Abstract
A cellulolytic enzyme was isolated from a commercial cellulase preparation form Aspergillus niger. A yield of about 50mg of enzyme was obtained per 100g of commerial cellulase. The isolated enzyme was homogeneous in the ultracentrifuge at pH 4.0 and 8.0, and in sodium dodecyl sulphate/polyacrylamide-gel electrophoresis but showed one major and two minor bands in disc gel electrophoresis. No carbohydrate was associated with the protein. Amino acid analysis revealed that the enzyme was rich in acidic and aromatic amino acids. Data from the amino acid composition and dodecyl sulphate/polyacrylamide-gel electrophoresis indicated a molecular weight of 26000. The purified enzyme was active towards CM-cellulose, but no activity towards either cellobiose or p-nitrophenyl beta-D-glucoside was detected under the assay conditions used. The pH optimum for the enzyme was pH 3.8-4.0, and it was stable at 25 degrees C over the range pH 1-9; maximum activity (at pH 4.0) was obtained at 45 degrees C. The cellulase was more stable to heat treatment at pH 8.0 than at 4.0. Kinetic studies gave pK values between 4.2 and 5.3 for groups involved in the enzyme-substrate complex.
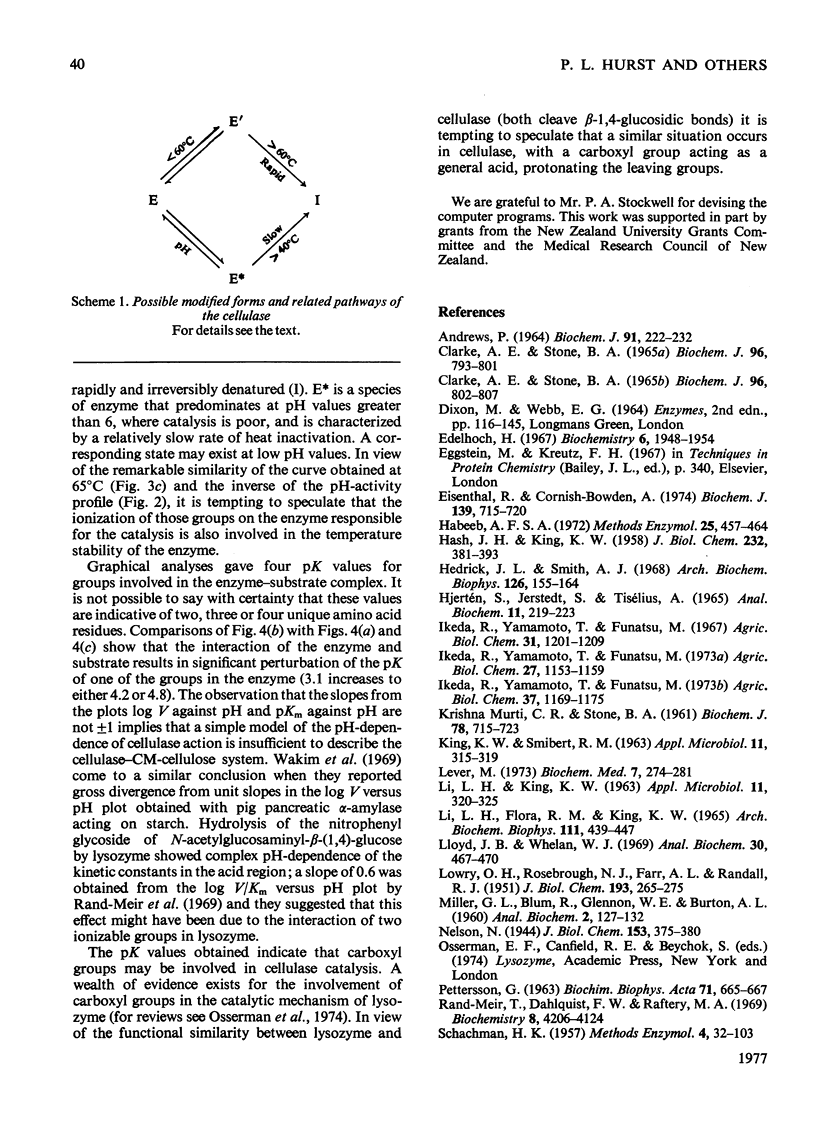
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. E., Stone B. A. Properties of a beta-(1-4)-glucan hydrolase from Aspergillus niger. Biochem J. 1965 Sep;96(3):802–807. doi: 10.1042/bj0960802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. E., Stone B. A. beta-glucan hydrolases from Aspergillus niger. Isolation of a beta-(1-4)-glucan hydrolase and some properties of the beta-(1-3)-glucan-hydrolase components. Biochem J. 1965 Sep;96(3):793–801. doi: 10.1042/bj0960793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASH J. H., KING K. W. On the nature of the beta-glucosidases of Myrothecium verrucaria. J Biol Chem. 1958 May;232(1):381–393. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hjertén S., Jerstedt S., Tiselius A. Some aspects of the use of "continuous" and "discontinuous" buffer systems in polyacrylamide gel electrophoresis. Anal Biochem. 1965 May;11(2):219–223. doi: 10.1016/0003-2697(65)90008-4. [DOI] [PubMed] [Google Scholar]
- KING K. W., SMIBERT R. M. Distinctive properties of beta-glucosidases and related enzymes derived from a commercial Aspergillus niger cellulase. Appl Microbiol. 1963 Jul;11:315–319. doi: 10.1128/am.11.4.315-319.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHNA MURTI C. R., STONE B. A. Fractionation of the beta-glucosidases from Aspergillus niger. Biochem J. 1961 Apr;78:715–723. doi: 10.1042/bj0780715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI L. H., KING K. W. Fractionation of beta-glucosidases and related extracellular enzymes from Aspergillus niger. Appl Microbiol. 1963 Jul;11:320–325. doi: 10.1128/am.11.4.320-325.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lever M. Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem Med. 1973 Apr;7(2):274–281. doi: 10.1016/0006-2944(73)90083-5. [DOI] [PubMed] [Google Scholar]
- Li L. H., Flora R. M., King K. W. Individual roles of cellulase components derived from Trichoderma viride. Arch Biochem Biophys. 1965 Aug;111(2):439–447. doi: 10.1016/0003-9861(65)90207-9. [DOI] [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Rand-Meir T., Dahlquist F. W., Raftery M. A. Use of synthetic substrates to study binding and catalysis by lysozyme. Biochemistry. 1969 Oct;8(10):4206–4214. doi: 10.1021/bi00838a044. [DOI] [PubMed] [Google Scholar]
- SELBY K., MAITLAND C. C. THE FRACTIONATION OF MYROTHECIUM VERRUCARIA CELLULASE BY GEL FILTRATION. Biochem J. 1965 Mar;94:578–583. doi: 10.1042/bj0940578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- SVENNERHOLM L. The quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956 May;1(1):42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- Scoffone E., Fontana A., Rocchi R. Sulfenyl halides as modifying reagents for polypeptides and proteins. I. Modification of tryptophan residues. Biochemistry. 1968 Mar;7(3):971–979. doi: 10.1021/bi00843a014. [DOI] [PubMed] [Google Scholar]
- WOLF M. J., JURKOVICH V., MACMASTERS M. M. Fractionation of a crude polysaccharase system by preparative paper chromatography and paper electrophoresis. Arch Biochem Biophys. 1959 Mar;81(1):15–24. doi: 10.1016/0003-9861(59)90171-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


