Abstract
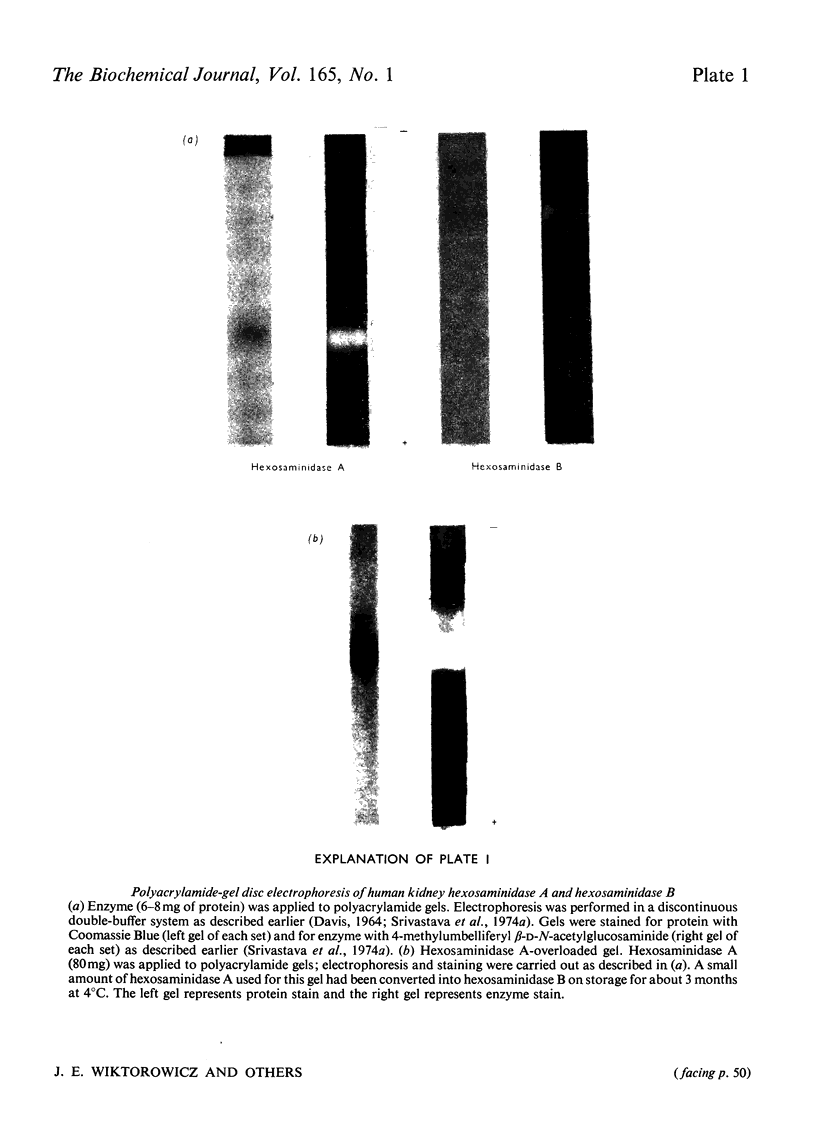
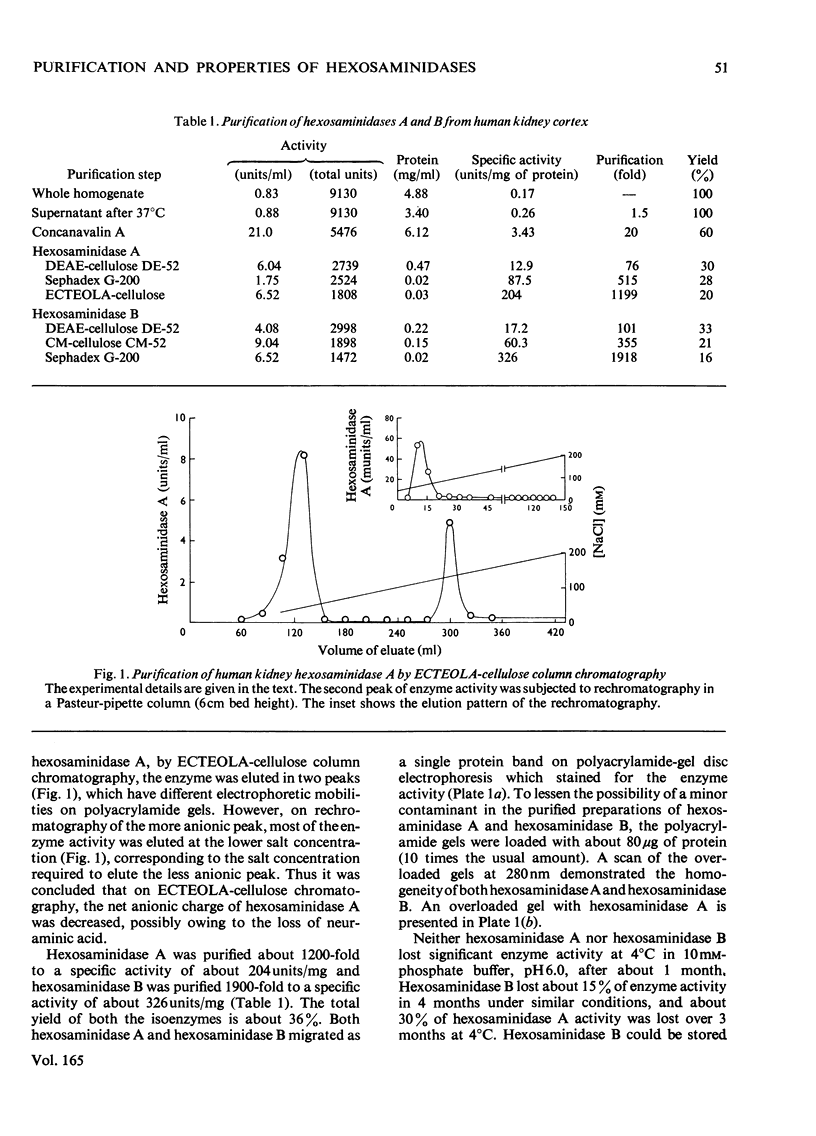
Hexosaminidases (EC 3.2.1.30) A and B from human kidney cortex were purified to homogeneity by using concanavalin A affinity chromatography, ion-exchange chromatography and gel filtration. The yield of homogeneous isoenzymes improved approx. 20-fold, giving preparations of hexosaminidases A and B with specific activities of about 200 and 325 units/mg of protein respectively. The kinetic and structural properties of kidney hexosaminidase isoenzymes were studied and compared with the hexosaminidase isoenzymes from human placenta. The amino acid composition of hexosaminidase A was significantly different from that of hexosaminidase B. In the event of success in developing enzyme-replacement therapy for Tay-Sachs and Sandhoff's diseases, this modified procedure can furnish larger amounts of homogeneous isoenzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Yoshida A., Kuhl W., Lee J. E. The subunits of human hexosaminidase A. Biochem J. 1976 Dec 1;159(3):541–543. doi: 10.1042/bj1590541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Weissmann G., Hoffstein S., Awasthi Y. C., Srivastava S. K. Introduction of purified hexosaminidase A into Tay-Sachs leukocytes by means of immunoglobulin-coated liposomes. Biochemistry. 1976 Jan 27;15(2):452–460. doi: 10.1021/bi00647a034. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Arnon R. Chemical characterization and subunit structure of human N-acetylhexosaminidases A and B. Biochemistry. 1976 Aug 10;15(16):3484–3493. doi: 10.1021/bi00661a014. [DOI] [PubMed] [Google Scholar]
- Hultberg B. N-acetylhexosaminidase activities in Tay-Sachs disease. Lancet. 1969 Nov 29;2(7631):1195–1195. doi: 10.1016/s0140-6736(69)92520-3. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Langerman N. R., Darnall D. W. Quaternary structure of proteins. Annu Rev Biochem. 1970;39:25–62. doi: 10.1146/annurev.bi.39.070170.000325. [DOI] [PubMed] [Google Scholar]
- Kolodny E. H., Brady R. O., Volk B. W. Demonstration of an alteration of ganglioside metabolism in Tay-Sachs disease. Biochem Biophys Res Commun. 1969 Oct 22;37(3):526–531. doi: 10.1016/0006-291x(69)90947-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. E., Yoshida A. Purification and chemical characterization of human hexosaminidases A and B. Biochem J. 1976 Dec 1;159(3):535–539. doi: 10.1042/bj1590535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega T., Ikenaka T., Matsushima Y. Studies on N-acetyl-beta-D-glucosaminidase of Aspergillus oryzae. I. Purification and characterization of N-acetyl-beta-D-glucosaminidase obtained from Takadiastase. J Biochem. 1970 Jul;68(1):109–117. [PubMed] [Google Scholar]
- Miller S. P., Awasthi Y. C., Srivastava S. K. Studies of human kidney gamma-glutamyl transpeptidase. Purification and structural, kinetic and immunological properties. J Biol Chem. 1976 Apr 25;251(8):2271–2278. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The cellular distribution of some rat-kidney glycosidases. Biochem J. 1967 Nov;105(2):877–883. doi: 10.1042/bj1050877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K., Andreae U., Jatzkewitz H. Deficient hexozaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Life Sci. 1968 Mar 15;7(6):283–288. doi: 10.1016/0024-3205(68)90024-6. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Wässle W. Anreicherung und Charakterisierung zweier Formen der menschlichen N-acetyl- -D-hexosaminidase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1119–1133. [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Antibody against purified human hexosaminidase B cross-reacting with human hexosaminidase A. Biochem Biophys Res Commun. 1972 May 26;47(4):753–759. doi: 10.1016/0006-291x(72)90556-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. 3. Biochemical genetics of Tay-Sachs and Sandhoff's diseases. J Biol Chem. 1974 Apr 10;249(7):2054–2057. [PubMed] [Google Scholar]
- Srivastava S. K., Wiktorowicz J. E., Awasthi Y. C. Interrelationship of hexosaminidases A and B: conformation of the common and the unique subunit theory. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2833–2837. doi: 10.1073/pnas.73.8.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Verpoorte J. A. Purification of two -N-acetyl-D-glucosaminidases from beef spleen. J Biol Chem. 1972 Aug 10;247(15):4787–4793. [PubMed] [Google Scholar]
- Wetmore S. J., Verpoorte J. A. The partial purification of two -N-acetyl-D-hexosaminidases from porcine kidney. Can J Biochem. 1972 May;50(5):563–573. doi: 10.1139/o72-078. [DOI] [PubMed] [Google Scholar]



