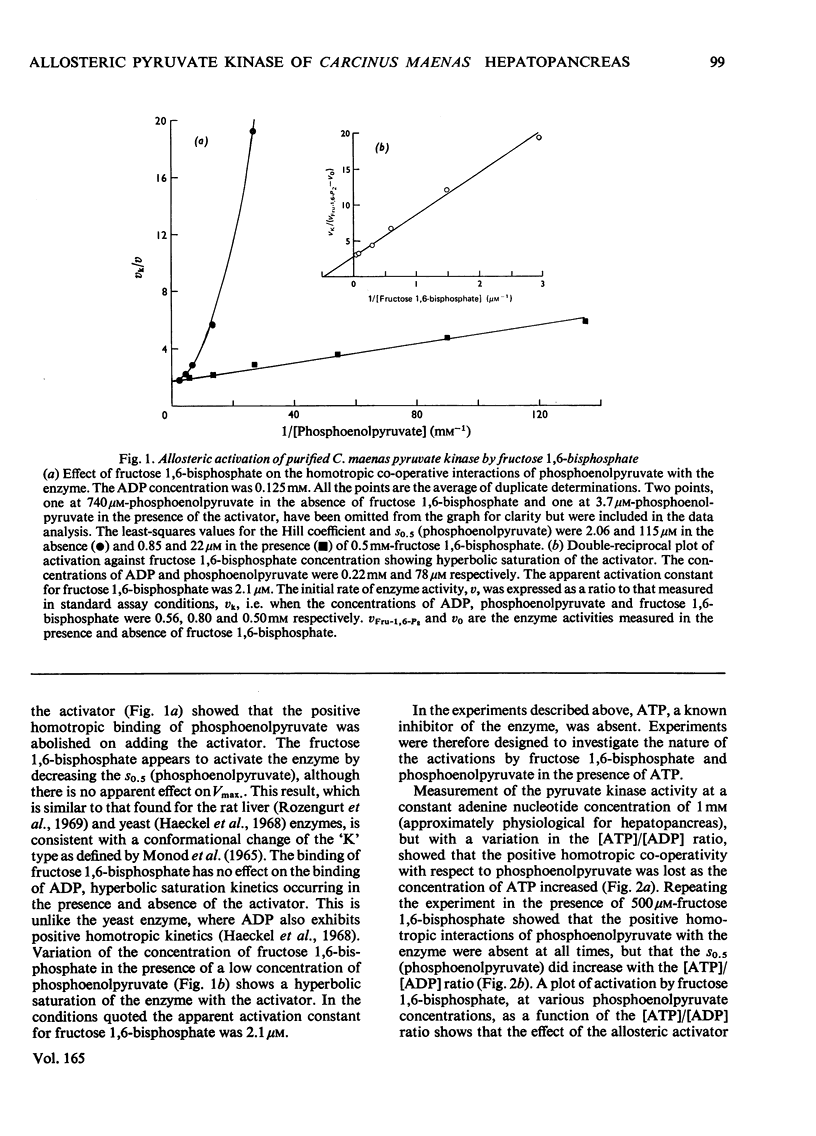
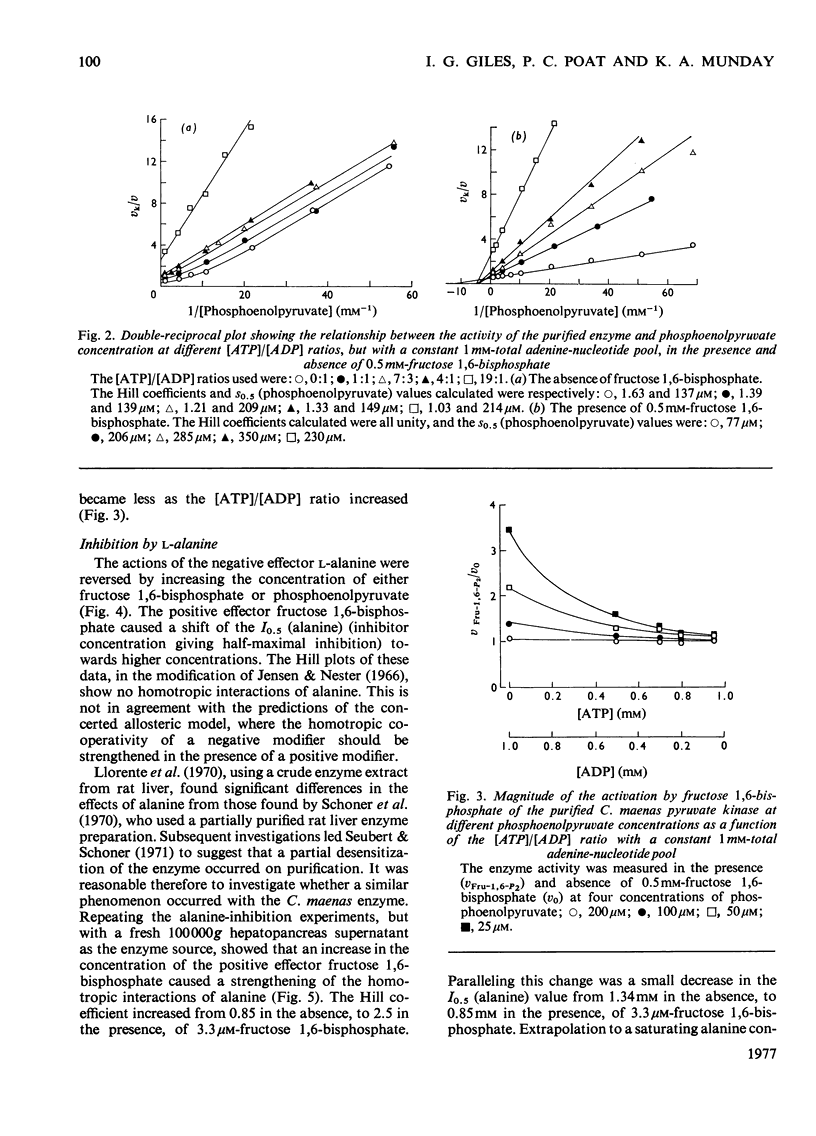
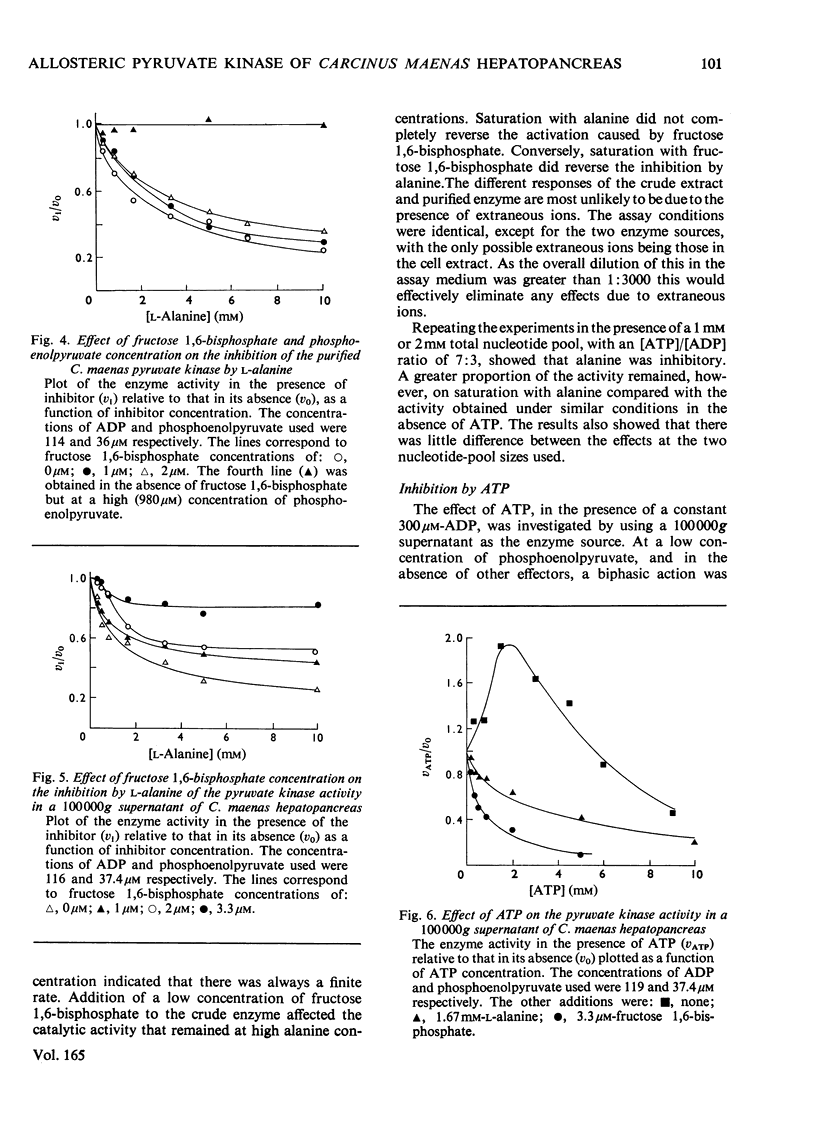
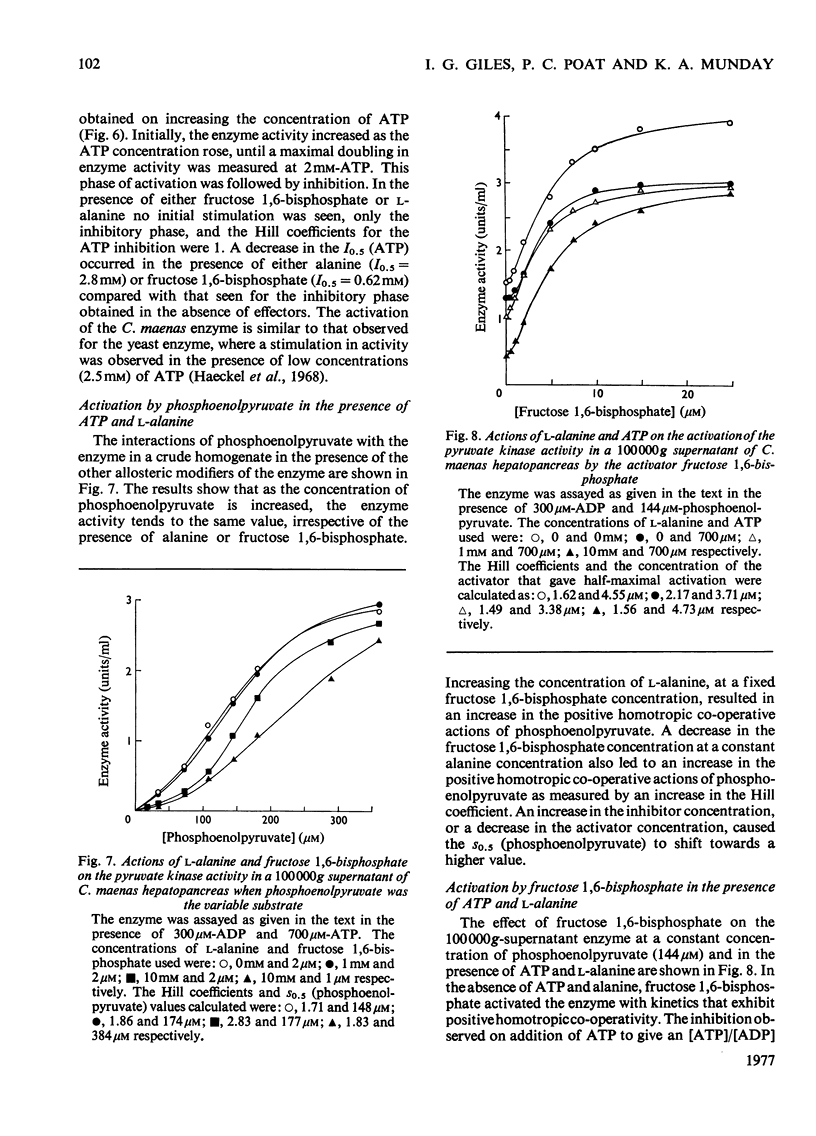
Abstract
1. Pyruvate kinase purified from the hepatopancrease of Carcinus maenas exhibited sigmoidal saturation kinetics with respect to the substrate phosphoenolpyruvate in the absence of the allosteric activator fructose 1,6-bisphosphate, but normal hyperbolic saturation was seen in the presence of this activator. The activation appears to be the result of a decrease in the s0.5 (phosphoenolpyruvate) and not to a change in Vmax. 2. In the presence of ADP and ATP at a constant nucleotide-pool size the results indicate that phosphoenolpyruvate co-operativity is lost on increasing the [ATP]/[ADP] ratio. 3. Paralleling this change is the observation that the fructose 1,6-bisphosphate activation became less at the [ATP]/[ATP] ratio was increased. This was due to the enzyme exhibiting a near-maximal activity in the absence of activator. 4. L-Alanine inhibited the enzyme, but homotropic co-operative interactions were only seen with a cruder (1000000g supernatant) enzyme preparation. The inhibition by alanine could be overcome by increasing the concentration of either phosphoenolpyruvate or fructose 1,6-bisphosphate, although increasing the L-alanine concentration did not appear to be able to reverse the activation by fructose 1,6-bisphosphate. 5. In the presence of a low concentration of phosphoenolpyruvate, increasing the concentration of the product, ATP, caused an initial increase in enzyme activity, followed by an inhibitory phase. In the presence of either fructose 1,6-bisphosphate or L-alanine only inhibition was seen. 6. The inhibition by ATP could not be completely reversed by fructose 1,6-bisphosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L. A simple digital-computer program for estimating the parameters of the hill equation. Eur J Biochem. 1973 Feb 15;33(1):175–180. doi: 10.1111/j.1432-1033.1973.tb02667.x. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy D., Buc H., Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Boyer P. D. The inhibition of pyruvate kinase by ATP: a Mg++ buffer system for use in enzyme studies. Biochem Biophys Res Commun. 1969 Mar 10;34(5):702–706. doi: 10.1016/0006-291x(69)90795-5. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A. Ionized magnesium concentration in axoplasm of dialyzed squid axons. FEBS Lett. 1975 Jan 15;50(1):82–85. doi: 10.1016/0014-5793(75)81046-5. [DOI] [PubMed] [Google Scholar]
- CHANGEUX J. P. [Effect of L-threonine and L-isoleucine analogs on L-threonine desaminase]. J Mol Biol. 1962 Mar;4:220–225. doi: 10.1016/s0022-2836(62)80054-0. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Dalziel K. Kinetic studies of glutamate dehydrogenase with glutamate and norvaline as substrates. Coenzyme activation and negative homotropic interactions in allosteric enzymes. Biochem J. 1969 Dec;115(4):621–631. doi: 10.1042/bj1150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles I. G., Poat P. C., Munday K. A. Purification and properties of pyruvate kinase from the hepatopancreas of Carcinus maenas. Biochem J. 1976 Jan 1;153(1):127–134. doi: 10.1042/bj1530127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles I. G., Poat P. C., Munday K. A. Regulation of pyruvate kinase from the hepatopancreas of the crab Carcinus maenas. Biochem Soc Trans. 1975;3(3):400–402. doi: 10.1042/bst0030400. [DOI] [PubMed] [Google Scholar]
- Giles I. G., Poat P. C., Munday K. A. The regulation of pyruvate kinase in the hepatopancreas of Carcinus maenas. Comp Biochem Physiol B. 1976;55(3B):423–427. doi: 10.1016/0305-0491(76)90315-1. [DOI] [PubMed] [Google Scholar]
- Haeckel R., Hess B., Lauterborn W., Wüster K. H. Purification and allosteric properties of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):699–714. doi: 10.1515/bchm2.1968.349.1.699. [DOI] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. II. The enzymology of feedback inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3373–3380. [PubMed] [Google Scholar]
- Jiménez de Asúa L., Rozengurt E., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). 3. Effect of monovalent cations on its allosteric behavior. J Biol Chem. 1970 Aug 10;245(15):3901–3905. [PubMed] [Google Scholar]
- KRIMSKY I. Phosphorylation of pyruvate by the pyruvate kinase reaction and reversal of glycolysis in a reconstructed system. J Biol Chem. 1959 Feb;234(2):232–236. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur J Biochem. 1970 Mar 1;13(1):45–54. doi: 10.1111/j.1432-1033.1970.tb00897.x. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- McQUATE J. T., UTTER M. F. Equilibrium and kinetic studies of the pyruvic kinase reaction. J Biol Chem. 1959 Aug;234(8):2151–2157. [PubMed] [Google Scholar]
- Rozengurt E., Jiménez de Asúa L., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). II. Effect of pH on its allosteric behavior. J Biol Chem. 1969 Jun 25;244(12):3142–3147. [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- Schoner W., Haag U., Seubert W. On the mechanism of gluconeogenesis and its regulation. VI. Regulation of carbohydrate metabolism by cortisol independent of the de novo synthesis of enzymes in rat liver. Hoppe Seylers Z Physiol Chem. 1970 Sep;351(9):1071–1088. doi: 10.1515/bchm2.1970.351.2.1071. [DOI] [PubMed] [Google Scholar]
- Seubert W., Henning H. V., Schoner W., L'age M. Effects of cortisol on the levels of metabolites and enzymes controlling glucose production from pyruvate. Adv Enzyme Regul. 1968;6:153–187. doi: 10.1016/0065-2571(68)90012-5. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Kleineke J., Willms B., Janson G., Kuhn A. Relationship between intracellular distribution of phosphoenolpyruvate carboxykinase, regulation of gluconeogenesis, and energy cost of glucose formation. Eur J Biochem. 1973 Aug 17;37(2):233–243. doi: 10.1111/j.1432-1033.1973.tb02980.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Viratelle O. M., Seydoux F. J. Pseudoconservative transition: a two-state model for the co-operative behavior of oligomeric proteins. J Mol Biol. 1975 Feb 25;92(2):193–205. doi: 10.1016/0022-2836(75)90223-5. [DOI] [PubMed] [Google Scholar]
- Wieker H. J., Hess B. Allosteric interactions of yeast pyruvate kinase as a function of pH. Biochemistry. 1971 Mar 30;10(7):1243–1248. doi: 10.1021/bi00783a022. [DOI] [PubMed] [Google Scholar]


