Abstract
The rubber antioxidant 6PPD has gained significant attention due to its highly toxic transformation product, 6PPD-quinone (6PPDQ). Despite their detection in urines of pregnant women, the placental transfer and developmental toxicity of 6PPD and 6PPDQ are unknown. Here, we treated C57Bl/6 mice with 4mg/kg 6PPD or 6PPDQ to investigate their urine excretion and placental transfer. Female and male mice exhibited sex difference in excretion profiles of 6PPD and 6PPDQ. Urine concentrations of 6PPDQ were one order of magnitude lower than those of 6PPD, suggesting lower excretion and higher bioaccumulation of 6PPDQ. In pregnant mice treated with 6PPD or 6PPDQ from embryonic day 11.5 to 15.5, 6PPDQ showed ~1.5–8 times higher concentrations than 6PPD in placenta, embryo body, and embryo brain, suggesting higher placental transfer of 6PPDQ. Using in vitro dual-luciferase reporter assays, we revealed that 6PPDQ activated the human retinoic acid receptor α (RARα) and retinoid X receptor α (RXRα) at concentrations as low as 0.3 μM, which was ~10-fold higher than the concentrations detected in human urines. 6PPD activated the RXRα at concentrations as low as 1.2 μM. These results demonstrate the exposure risks of 6PPD and 6PPDQ during pregnancy and emphasize the need for further toxicological and epidemiological investigations.
Keywords: 6PPD, 6PPDQ, placental transfer, RARα, RXRα
Graphic Abstract
INTRODUCTION
N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) is widely used in tire rubber as an antioxidant at ~0.4–2% by weight.1 Recently, 6PPD raised significant concerns due to its transformation product, 6PPD-quinone (6PPDQ), which showed extremely high acute toxicity in several aquatic species at environmentally-relevant concentrations.2–6 6PPDQ is generated through the reaction of 6PPD with atmospheric ozone and discharged largely through tire wear particles and stormwater runoff.2,7 6PPDQ has been reported to induce acute mortality in coho salmon Oncorhynchus kisutch, brook trout Salvelinus fontinalis, rainbow trout Oncorhynchus mykiss, and white-spotted char Salvelinus leucomaenis Pluvius, with 24/96-hr median lethal concentrations (LC50) ranging from 0.095 to 1.0 μg/L.2–6 Global studies have now reported 6PPD and 6PPDQ ubiquitously in aquatic systems.8–16
Beyond ecological risks, emerging evidence suggests that humans also have considerable exposure risks to 6PPD and 6PPDQ. 6PPD and 6PPDQ have been detected in air and atmospheric particles,8,17–21 indoor dust,18,22 playground dust,23 as well as crumb rubber and consumer products.24 More importantly, 6PPD and 6PPDQ were recently reported in human urine, with pregnant women showing the highest exposure (median concentrations: 6PPD, 0.068 ng/mL; 6PPDQ, 2.91 ng/mL), followed by non-pregnant adults (6PPD, 0.018 ng/mL; 6PPDQ, 0.40 ng/mL) and children (6PPD, 0.015 ng/mL; 6PPDQ, 0.076 ng/mL).25 These results raise concerns about the potential health effects of 6PPD and 6PPDQ, especially during gestation. Studies have suggested that 6PPD and 6PPDQ can cause developmental and neurotoxicity in vitro and in zebrafish and elegan models.26–29 For example, 6PPDQ can form DNA adducts in mammalian cells, indicating potential for teratogenic effects.26 Exposure to 6PPDQ caused abnormal locomotion behaviors in Caenorhabditis elegans at 0.1–10 μg/L and neurodegeneration at 10 μg/L.27 6PPD and 6PPDQ exposure to zebrafish embryos (10 and 25 μg/L) during the developmental period (1–96 hours post fertilization) caused morphological changes, behavioral toxicity, and cardiotoxicity in zebrafish larvae.28 Exposure of zebrafish embryos to 6PPD (0.22 mg/L; 2–120 hours post fertilization) caused developmental toxicity and altered hormone levels and gene expressions in embryos.29 Although developmental effects have not been observed in mammals for 6PPD and 6PPDQ, these results suggest that the mammalian embryonic stage may also be sensitive to these chemicals due to the physiologic similarities to the model organisms.30,31 In mammalian systems, the placenta serves as a barrier to protect the fetus from circulating environmental contaminants.32,33 However, some contaminants with specific physicochemical properties or binding activities can cross the placental barrier,34–36 leading to fetal exposure.37–39 It is currently unclear whether 6PPD and 6PPDQ can be transferred from exposed mothers to their embryos in mammalian systems. Moreover, the impact of 6PPD and 6PPDQ on nuclear receptor pathways such as thyroid hormone receptors (TRs), retinoic acid receptor (RAR), and retinoid X receptor (RXR), which are essential for embryonic development, remains unknown. According to U.S. Environmental Protection Agency, chemicals that pose risks to children’s health should be prioritized for assessment under the Toxic Substances Control Act.40,41 Therefore, understanding the maternal transfer and developmental effects of 6PPD and 6PPDQ is crucial for future toxicological investigations and risk assessments.
Here, we seek to fill these knowledge gaps by investigating the gestational transfer of 6PPD and 6PPD-Q in mice. Female and male C57Bl/6 mice were first treated with 6PPD and 6PPDQ by oral gavage to determine urine excretion kinetics and relevant dosing concentrations. Pregnant mice were then exposed to 6PPD and 6PPDQ (4 mg/kg by oral gavage) from embryonic day (E) 11.5 to E15.5, and 6PPD and 6PPDQ concentrations in dam liver, brain, and placenta, as well as fetus body and brain, were measured with internal standard calibration using liquid chromatography coupled to an orbitrap high resolution mass spectrometer (LC-HRMS). To investigate the potential developmental toxicity of 6PPD and 6PPDQ, we further evaluated the agonistic and antagonistic potency of 6PPD (0.005–20 μM) and 6PPDQ (0.005–20 μM) towards TRs, RARα, and RXRα by in vitro dual-luciferase reporter assay.
MATERIALS AND METHODS
Chemicals.
6PPD (>95% purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). 6PPDQ (97% purity) and 6PPDQ-d5 (96% purity) were purchased from Toronto Research Chemicals (Toronto, ON, Canada). All solid standards were stored in airtight bottles at −20 °C. OPTIMA® grade acetone, chloroform, methanol (MeOH), acetonitrile (ACN), water, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA).
Urinary Excretion in Mice.
Animal protocols in this study were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. The C57Bl/6 mice were maintained under a 12-hour light and 12-hour dark cycle and provided with ad libitum access to food and water. To investigate urine excretion kinetics, we treated female and male mice at age of 10–15 weeks with 6PPD or 6PPDQ (n = 12/group, with 6 females and 6 males) at 4 mg/kg by oral gavage, which mimics the primary exposure pathway of 6PPD and 6PPDQ through oral ingestion reported in a previous study.8 Chemicals were dissolved in corn oil. The volume used for treatment was adjusted based on the body weight of the mouse (5 mL/kg body weight). Mouse urine samples (10–60 μL) were collected prior to treatment and 1, 5, and 24 hours after oral gavage, if available (n = 3–6 urine samples collected for each sex at each time point; Table S3). Mice were gently held from behind and positioned over foil to collect urine. Urine samples were collected into glass vials to avoid sorption loss onto plastics,42 and were spiked immediately with 150 μL MeOH and 50 μL of 500 μg/L 6PPDQ-d5 in MeOH. The MeOH spike was employed because 6PPD reacts fast in water (6PPD aqueous half-life at pH 7: ~5 hours),5,43,44 and our previous study has suggested acceptable stability of 6PPD in MeOH when stored at −20 °C (69–96% recovery after three months of storage).24 Samples were vortexed (1 min), frozen (-20 °C, 2 hours), and centrifuged (2000 rpm, 4 °C, 10 min). The supernatants were collected in 200 μL glass inserts and frozen at −80 °C until LC-HRMS analysis.
Maternal Transfer Mice Experiments.
C57Bl/6 mice were set up with one male and two females per breeding cage. After overnight breeding, male and female mice were separated, and the copulatory plug-positive females were identified as E0.5. Pregnant mice were randomly assigned to control (n = 3; mice treated with corn oil) and exposure groups (n = 3 per group; mice treated with 6PPD or 6PPDQ dissolved in corn oil) and were treated with vehicle control or chemicals (4 mg/kg) from E11.5 to E15.5 once per day by oral gavage. On E15.5, pregnant mice were sacrificed. Dam brain, liver, placenta, embryo body, and embryo brain samples were collected. Six to eight placentae and embryos were collected from each dam and analyzed separately. All tissue samples were contained in 2 mL sterile Eppendorf Safe-Lock tubes (Eppendorf, Hamburg, Germany), wet tissue mass recorded, and then frozen at −80°C until chemical extraction.
Tissue Extraction.
Tissue samples were thawed on ice for 30 min. Samples were spiked with 50 μL methanol solution of 500 μg/L 6PPDQ-d5 and equilibrated under room temperature for 30 min. Preliminary data showed that MeOH had the highest extraction efficiency for 6PPD and 6PPDQ compared with MeOH:water 1:1 (v/v) and chloroform (data not shown). Therefore, pure methanol was selected for tissue extraction. A pre-cleaned 5 mm stainless steel bead (Qiagen Inc., Valencia, CA) and 0.8 mL of MeOH were added to each sample tube. Samples were homogenized on a TissueLyzer (Qiagen Inc., Valencia, CA; 25 Hz, 5 min), centrifuged (16000 rpm, 15 min), and the supernatants were collected into 2 mL glass vials. After the extraction was repeated once, the combined supernatants for each sample were dried on a vacuum concentrator (liquid volume monitored constantly to avoid over-drying and potential chemical loss), redissolved in 200 μL ACN, and transferred into glass vial inserts. Because precipitates were observed in the re-dissolved samples, they were centrifuged again at 4 °C (2000 rpm, 10 min). The supernatants were then transferred into new vial inserts and frozen at −80 °C until LC-HRMS analysis. Triplicate method blanks (empty Eppendorf tubes without tissue samples) were included for each sample extraction batch (three extraction batches, n = 9 method blanks total). 6PPD and 6PPDQ were not detected in method blanks.
Instrumental Analysis.
Urine samples and tissue extracts were injected (5 μL) into a Vanquish ultra-high performance liquid chromatography (UHPLC) system coupled to a Q Exactive quadrupole orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Kinetex polar C18 column (150 × 2.1 mm, 2.6 μm particle size, 100 A pore size; Phenomenex, Torrance, USA) was employed with SecurityGuard C18 column (2.1 mm ID) at 30 °C column temperature. The mobile phases (0.5 mL/min) were 0.1 % formic acid in both water (A) and ACN (B) with the following gradient: 0–1 min 5 % B, 1–11 min 5–100 % B, 11–12 min 100 % B, 12–12.5 min 100–5% B, 12.5–15 min 5% B. The mass spectrometer was performed in positive heated electrospray ionization (see Text S1 for detailed instrumental parameters). The raw spectra were converted to .mzML files using MSconvert (ProteoWizard)45 followed by feature extraction with MZmine 3.2.846 (see Table S1 for feature extraction parameters). Peak areas of the chemical features for 6PPD (m/z 269.2013 at 6.0 min, mass error 2 ppm), 6PPDQ (m/z 299.1752 at 8.4 min, mass error 2 ppm), and 6PPDQ-d5 (m/z 304.2067 at 8.4 min, mass error <1 ppm) were used for quantification. LC–HRMS data files are available through the MassIVE repository (massive.uscd.edu) under the following identifier: MSV000091363.
6PPD and 6PPDQ were quantified with 7-point calibration curves (0.5, 1, 5, 10, 50, 100, 500 μg/L with 125 μg/L 6PPDQ-d5, R2 >0.997) based on their peak area ratios to 6PPDQ-d5 as the internal standard. Fresh stock solutions of 6PPD (1 g/L in acetone) were prepared for each analytical batch from solid standards to mitigate the stock solution instability.24,43 Stock solutions of 6PPDQ and 6PPDQ-d5 (both at 100 mg/L in acetone) were prepared once and stored at −20 °C until use. The limits of detection (LODs) and quantification (LOQs) were defined as the analyte concentrations with signal-to-noise ratios of 3 and 10, respectively. The LOD and LOQ of 6PPD in urine samples were 0.28 and 0.92 ng/mL, and those for 6PPDQ were 0.19 and 0.63 ng/mL, respectively. In fetus brain samples (sample weight <0.05 g), the LOD and LOQ of 6PPD were 0.43 and 1.4 ng/g, and those for 6PPDQ were 0.13 and 0.43 ng/g, respectively. In all other tissue samples (sample weight ~0.1–0.5 g), the LOD and LOQ of 6PPD were 0.07–0.18 and 0.23–0.60 ng/g, and those for 6PPDQ were 0.08–0.43 and 0.27–1.4 ng/g, respectively (Table S2). All the detected concentrations in samples were >LOQs, except one urine sample in the 6PPD exposure group (n = 1/10) and five urine samples in the 6PPDQ exposure group (n = 5/9) collected at 24 hours after oral gavage. The 6PPD concentration between the LOD and LOQ was estimated as half of the LOQ in statistical analysis, following previous studies.47–51 The 6PPDQ urine concentration at 24-hours were not estimated (and noted as <LOQs throughout the manuscript) due to the high frequency of samples between the LOD and LOQ. To evaluate method recovery, 6PPD and 6PPDQ were spiked in triplicate into ~0.1 g of control fetus body samples (i.e., fetus body tissue without 6PPD and 6PPDQ) at three levels (5, 10, 50 μg/kg) and extracted together with the tissue samples. Recovery was defined as the detected concentrations in spiked samples over nominal spike concentrations and was 63–110% for 6PPD and 89–110% for 6PPDQ across the three spiking levels. The standard deviations of the measured spike recoveries were <17% for both analytes at three spiking levels. The absolute recoveries of 6PPDQ-d5 in samples of exposed mice, defined as the peak areas in samples over the peak areas in analytical standards, were 81 ± 37% for urine samples, 91 ± 22% for fetus brain samples, and from 40 ± 15% to 58 ± 14% in dam brain, liver, placenta, and fetus body samples (Table S2).
Dual-luciferase Reporter Assays.
The dual-luciferase reporter assays for TRs, RXRα and RARα were carried out as described previously with some modifications.38,52,53 The plasmids used for RARα and RXRα assays were pGAL4-(UAS)5-TK-LUC, pCMX-GAL4- hRARα-LBD, and pCMX-GAL4- hRXRα-LBD, kindly provided by Professor Albert Braeuning (German Federal Institute for Risk Assessment). Plasmids used for TRs assays were pcDNA3-TRα or pcDNA3-TRβ, and DR-4-TKLuc-reporter plasmid, kindly provided by Professor Lars C. Moeller (University of Duisburg-Essen Germany). Following previous methods,52,53 we employed Human Embryonic Kidney 293 (HEK293) cells due to their high transfection efficiency, high reproducibility, and absence of interfering bioluminescence signals. In brief, HEK 293 cells were seeded in 96 well plate in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, and incubated for 24 hours before transfection with Fugene 6 (Promega, Madison, WI, USA) according to the manufacturer’s protocol. pGL4.74, which contains renilla luciferase gene, was used as an internal control for transfection efficiency. After incubating for another 24 hours, cells were treated with DMSO (0.2%), 6PPD (0.005–20 μM, 4-fold serial dilution), or 6PPDQ (0.005–20 μM, 4-fold serial dilution) for 48 hours. The activities of the firefly luciferase and renilla luciferase were measured following the protocol in the dual-luciferase reporter assay kit (Promega, Madison, WI, USA). The results were expressed as the average relative firefly luciferase activity.
Statistical Analysis.
Statistics were performed using Microsoft Excel and R (version 4.2.1). Results are presented as mean ± standard error of the mean. For animal experiments, the differences between groups were compared using the Mann-Whitney U test. For in vitro cell assays, the Kruskal-Wallis test followed by the Dunn’s post-hoc test was performed to determine statistical significance. Results with p <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Urine Excretion Tests.
To test the urine excretion kinetics of 6PPD and 6PPDQ, we treated female (n=6) and male (n=6) mice with 6PPD or 6PPDQ at 4 mg/kg. Urine samples were collected pre-exposure and 1, 5, and 24 hours after oral gavage; urine was available for 3–6 mice for each sex at each time point (Table S3). 6PPD and 6PPDQ were not detected in the pre-exposure samples (Figure 1a, Table S3). After oral gavage, urine concentrations of 6PPD and 6PPDQ in treated mice varied in a time-dependent manner (Figure 1a; Table S3). Urine concentrations of 6PPD were 45 ± 45 ng/mL after 1 hour of treatment, increased to 110 ± 48 ng/mL at 5 hours, and then decreased to 5.9 ± 3.6 ng/mL at 24 hours. For 6PPDQ, urine concentrations were 7.9 ± 2.2 ng/mL, 4.3 ± 1.1 ng/mL, and <LOQ at 1, 5, and 24-hours post-treatment, respectively (Figure 1a). We do note that there is a potential low bias in urine 6PPD concentrations due to the short aqueous half-life of 6PPD (~5 h aqueous half-life at pH 7).5,43,44 Although we spiked methanol (200 μL; ~3–20 times the volume of urine) immediately into urine samples to mitigate aqueous reactions, the stability of 6PPD in water/methanol mixture remains to be tested. Therefore, 6PPD urine concentrations should be considered semi-quantitative. Despite these quantification uncertainties, 6PPD concentrations in mice urine were one order of magnitude higher than those of 6PPDQ (p < 0.05 in Mann-Whitney U test). This was consistent with the higher water solubility of 6PPD (6PPD solubility, 560–1000 μg/L;43,44 6PPDQ solubility, 38–67 μg/L42,44) and suggests higher excretion and lower bioaccumulation of 6PPD compared with 6PPDQ. Interestingly, the peak urine concentrations of 6PPD appeared later than 6PPDQ (5 hours for 6PPD vs 1 hour for 6PPDQ). This observation possibly indicates different binding affinities of 6PPD and 6PPDQ to mice kidney transporters, which could lead to altered reabsorption or secretion efficiencies.54 Nevertheless, a comprehensive understanding of this phenomenon requires further in-depth investigations.
Figure 1.
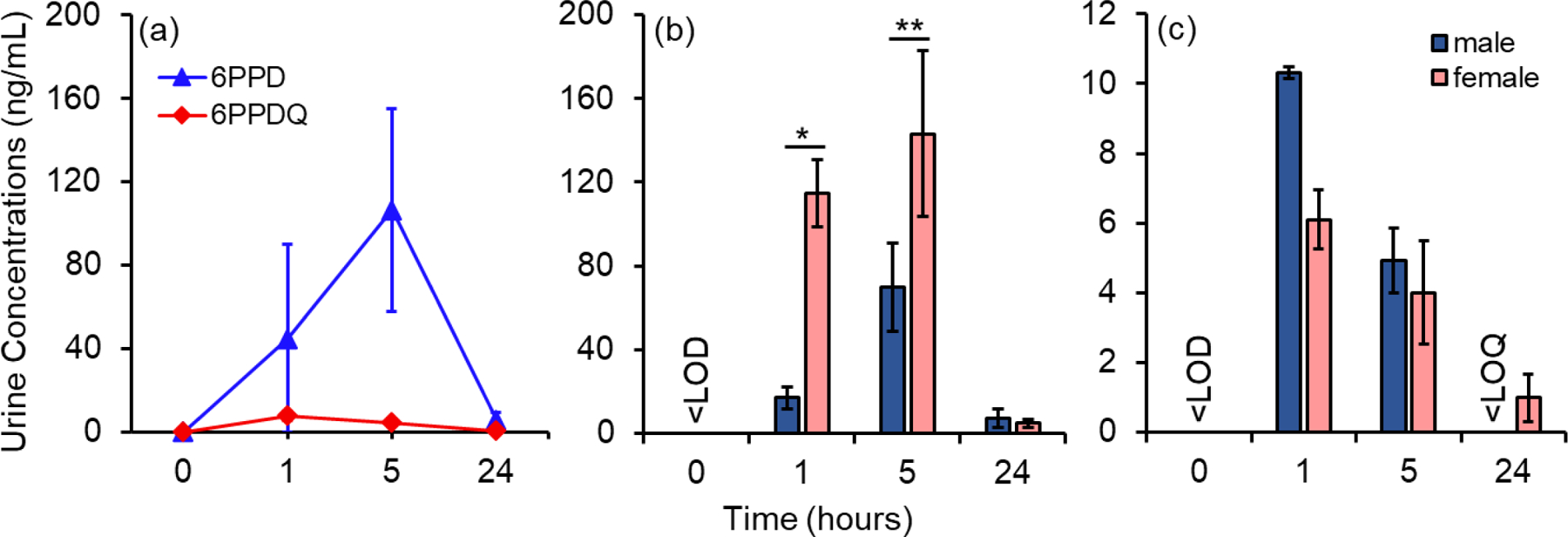
Urine concentrations of 6PPD and 6PPDQ in mice at different time points after oral gavage. (a) Concentrations of 6PPD and 6PPDQ in all mice (n = 12 per treatment group, with 6 male and 6 female; urine samples were available for 7–12 mice at each time point). (b) 6PPD and (c) 6PPDQ urine concentrations separated by sex. Male (n = 6) and female (n = 6) mice were orally gavaged with 4 mg/kg of 6PPD or 6PPDQ in corn oil. Results are expressed as means ± SEM. SEM, standard error of the mean. Concentrations <LOQs were estimated as LOQ/2 in statistical analysis. * (p < 0.05), ** (p < 0.01) indicate concentrations significantly different between male and female mice in Mann-Whitney U test. <LOD, 6PPD and 6PPDQ were not detected in urine samples pre-exposure. <LOQ, 6PPDQ concentrations were not estimated in urines of male mice at 24-hr because four out of six samples were detected at concentrations <LOQ.
Since 6PPD can undergo abiotic transformation to 6PPDQ in the environment, we investigated whether this transformation process also occurs in vivo by screening for 6PPDQ in urine samples from mice treated with 6PPD. 6PPDQ was only detected in five urine samples from the 6PPD exposure group at 24 hours post-exposure (10 samples total; Table S3). The detected concentrations were all <LOQs, suggesting that 6PPDQ is not the major biotransformation product of 6PPD in mice. This is consistent with previous observations that the 6PPD to 6PPDQ transformation ratio was less than 2% in human liver microsomes,25 suggesting that human are exposed to 6PPDQ mainly through direct environmental exposure rather than through 6PPD metabolism.
Many chemicals exhibit sex-related differences in metabolism and excretion.55,56 When the concentrations of 6PPD and 6PPDQ were evaluated separately in male and female samples, 6PPD were significantly higher in female urine samples than 6PPD at 1 hour (110 ± 20 vs 17 ± 5 ng/mL) and 5 hours post treatment (140 ± 40 vs 70 ± 21 ng/mL; p < 0.05 in Mann-Whitney U test; Table S3, Figure 1b). In contrast, the 6PPDQ concentrations were higher in male urine samples at 1 hour (10 ± 0.2 vs 6.1 ± 0.8 ng/mL Table S3, Figure 1c), despite the lack of statistical significance (p = 0.057 in Mann-Whitney U test) potentially due to the low sample sizes (n = 3 for male and n = 4 for female at 1 hour; Table S3). The mechanisms for sex specific chemical excretion are complicated. Gender differences in body weight, size, blood and organ volumes, perfusion rates, body water and fat content, and enzyme activities could all contribute to the divergent absorption, distribution, and metabolism profiles between males and females, and ultimately affect the elimination rates.55,57,58 Although detailed mechanisms remain unclear, our results underscore the sex difference in the toxicokinetics of 6PPD and 6PPDQ.
Overall, the concentrations of 6PPDQ in urine samples of mice exposed acutely at 4 mg/kg were similar to those detected in human urine (<LOQ-4.3 ng/mL at 5–24 hour post exposure in mice, 0.08–2.9 ng/mL in humans), while the urine concentrations of 6PPD in mice were two orders of magnitude higher than those in human (5.9 ng/mL at 24 hour post exposure in mice, 0.015–0.068 ng/mL in human).25 We considered the discrepancy for 6PPD acceptable due to the sample collection and analytical uncertainties of 6PPD (e.g., this study: immediate methanol spike after urine collection, immediate sample freezing at −80 °C; population survey: at-home or in-hospital urine collection, sample frozen at −40 °C without methanol spike).25 We used the 4 mg/kg/d exposure for subsequent placental transfer experiments to maintain 6PPDQ within environmentally relevant concentrations.
Organ Distribution and Placental Transfer of 6PPD and 6PPDQ.
E11.5 to E15.5 is a crucial period for embryonic development in mice, during which many developmental processes occur that are essential for the proper growth and differentiation of various tissues and organs, including the heart, lungs, liver, and nervous system.59–61 Any disruptions during this period may lead to various developmental abnormalities, such as neurological disorders or birth defects.62,63 To evaluate whether 6PPD and 6PPDQ can cross the placenta and enter the developing embryo, we treated pregnant dams with vehicle control, 6PPD, or 6PPDQ from E11.5 to E15.5. Samples collected included dam liver, dam brain, placenta, and the body and brain of the embryos.
After treatment for 5 days, 6PPD and 6PPDQ did not alter the body weight, placenta weight, or brain weight of the embryos compared to the vehicle-only controls (Figure 2). 6PPD and 6PPDQ were not detected in the method blanks or the vehicle-only group (Table S4). In contrast, 6PPD and 6PPDQ were detected in all tissue types in the corresponding exposure groups, demonstrating the bioaccumulation, placental transfer, and blood-brain barrier permeation potentials of 6PPD and 6PPDQ. In the dams, liver appeared to be the major target organ for both 6PPD and 6PPDQ after oral ingestion (6PPD, 200 ± 180 μg/kg; 6PPDQ, 470 ± 90 μg/kg), followed by brain (6PPD, 11 ± 4 μg/kg; 6PPDQ, 84 ± 6 μg/kg) and placenta (6PPD, 9.2 ± 5.3 μg/kg; 6PPDQ, 77 ± 14 μg/kg) (Figure 3, Table S4). In the fetuses, 6PPD and 6PPDQ were higher in body tissues (6PPD, 23 ± 9 μg/kg; 6PPDQ, 34 ± 5 μg/kg) than in the brain (6PPD, 8.6 ± 5.0 μg/kg; 6PPDQ, 15 ± 3 μg/kg). The mechanisms for xenobiotics to cross the placenta typically involve passive diffusion, active transport, and pinocytosis.34,64 Chemicals with logKow values of −0.9 to 5 are suggested to have higher cross-placental potentials through passive diffusion, while the placental transfer of more lipophilic chemicals are slow or limited.65,66 The measured logKow values of 6PPD (4.84)43 and 6PPDQ (4.30 ± 0.02)42 suggested that placental transfer through passive diffusion is possible; however, the actual transfer mechanisms of 6PPD and 6PPDQ merit further investigation. A previous study has shown that exposure to roadway runoff can disrupt the blood-brain barrier of juvenile coho salmons, a potentially key mechanism for the acute lethality of 6PPDQ to salmonids.67 Here, we corroborate this result by demonstrating that 6PPD and 6PPDQ can cross the blood-brain barrier in mammalian systems. In the fetal brain, lower concentrations of 6PPD and 6PPDQ were observed compared with the dam brain (p < 0.05 in Mann-Whitney U test), indicating a partial protection effect of the placenta.
Figure 2.
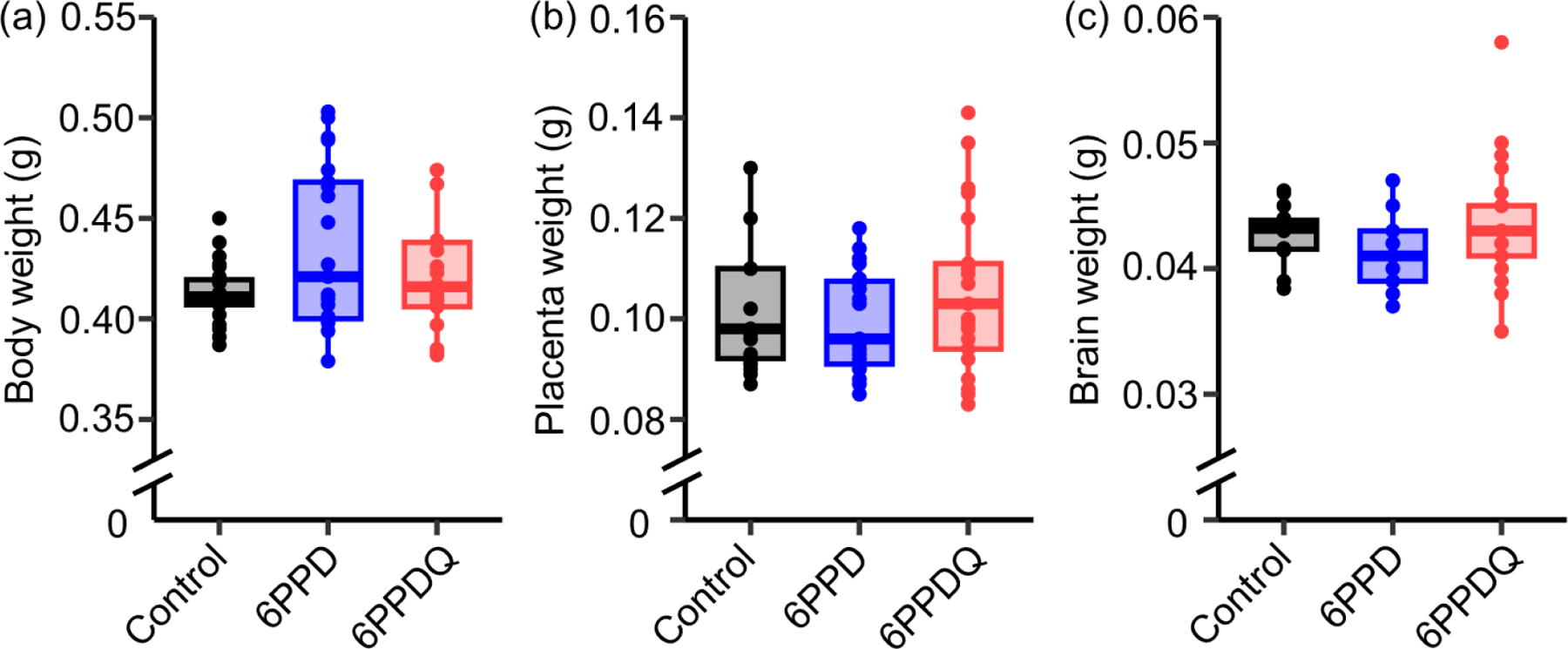
(a) Body, (b) placenta, and (c) brain weights for the embryos at E15.5. Pregnant mice (n = 3 per group) were treated with vehicle control (corn oil, n = 23 embryos), 6PPD (4 mg/kg in corn oil, n = 23 embryos), or 6PPDQ (4 mg/kg in corn oil, n = 21 embryos) from E11.5 to E15.5 by oral gavage.
Figure 3.
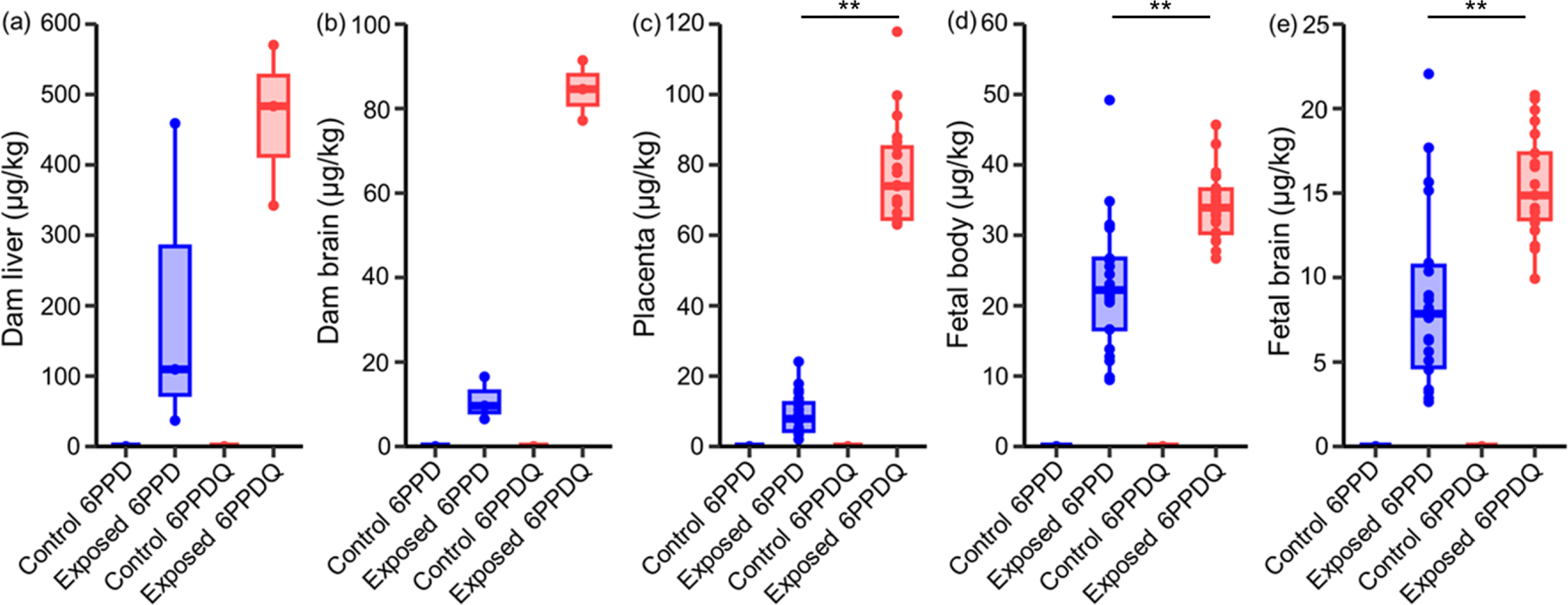
Concentrations (μg/kg) of 6PPD and 6PPDQ in (a) dam liver, (b) dam brain, (c) placenta, (d) fetal body, and (e) fetal brain on E15.5. Pregnant mice (n = 3 per group) were treated with vehicle control (corn oil, n= 23 embryos), 6PPD (4 mg/kg in corn oil, n=23 embryos), or 6PPDQ (4 mg/kg in corn oil, n= 21 embryos) from E11.5 to E15.5 by oral gavage. ** (p < 0.01) indicates 6PPD and 6PPDQ concentrations were significantly different in Mann-Whitney U test.
Notably, 6PPDQ showed higher bioaccumulation than 6PPD in all tissue types with concentrations ~1.5–8 times higher (p < 0.05 for placenta, fetal body, and fetal brain; p = 0.1 for dam brain; p = 0.2 for dam liver in Mann-Whitney U test; Figure 3). This is consistent with the higher excretion of 6PPD than 6PPDQ in our toxicokinetics tests, and the rapid depletion of 6PPD in human liver microsomes (65% depletion in 3 hours).25 In the 6PPD exposure group, 6PPDQ was detected in only one dam liver sample (n = 1/3, 6PPDQ of 5.5 μg/kg) at 5% of the detected 6PPD concentration, consistent with previous results that 6PPDQ is not the main metabolite of 6PPD in liver metabolism.25 6PPD was not detected in tissues from the 6PPDQ exposure group (Table S4).
Human RAR and RXR Activation.
Since both 6PPD and 6PPDQ were detected in the embryo body and brain tissues, we next sought to determine whether they could disrupt TRs, RARα, and RXRα pathways with a dual-luciferase assay. Triiodothyonine (T3), all trans retinoic acid (atRA), and 9-cis retinoic acid (9-cis-RA), the endogenous TRs, RARα and RXRα agonists, were used as positive controls, and respectively activated TRs, RARα, and RXRα in a dose dependent manner (Figure S1 and S2). For both 6PPD and 6PPDQ, no significant changes in relative luciferase activity were observed in the TRα and TRβ assays after treatment at the concentration of 20 μM, indicating no agonistic or antagonistic activity towards TRα or TRβ at 20 μM (Figure S2). In contrast, 6PPDQ activated the RARα in a dose dependent manner, with significant changes observed at concentrations as low as 0.3 μM (p < 0.05 in Kruskal-Wallis test). 6PPDQ showed strong agonistic activity towards RARα, with a maximum fold change of 2.3 ± 0.1 fold over DMSO control (Figure 4). No significant changes in relative luciferase activity were observed following 6PPD exposure to RARα, suggesting that 6PPD does not behave as RARα agonist. RARα is an important transcription factor that plays crucial roles in many biological processes, including cell growth, differentiation, and embryonic development, which has been extensively studied as a potential drug target for various diseases.68–70 While there are many endogenous and synthetic drugs that can bind to RARs, the identification of environmental chemicals as RAR ligands is relatively limited. Certain polybrominated diphenyl ethers (PBDEs) - flame retardant chemicals used in textiles, furniture, and electronics, have been reported to have RAR agonistic activity. Among the 11 PBDEs and 6 OH-PBDEs tested, only one PBDE (BDE154) and three OH-PBDEs (2’-OH-BDE7, 4’-OH-BDE17, and 2’-OH-BDE68) showed RAR agonistic activity.71 Significant RAR effects were observed at minimum concentrations of 50 or 500 μg/L for the PBDEs,71 similar to that for 6PPDQ (lowest effects seen at 0.3 μM (~80 μg/L)).
Figure 4.
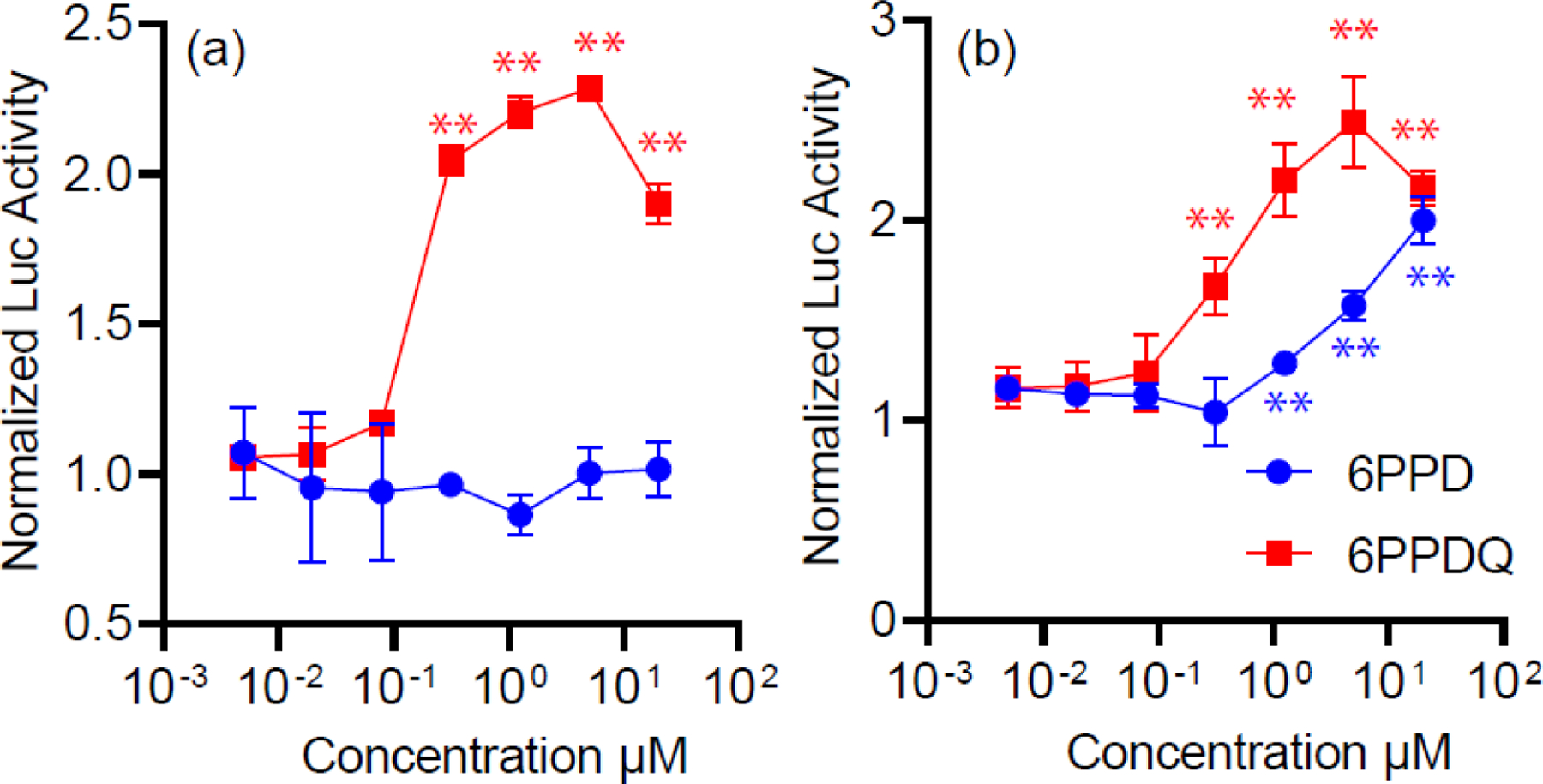
RAR and RXR agonistic activities of 6PPD and 6PPDQ. (a) Luciferase activities induced by 6PPD and 6PPDQ at the concentrations ranged from 0.005–20 μM to test RAR agonistic activity. (b) Luciferase activities induced by 6PPD and 6PPDQ at the concentrations ranged from 0.005–20 μM to test RXR agonistic activity. *(p < 0.05), **(p < 0.01) indicate values significantly different compared with the untreated group (control, 0.2% DMSO) in the Kruskal-Wallis test followed by a Dunn’s post-hoc test. The results were expressed as means ± SEM (n = 3). SEM, standard error of the mean.
Both 6PPD and 6PPDQ were found to exhibit agonistic activity towards RXRα (p < 0.05 in Kruskal-Wallis test; Figure 4). 6PPD activated RXRα in a dose-dependent manner, with significant increases observed at concentrations as low as 1.2 μM, and a maximum increase of 2.0 ± 0.2 fold over control. 6PPDQ had a higher agonistic activity towards RXRα, with a significant increase first observed at 0.3 μM and a maximum increase of 2.5 ± 0.4 fold over the control. Studies have shown that RXRs play important roles in embryonic development, and postnatal treatment with RXR agonists can disrupt skeletal morphogenesis in rats and enhance the differentiation of cardiomyocytes derived from embryonic stem cells.72,73 However, there is limited information on environmental chemicals that exhibit RXR agonistic activity. Organotin compounds are known to activate RXRs at nanomolar concentrations and have been identified as the causal agents for the malformations in Chinese sturgeon and the imposex in gastropods.74,75 Our study identifies 6PPD and 6PPDQ as new ligands that contribute to RXR agonistic activities in the environment.
ENVIRONMENTAL IMPLICATIONS
The ubiquitous usage of 6PPD and the detection of 6PPD and 6PPDQ in pregnant women raise significant concerns about their health risks, particularly during the vulnerable developmental periods. In this study, we reported for the first time that both 6PPD and 6PPDQ can cross the placental blood barrier and enter the developing embryo and brain. We also report for the first time that 6PPDQ is a RAR agonist, and that both 6PPD and 6PPDQ are RXR agonists. These nuclear receptors play crucial roles in embryonic development. Consequently, when environmental chemicals with RAR and/or RXR agonistic activities are maternally transferred to the embryos, there is a serious risk of developmental abnormalities or birth defects.76–78 Both 6PPDQ and 6PPD have been detected in human urine samples with concentrations of 6PPDQ up to 8.58 μg/L (~0.03 μM) in pregnant women.25 Significant increases in both the RARα and RXRα pathways were observed upon exposure to 6PPDQ at 0.3 μM, which was approximately 10-fold higher than the concentrations found in human urine samples. While this study focused on the effects of short-term exposure to 6PPDQ and 6PPD, humans are exposed to these chemicals over the lifespan. Given this prolonged exposure, it is possible that these chemicals can disrupt the RARα and RXRα pathways, leading to potential adverse health effects at exposure-relevant levels. Therefore, there is an urgent need for continued research into the developmental toxicity of 6PPD and 6PPDQ using both chronic exposure animal studies and epidemiological investigations. Notably, we observed sex-specific excretion of 6PPD and 6PPDQ in mice urine samples, adding to the increasing evidence that sex can have strong influences on the toxicokinetics, toxicodynamics, and outcomes of environmental pollutants.55,79,80 In future toxicology and epidemiological studies of 6PPD and 6PPDQ, separate considerations for males and females are merited.
We note that the statistical analysis in this study is limited by the sex differences and sample sizes. The distinct excretion profiles of male and female mice have led to the apparently large variations in urine concentrations when both sexes are considered together. Additionally, although six animals were included for each sex in the urine excretion tests, urine was not always available for all the animals at each time point (n = 3–6 urine samples for each sex at each time). The reduced sample sizes also led to sometimes large variations in the urine concentrations, reflecting the inherent biological variations in animal models.81 Additional replicates would improve the statistical power of this study and establish more representative data on the urine concentrations of 6PPD and 6PPDQ. Here, we aimed to explore the excretion, bioaccumulation, placental transfer, and blood-brain barrier permeation potentials of 6PPD and 6PPDQ, without attempting to benchmark the toxicokinetic parameters of these chemicals.
Previous reports have highlighted the drastically increased aquatic toxicity and higher environmental persistence of 6PPDQ compared with 6PPD.2,4,6,24,82 Here, we demonstrated that 6PPDQ is ~1.5–8 times more bioaccumulative in pregnant mice than 6PPD in both dam and embryo tissues. More importantly, we also observed that 6PPDQ has higher RAR and RXR agonistic potencies than 6PPD. Our results highlighted again that the inadvertent formation of 6PPDQ from 6PPD is causing much higher ecological and human health risks than previously appreciated. This study continues to stress the urgent need for more careful consideration of environmental transformation products during the safety assessments of high-production-volume commercial chemicals.
Supplementary Material
Synopsis:
6PPD and 6PPDQ can cross the placentae, enter the brains of developing embryos during gestation, and act as RARα and RXRα agonists.
Acknowledgements
M.J.Z. is supported by grants from The National Institute of Environmental Health Sciences (NIEHS; R35ES028366). We thank Dr. Edward P. Kolodziej for valuable feedbacks on the manuscript. We thank Dr. Yuyin Zhou for help on tissue extraction method development. We thank Dr. Shirley Tsunoda for insights on urine excretion kinetics.
Footnotes
Conflict of Interest Disclosure
PCD is an advisor to Cybele and co-founder and scientific advisor to Ometa and Enveda, with prior approval by UC San Diego.
SI statement
Supplementary texts on instrument parameters (Text S1). Supplementary tables on MZmine feature extraction parameters (Table S1); limits of detection/quantification of 6PPD and 6PPDQ (Table S2); urine concentrations of 6PPD and 6PPDQ (Table S3); and tissue concentrations of 6PPD and 6PPDQ (Table S4). Supplementary figures on dose-response curves of endogenous TRs, RARα and RXRα agonists (Figure S1); and TRs activity of 6PPD and 6PPDQ (Figure S2).
References
- (1).R.T. Vanderbilt Company. The Vanderbilt Rubber Handbook; 2010.
- (2).Tian Z; Zhao H; Peter KT; Gonzalez M; Wetzel J; Wu C; Hu X; Prat J; Mudrock E; Hettinger R; Cortina AE; Biswas RG; Kock FVC; Soong R; Jenne A; Du B; Hou F; He H; Lundeen R; Gilbreath A; Sutton R; Scholz NL; Davis JW; Dodd MC; Simpson A; McIntyre JK; Kolodziej EP A Ubiquitous Tire Rubber–Derived Chemical Induces Acute Mortality in Coho Salmon. Science 2021, 371 (6525), 185–189. [DOI] [PubMed] [Google Scholar]
- (3).Tian Z; Gonzalez M; Rideout CA; Zhao HN; Hu X; Wetzel J; Mudrock E; James CA; McIntyre JK; Kolodziej EP 6PPD-Quinone: Revised Toxicity Assessment and Quantification with a Commercial Standard. Environ. Sci. Technol. Lett 2022, 9 (2), 140–146. [Google Scholar]
- (4).Brinkmann M; Montgomery D; Selinger S; Miller JGP; Stock E; Alcaraz AJ; Challis JK; Weber L; Janz D; Hecker M; Wiseman S Acute Toxicity of the Tire Rubber-Derived Chemical 6PPD-Quinone to Four Fishes of Commercial, Cultural, and Ecological Importance. Environ. Sci. Technol. Lett 2022, 9 (4), 333–338. [Google Scholar]
- (5).Di S; Liu Z; Zhao H; Li Y; Qi P; Wang Z; Xu H; Jin Y; Wang X Chiral Perspective Evaluations: Enantioselective Hydrolysis of 6PPD and 6PPD-Quinone in Water and Enantioselective Toxicity to Gobiocypris Rarus and Oncorhynchus Mykiss. Environ. Int 2022, 166, 107374. [DOI] [PubMed] [Google Scholar]
- (6).Hiki K; Yamamoto H The Tire-Derived Chemical 6PPD-Quinone is Lethally Toxic to the White-Spotted Char Salvelinus Leucomaenis Pluvius but Not to Two Other Salmonid Species. Environ. Sci. Technol. Lett 2022, 9 (12), 1050–1055. [Google Scholar]
- (7).Hu X; Zhao HN; Tian Z; Peter KT; Dodd MC; Kolodziej EP Transformation Product Formation upon Heterogeneous Ozonation of the Tire Rubber Antioxidant 6PPD (N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine). Environ. Sci. Technol. Lett 2022, 9 (5), 413–419. [Google Scholar]
- (8).Cao G; Wang W; Zhang J; Wu P; Zhao X; Yang Z; Hu D; Cai Z New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol 2022, 56 (7), 4142–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Johannessen C; Helm P; Metcalfe CD Runoff of the Tire-Wear Compound, Hexamethoxymethyl-Melamine into Urban Watersheds. Arch. Environ. Contam. Toxicol 2021, 2 (2), 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Challis JK; Popick H; Prajapati S; Harder P; Giesy JP; McPhedran K; Brinkmann M Occurrences of Tire Rubber-Derived Contaminants in Cold-Climate Urban Runoff. Environ. Sci. Technol. Lett 2021, 8 (11), 961–967. [Google Scholar]
- (11).Rauert C; Charlton N; Okoffo ED; Stanton RS; Agua AR; Pirrung MC; Thomas KV Concentrations of Tire Additive Chemicals and Tire Road Wear Particles in an Australian Urban Tributary. Environ. Sci. Technol 2022, 56 (4), 2421–2431. [DOI] [PubMed] [Google Scholar]
- (12).Seiwert B; Nihemaiti M; Troussier M; Weyrauch S; Reemtsma T Abiotic Oxidative Transformation of 6-PPD and 6-PPD Quinone from Tires and Occurrence of Their Products in Snow from Urban Roads and in Municipal Wastewater. Water Res. 2022, 212, 118122. [DOI] [PubMed] [Google Scholar]
- (13).Gasperi J; Le Roux J; Deshayes S; Ayrault S; Bordier L; Boudahmane L; Budzinski H; Caupos E; Caubrière N; Flanagan K; Guillon M; Huynh N; Labadie P; Meffray L; Neveu P; Partibane C; Paupardin J; Saad M; Varnede L; Gromaire M-C Micropollutants in Urban Runoff from Traffic Areas: Target and Non-Target Screening on Four Contrasted Sites. Water 2022, 14 (3), 394. [Google Scholar]
- (14).Rauert C; Vardy S; Daniell B; Charlton N; Thomas KV Tyre Additive Chemicals, Tyre Road Wear Particles and High Production Polymers in Surface Water at 5 Urban Centres in Queensland, Australia. Sci. Total Environ 2022, 852, 158468. [DOI] [PubMed] [Google Scholar]
- (15).Zhang H-Y; Huang Z; Liu Y-H; Hu L-X; He L-Y; Liu Y-S; Zhao J-L; Ying G-G Occurrence and Risks of 23 Tire Additives and Their Transformation Products in an Urban Water System. Environ. Int 2023, 171, 107715. [DOI] [PubMed] [Google Scholar]
- (16).Zhang R; Zhao S; Liu X; Thomes MW; Bong CW; Samaraweera ND, D.; Priyadarshana T; Zhong G; Li J; Zhang G Fates of Benzotriazoles, Benzothiazoles, and p-Phenylenediamines in Wastewater Treatment Plants in Malaysia and Sri Lanka. ACS EST Water 2023, 3 (6), 1630–1640. [Google Scholar]
- (17).Zhang Y; Xu C; Zhang W; Qi Z; Song Y; Zhu L; Dong C; Chen J; Cai Z P-Phenylenediamine Antioxidants in PM2.5: The Underestimated Urban Air Pollutants. Environ. Sci. Technol 2021, 56 (11), 6914–6921. [DOI] [PubMed] [Google Scholar]
- (18).Zhang Y-J; Xu T-T; Ye D-M; Lin Z-Z; Wang F; Guo Y Widespread N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine Quinone in Size-Fractioned Atmospheric Particles and Dust of Different Indoor Environments. Environ. Sci. Technol. Lett 2022, 9 (5), 420–425. [Google Scholar]
- (19).Wang W; Cao G; Zhang J; Chen Z; Dong C; Chen J; Cai Z p-Phenylenediamine-Derived Quinones as New Contributors to the Oxidative Potential of Fine Particulate Matter. Environ. Sci. Technol. Lett 2022, 9 (9), 712–717. [Google Scholar]
- (20).Wang W; Cao G; Zhang J; Wu P; Chen Y; Chen Z; Qi Z; Li R; Dong C; Cai Z Beyond Substituted p-Phenylenediamine Antioxidants: Prevalence of Their Quinone Derivatives in PM2.5. Environ. Sci. Technol 2022, 56 (15), 10629–10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Johannessen C; Saini A; Zhang X; Harner T Air Monitoring of Tire-Derived Chemicals in Global Megacities Using Passive Samplers. Environ. Pollut 2022, 314, 120206. [DOI] [PubMed] [Google Scholar]
- (22).Huang W; Shi Y; Huang J; Deng C; Tang S; Liu X; Chen D Occurrence of Substituted p-Phenylenediamine Antioxidants in Dusts. Environ. Sci. Technol. Lett 2021, 8 (5), 381–385. [Google Scholar]
- (23).Liu R; Li Y; Lin Y; Ruan T; Jiang G Emerging Aromatic Secondary Amine Contaminants and Related Derivatives in Various Dust Matrices in China. Ecotoxicol. Environ. Saf 2019, 170, 657–663. [DOI] [PubMed] [Google Scholar]
- (24).Zhao HN; Hu X; Gonzalez M; Rideout CA; Hobby GC; Fisher MF; McCormick CJ; Dodd MC; Kim KE; Tian Z; Kolodziej EP Screening p-Phenylenediamine Antioxidants, Their Transformation Products, and Industrial Chemical Additives in Crumb Rubber and Elastomeric Consumer Products. Environ. Sci. Technol 2023, 57 (7), 2779–2791. [DOI] [PubMed] [Google Scholar]
- (25).Du B; Liang B; Li Y; Shen M; Liu L-Y; Zeng L First Report on the Occurrence of N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine (6PPD) and 6PPD-Quinone as Pervasive Pollutants in Human Urine from South China. Environ. Sci. Technol. Lett 2022, 9 (12), 1056–1062. [Google Scholar]
- (26).Wu J; Cao G; Zhang F; Cai Z A New Toxicity Mechanism of N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine Quinone: Formation of DNA Adducts in Mammalian Cells and Aqueous Organisms. Sci. Total Environ 2023, 866, 161373. [DOI] [PubMed] [Google Scholar]
- (27).Hua X; Feng X; Liang G; Chao J; Wang D Exposure to 6-PPD Quinone at Environmentally Relevant Concentrations Causes Abnormal Locomotion Behaviors and Neurodegeneration in Caenorhabditis Elegans. Environ. Sci. Technol 2023, 57 (12), 4940–4950. [DOI] [PubMed] [Google Scholar]
- (28).Varshney S; Gora AH; Siriyappagouder P; Kiron V; Olsvik PA Toxicological Effects of 6PPD and 6PPD Quinone in Zebrafish Larvae. J. Hazard. Mater 2022, 424, 127623. [DOI] [PubMed] [Google Scholar]
- (29).Peng W; Liu C; Chen D; Duan X; Zhong L Exposure to N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine (6PPD) Affects the Growth and Development of Zebrafish Embryos/Larvae. Ecotoxicol. Environ. Saf 2022, 232, 113221. [DOI] [PubMed] [Google Scholar]
- (30).Veldman MB; Lin S Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res 2008, 64 (5), 470–476. [DOI] [PubMed] [Google Scholar]
- (31).Kaletta T; Hengartner MO Finding Function in Novel Targets: C. Elegans as a Model Organism. Nat. Rev. Drug Discov 2006, 5 (5), 387–399. [DOI] [PubMed] [Google Scholar]
- (32).Prouillac C; Lecoeur S The Role of the Placenta in Fetal Exposure to Xenobiotics: Importance of Membrane Transporters and Human Models for Transfer Studies. Drug Metab. Dispos 2010, 38 (10), 1623–1635. [DOI] [PubMed] [Google Scholar]
- (33).Hu W; Liu C-W; Jiménez JA; McCoy ES; Hsiao Y-C; Lin W; Engel SM; Lu K; Zylka MJ Detection of Azoxystrobin Fungicide and Metabolite Azoxystrobin-Acid in Pregnant Women and Children, Estimation of Daily Intake, and Evaluation of Placental and Lactational Transfer in Mice. Environ. Health Perspect 2022, 130 (2), 27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ma D; Lu Y; Liang Y; Ruan T; Li J; Zhao C; Wang Y; Jiang G A Critical Review on Transplacental Transfer of Per- and Polyfluoroalkyl Substances: Prenatal Exposure Levels, Characteristics, and Mechanisms. Environ. Sci. Technol 2022, 56 (10), 6014–6026. [DOI] [PubMed] [Google Scholar]
- (35).Tetro N; Moushaev S; Rubinchik-Stern M; Eyal S The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res 2018, 35 (4), 71. [DOI] [PubMed] [Google Scholar]
- (36).Zhang X; Cheng X; Lei B; Zhang G; Bi Y; Yu Y A Review of the Transplacental Transfer of Persistent Halogenated Organic Pollutants: Transfer Characteristics, Influential Factors, and Mechanisms. Environ. Int 2021, 146, 106224. [DOI] [PubMed] [Google Scholar]
- (37).Li J; Sun X; Xu J; Tan H; Zeng EY; Chen D Transplacental Transfer of Environmental Chemicals: Roles of Molecular Descriptors and Placental Transporters. Environ. Sci. Technol 2021, 55 (1), 519–528. [DOI] [PubMed] [Google Scholar]
- (38).Hu W; Gao F; Zhang H; Hiromori Y; Arakawa S; Nagase H; Nakanishi T; Hu J Activation of Peroxisome Proliferator-Activated Receptor Gamma and Disruption of Progesterone Synthesis of 2-Ethylhexyl Diphenyl Phosphate in Human Placental Choriocarcinoma Cells: Comparison with Triphenyl Phosphate. Environ. Sci. Technol 2017, 51 (7), 4061–4068. [DOI] [PubMed] [Google Scholar]
- (39).Jiménez JA; Simon JM; Hu W; Moy SS; Harper KM; Liu C-W; Lu K; Zylka MJ Developmental Pyrethroid Exposure and Age Influence Phenotypes in a Chd8 Haploinsufficient Autism Mouse Model. Sci. Rep 2022, 12 (1), 5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).U.S. Environmental Protection Agency. TSCA Work Plan for Chemical Assessments: 2014 Update. Available at http://www.epa.gov/sites/production/files/2015-01/documents/tsca_work_plan_chemicals_2014_update-final.pdf. Accessed June, 2023.
- (41).Phillips AL; Chen A; Rock KD; Horman B; Patisaul HB; Stapleton HM Editor’s Highlight: Transplacental and Lactational Transfer of Firemaster® 550 Components in Dosed Wistar Rats. Toxicol. Sci. Off. J. Soc. Toxicol 2016, 153 (2), 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Hu X; Zhao HN; Tian Z; Peter KT; Dodd MC; Kolodziej EP Chemical Characteristics, Leaching, and Stability of the Ubiquitous Tire Rubber-Derived Toxicant 6PPD-Quinone. Environ. Sci. Process. Impacts 2023, 25, 901. [DOI] [PubMed] [Google Scholar]
- (43).European Union. OSPAR Background Document on 4-(Dimethylbutylamino)Diphenylamine (6PPD), 2006.
- (44).Hiki K; Asahina K; Kato K; Yamagishi T; Omagari R; Iwasaki Y; Watanabe H; Yamamoto H Acute Toxicity of a Tire Rubber-Derived Chemical, 6PPD Quinone, to Freshwater Fish and Crustacean Species. Environ. Sci. Technol. Lett 2021, 8 (9), 779–784. [Google Scholar]
- (45).Chambers MC; Maclean B; Burke R; Amodei D; Ruderman DL; Neumann S; Gatto L; Fischer B; Pratt B; Egertson J; Hoff K; Kessner D; Tasman N; Shulman N; Frewen B; Baker TA; Brusniak M-Y; Paulse C; Creasy D; Flashner L; Kani K; Moulding C; Seymour SL; Nuwaysir LM; Lefebvre B; Kuhlmann F; Roark J; Rainer P; Detlev S; Hemenway T; Huhmer A; Langridge J; Connolly B; Chadick T; Holly K; Eckels J; Deutsch EW; Moritz RL; Katz JE; Agus DB; MacCoss M; Tabb DL; Mallick P A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol 2012, 30 (10), 918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Schmid R; Heuckeroth S; Korf A; Smirnov A; Myers O; Dyrlund TS; Bushuiev R; Murray KJ; Hoffmann N; Lu M; Sarvepalli A; Zhang Z; Fleischauer M; Dührkop K; Wesner M; Hoogstra SJ; Rudt E; Mokshyna O; Brungs C; Ponomarov K; Mutabdžija L; Damiani T; Pudney CJ; Earll M; Helmer PO; Fallon TR; Schulze T; Rivas-Ubach A; Bilbao A; Richter H; Nothias L-F; Wang M; Orešič M; Weng J-K; Böcker S; Jeibmann A; Hayen H; Karst U; Dorrestein PC; Petras D; Du X; Pluskal T Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol 2023, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hornung RW; Reed LD Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 1990, 5 (1), 46–51. [Google Scholar]
- (48).Antweiler RC; Taylor HE Evaluation of Statistical Treatments of Left-Censored Environmental Data Using Coincident Uncensored Data Sets: I. Summary Statistics. Environ. Sci. Technol 2008, 42 (10), 3732–3738. [DOI] [PubMed] [Google Scholar]
- (49).Song S; Zhang T; Huang Y; Zhang B; Guo Y; He Y; Huang X; Bai X; Kannan K Urinary Metabolites of Neonicotinoid Insecticides: Levels and Recommendations for Future Biomonitoring Studies in China. Environ. Sci. Technol 2020, 54 (13), 8210–8220. [DOI] [PubMed] [Google Scholar]
- (50).Schettgen T; Esser A; Alt A; Randerath I; Kraus T; Ziegler P Decomposition Products of the Initiator Bis(2,4-Dichlorobenzoyl)Peroxide in the Silicone Industry: Human Biomonitoring in Plasma and Urine of Workers. Environ. Sci. Technol 2022, 56 (12), 8518–8527. [DOI] [PubMed] [Google Scholar]
- (51).Fromme H; Fuchs V; Albrecht M; Aschenbrenner B; Röhl C; Janitzki N; Herber-Jonat S; Wöckner M; Völkel W; Flemmer AW; Schober W Polychlorinated Dioxins and Dibenzofurans (PCDD/F), Polybrominated Dioxins and Dibenzofurans (PBDD/F), Polychlorinated Biphenyls (PCB), Polybrominated Diphenyl Ethers (PBDE), and per- and Polyfluoroalkyl Substances (PFAS) in German Breast Milk Samples (LUPE 8). Sci. Total Environ 2022, 825, 154066. 10.1016/j.scitotenv.2022.154066. [DOI] [PubMed] [Google Scholar]
- (52).Luckert C; Braeuning A; de Sousa G; Durinck S; Katsanou ES; Konstantinidou P; Machera K; Milani ES; Peijnenburg AACM; Rahmani R; Rajkovic A; Rijkers D; Spyropoulou A; Stamou M; Stoopen G; Sturla S; Wollscheid B; Zucchini-Pascal N; Lampen A Adverse Outcome Pathway-Driven Analysis of Liver Steatosis in Vitro: A Case Study with Cyproconazole. Chem. Res. Toxicol 2018, 31 (8), 784–798. [DOI] [PubMed] [Google Scholar]
- (53).Hönes GS; Rakov H; Logan J; Liao X-H; Werbenko E; Pollard AS; Præstholm SM; Siersbæk MS; Rijntjes E; Gassen J; Latteyer S; Engels K; Strucksberg K-H; Kleinbongard P; Zwanziger D; Rozman J; Gailus-Durner V; Fuchs H; Hrabe de Angelis M; Klein-Hitpass L; Köhrle J; Armstrong DL; Grøntved L; Bassett JHD; Williams GR; Refetoff S; Führer D; Moeller LC Noncanonical Thyroid Hormone Signaling Mediates Cardiometabolic Effects in Vivo. Proc. Natl. Acad. Sci 2017, 114 (52), E11323–E11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Regårdh CG Factors Contributing to Variability in Drug Pharmacokinetics. Iv. Renal Excretion. J. Clin. Pharm. Ther 1985, 10 (4), 337–349. [DOI] [PubMed] [Google Scholar]
- (55).Gochfeld M Sex Differences in Human and Animal Toxicology: Toxicokinetics. Toxicol. Pathol 2017, 45 (1), 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Hu W; Gao P; Wang L; Hu J Endocrine Disrupting Toxicity of Aryl Organophosphate Esters and Mode of Action. Crit. Rev. Environ. Sci. Technol 2023, 53 (1), 1–18. [Google Scholar]
- (57).Soldin O; Mattison D Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet 2009, 48 (3), 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Tanaka E Gender-Related Differences in Pharmacokinetics and Their Clinical Significance. J. Clin. Pharm. Ther 1999, 24 (5), 339–346. [DOI] [PubMed] [Google Scholar]
- (59).Greig LC; Woodworth MB; Galazo MJ; Padmanabhan H; Macklis JD Molecular Logic of Neocortical Projection Neuron Specification, Development and Diversity. Nat. Rev. Neurosci 2013, 14 (11), 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Watson CJ; Khaled WT Mammary Development in the Embryo and Adult: A Journey of Morphogenesis and Commitment. Dev. Camb. Engl 2008, 135 (6), 995–1003. [DOI] [PubMed] [Google Scholar]
- (61).Hata S; Namae M; Nishina H Liver Development and Regeneration: From Laboratory Study to Clinical Therapy. Dev. Growth Differ 2007, 49 (2), 163–170. [DOI] [PubMed] [Google Scholar]
- (62).Chen VS; Morrison JP; Southwell MF; Foley JF; Bolon B; Elmore SA Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. Toxicol. Pathol 2017, 45 (6), 705–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Brust V; Schindler PM; Lewejohann L Lifetime Development of Behavioural Phenotype in the House Mouse (Mus Musculus). Front. Zool 2015, 12 (Suppl 1), S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Griffiths SK; Campbell JP Placental Structure, Function and Drug Transfer. Contin. Educ. Anaesth. Crit. Care Pain 2015, 15 (2), 84–89. [Google Scholar]
- (65).Wan Y; Choi K; Kim S; Ji K; Chang H; Wiseman S; Jones PD; Khim JS; Park S; Park J; Lam MHW; Giesy JP Hydroxylated Polybrominated Diphenyl Ethers and Bisphenol A in Pregnant Women and Their Matching Fetuses: Placental Transfer and Potential Risks. Environ. Sci. Technol 2010, 44 (13), 5233–5239. [DOI] [PubMed] [Google Scholar]
- (66).Takahashi O; Oishi S Disposition of Orally Administered 2,2-Bis(4-Hydroxyphenyl)Propane (Bisphenol A) in Pregnant Rats and the Placental Transfer to Fetuses. Environ. Health Perspect 2000, 108 (10), 931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Blair SI; Barlow CH; McIntyre JK Acute Cerebrovascular Effects in Juvenile Coho Salmon Exposed to Roadway Runoff. Can. J. Fish. Aquat. Sci 2021, 78 (2), 103–109. [Google Scholar]
- (68).Janosek J; Hilscherová K; Bláha L; Holoubek I Environmental Xenobiotics and Nuclear Receptors--Interactions, Effects and in Vitro Assessment. Toxicol. In Vitro 2006, 20 (1), 18–37. [DOI] [PubMed] [Google Scholar]
- (69).Kastner P; Mark M; Chambon P Nonsteroid Nuclear Receptors: What Are Genetic Studies Telling Us about Their Role in Real Life? Cell 1995, 83 (6), 859–869. [DOI] [PubMed] [Google Scholar]
- (70).Jia Y; Zhang H; Hu W; Wang L; Kang Q; Liu J; Nakanishi T; Hiromori Y; Kimura T; Tao S; Hu J Discovery of Contaminants with Antagonistic Activity against Retinoic Acid Receptor in House Dust. J. Hazard. Mater 2022, 426, 127847. [DOI] [PubMed] [Google Scholar]
- (71).Zhao J; Zhu X; Xu T; Yin D Structure-Dependent Activities of Polybrominated Diphenyl Ethers and Hydroxylated Metabolites on Zebrafish Retinoic Acid Receptor. Environ. Sci. Pollut. Res 2015, 22 (3), 1723–1730. [DOI] [PubMed] [Google Scholar]
- (72).Honda M; Hamazaki TS; Komazaki S; Kagechika H; Shudo K; Asashima M RXR Agonist Enhances the Differentiation of Cardiomyocytes Derived from Embryonic Stem Cells in Serum-Free Conditions. Biochem. Biophys. Res. Commun 2005, 333 (4), 1334–1340. [DOI] [PubMed] [Google Scholar]
- (73).Dupuis H; Pest MA; Hadzic E; Vo TX; Hardy DB; Beier F Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model. Int. J. Mol. Sci 2019, 20 (20), 5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Hu J; Zhang Z; Wei Q; Zhen H; Zhao Y; Peng H; Wan Y; Giesy JP; Li L; Zhang B Malformations of the Endangered Chinese Sturgeon, Acipenser Sinensis, and Its Causal Agent. Proc. Natl. Acad. Sci 2009, 106 (23), 9339–9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Nishikawa J; Mamiya S; Kanayama T; Nishikawa T; Shiraishi F; Horiguchi T Involvement of the Retinoid X Receptor in the Development of Imposex Caused by Organotins in Gastropods. Environ. Sci. Technol 2004, 38 (23), 6271–6276. [DOI] [PubMed] [Google Scholar]
- (76).Janesick A; Wu SC; Blumberg B Retinoic Acid Signaling and Neuronal Differentiation. Cell. Mol. Life Sci 2015, 72 (8), 1559–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Morriss-Kay GM; Ward SJ Retinoids and Mammalian Development. Int. Rev. Cytol 1999, 188, 73–131. [DOI] [PubMed] [Google Scholar]
- (78).Maden M The Role of Retinoic Acid in Embryonic and Post-Embryonic Development. Proc. Nutr. Soc 2000, 59 (1), 65–73. [DOI] [PubMed] [Google Scholar]
- (79).Iwata H; Watanabe M; Okajima Y; Tanabe S; Amano M; Miyazaki N; Petrov EA Toxicokinetics of PCDD, PCDF, and Coplanar PCB Congeners in Baikal Seals, Pusa Sibirica: Age-Related Accumulation, Maternal Transfer, and Hepatic Sequestration. Environ. Sci. Technol 2004, 38 (13), 3505–3513. [DOI] [PubMed] [Google Scholar]
- (80).Narizzano AM; Bohannon ME; East AG; McDonough C; Choyke S; Higgins CP; Quinn MJ Jr Patterns in Serum Toxicokinetics in Peromyscus Exposed to Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem 2021, 40 (10), 2886–2898. [DOI] [PubMed] [Google Scholar]
- (81).Voelkl B; Altman NS; Forsman A; Forstmeier W; Gurevitch J; Jaric I; Karp NA; Kas MJ; Schielzeth H; Van de Casteele T; Würbel H Reproducibility of Animal Research in Light of Biological Variation. Nat. Rev. Neurosci 2020, 21 (7), 384–393. [DOI] [PubMed] [Google Scholar]
- (82).Zeng L; Li Y; Sun Y; Liu L-Y; Shen M; Du B Widespread Occurrence and Transport of p-Phenylenediamines and Their Quinones in Sediments across Urban Rivers, Estuaries, Coasts, and Deep-Sea Regions. Environ. Sci. Technol 2023, 57 (6), 2393–2403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


