitutive and was not subject to multivalent repression by l-isoleucine or other branched-chain amino acids either singly or in combination. 5. The catabolism of l-threonine, apparently initiated by a `biosynthetic' l-threonine dehydratase in the isolates studied, depended on the concomitant catabolism of branched-chain amino acids. The biochemical basis of this dependence appeared to lie in the further catabolism of 2-oxobutyrate by enzymes which required branched-chain 2-oxo acids for their induction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrtt G. J. Biosynthesis of isoleucine and valine in Rhodopseudomonas spheroides: regulation of threonine deaminase activity. J Bacteriol. 1971 Mar;105(3):718–721. doi: 10.1128/jb.105.3.718-721.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. Bacterial catabolism of threonine. Threonine degradation initiated by L-threonine-NAD+ oxidoreductase. Biochem J. 1976 May 15;156(2):449–458. doi: 10.1042/bj1560449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. L-Threonine catabolism via aminoacetone: a search for a pathway in bacteria. Biochem Soc Trans. 1976;4(3):497–500. doi: 10.1042/bst0040497. [DOI] [PubMed] [Google Scholar]
- Datta P. Purification and feedback control of threonine deaminase activity of Rhodopseudomonas spheroides. J Biol Chem. 1966 Dec 25;241(24):5836–5844. [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y. P. Threonine deaminase (dehydratase) in Azotobacter chroococcum. Enzymologia. 1971 Aug 31;41(2):91–98. [PubMed] [Google Scholar]
- Hall T. C., Cocking E. C. High-efficiency liquid-scintillation counting of 14C-labelled material in aqueous solution and determination of specific activity of labelled proteins. Biochem J. 1965 Sep;96(3):626–633. doi: 10.1042/bj0960626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T., Hirashima M., Ide S., Hamada M., Okabe K., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. I. Isolation, purification, and properties of pyruvate dehydrogenase complex of pig heart muscle. J Biol Chem. 1966 Oct 25;241(20):4694–4699. [PubMed] [Google Scholar]
- Higgins I. J., Pickard M. A., Turner J. M. Aminoacetone formation and utilization by pseudomonads grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):105–114. doi: 10.1099/00221287-54-1-105. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. I. The incorporation of [2-14C] acetate by Pseudomonas fluorescens, and by a Corynebacterium, grown on ammonium acetate. Biochem J. 1958 Mar;68(3):535–542. doi: 10.1042/bj0680535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsunuma T., Elsässer S., Holzer H. Purification and some properties of threonine dehydratase from yeast. Eur J Biochem. 1971 Dec 22;24(1):83–87. doi: 10.1111/j.1432-1033.1971.tb19657.x. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Nagate T., Yoshida H., Yamada H. Threonine aldolase from Candida humicola. II. Purification, crystallization and properties. Biochim Biophys Acta. 1972 Mar 8;258(3):779–790. doi: 10.1016/0005-2744(72)90179-9. [DOI] [PubMed] [Google Scholar]
- Marshall V. D., Sokatch J. R. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972 Jun;110(3):1073–1081. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey L. K., Conrad R. S., Sokatch J. R. Regulation of leucine catabolism in Pseudomonas putida. J Bacteriol. 1974 Apr;118(1):112–120. doi: 10.1128/jb.118.1.112-120.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey L. K., Sokatch J. R., Conrad R. S. Branched-chain amino acid catabolism in bacteria. Bacteriol Rev. 1976 Mar;40(1):42–54. doi: 10.1128/br.40.1.42-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima R., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. VI. Effects of isoleucine and valine on threonine dehydratase activity and its formation. J Biochem. 1972 Jun;71(6):951–960. doi: 10.1093/oxfordjournals.jbchem.a129866. [DOI] [PubMed] [Google Scholar]
- Morris J. G. Utilization of L-threnonine by a pseudomonad: a catabolic role for L-threonine aldolase. Biochem J. 1969 Nov;115(3):603–605. doi: 10.1042/bj1150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning C., Gest H. Regulation of L-isoleucine biosynthesis in the photosynthetic bacterium rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1823–1827. doi: 10.1073/pnas.56.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokushige M., Hayaishi O. Threonine metabolism and its regulation in Clostridium tetanomorphum. J Biochem. 1972 Aug;72(2):469–477. doi: 10.1093/oxfordjournals.jbchem.a129923. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Threonine deaminases. Adv Enzymol Relat Areas Mol Biol. 1973;37:349–395. doi: 10.1002/9780470122822.ch6. [DOI] [PubMed] [Google Scholar]
- Willetts A. J., Turner J. M. L-Threonine acetaldehyde-lyase in a strain of Bacillus subtilis. Biochim Biophys Acta. 1971 Oct;252(1):105–110. doi: 10.1016/0304-4165(71)90097-3. [DOI] [PubMed] [Google Scholar]


