Abstract
Diabetes mellitus is a prevalent metabolic disorder worldwide. A variety of antidiabetic medications have been developed to help manage blood glucose levels in diabetic patients, but adverse reactions and efficacy loss over time have spurred research into new therapeutic agents. In view of this, investigations into the antidiabetic effect of herbal products have been encouraged due to their potential availability, inexpensiveness, and relatively minimal side effects. This review explores the antidiabetic potentials of the eight most promising medicinal plants in terms of molecular mechanisms, phytochemistry, toxicology, and efficacy. These plant extracts have gone through clinical trials and demonstrated good control of blood glucose levels by increasing serum insulin levels, enhancing tissue glucose uptake, and/or decreasing intestinal glucose uptake. Yet, medicinal plants are far from being able to replace conventional antidiabetic drugs for patient management but they have the potential for further development if rigorous clinical trials on their mechanisms, delivery, and dose regimen are performed. To date, no study has been performed to isolate and characterize active compounds in these plant extracts, suggesting that further investigations in this area would be the next step to advance this field.
1. Introduction
Diabetes mellitus can cause chronic or acute problems [1] through deficient action or lack of insulin. This happens when the pancreas is unable to produce enough insulin, or when body tissues do not respond to insulin. In those cases, the body cells cannot metabolize sugar properly leading to the breakdown of body fat, protein, glycogen, and the generation of by-products such as ketones [2]. Environmental and genetic factors affect onset and progression of the disease [3]. Polyuria, thirst, weight loss, and blurring of vision are well-known symptoms [4]. Ultimately, diabetes causes organ failure leading to disability or death [5]. Impaired insulin secretion or action could lead to chronic hyperglycemia hence severity of damage is related to time with the disease and how well it has been controlled.
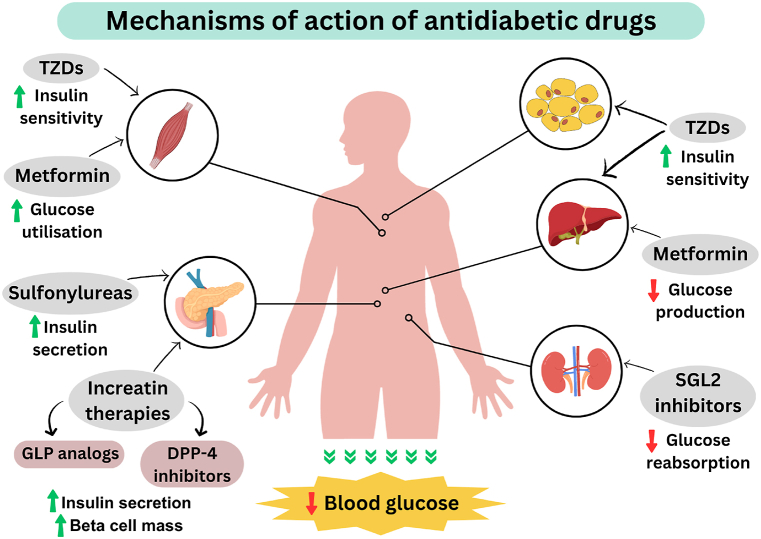
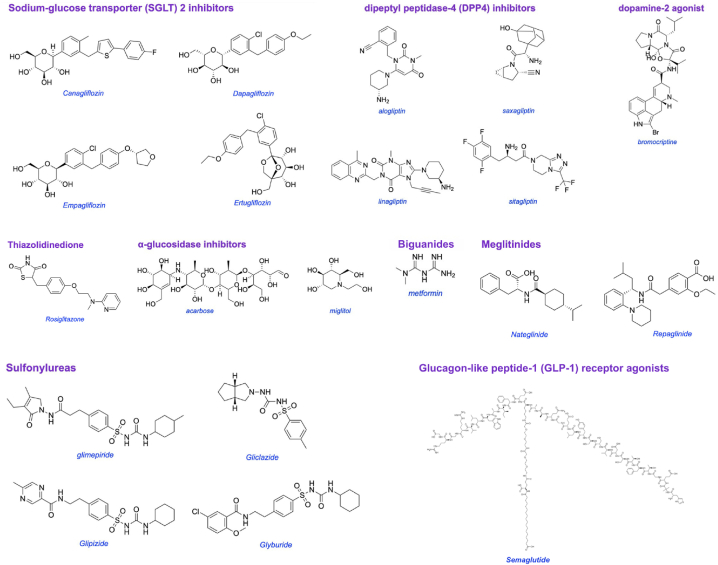
There are two common types of diabetes, 1 and 2: insulin-dependent and non-insulin-dependent, respectively. Type 1 occurs when pancreatic islet beta cells are destroyed so the body cannot produce insulin. This is an auto-immune disease with no cure. There is no medication other than insulin and its derivatives available for the controlling of type 1 diabetes. Type 1 patients need daily insulin to regulate their blood sugar levels [5]. Type 2 diabetes accounts for the vast majority of the diabetic people in the world, and accounts for the majority of treatable and preventable cases [6]. In type 2, insulin is produced and secreted but does not function properly due to the reduced responsiveness of the body tissue. Most currently available antidiabetic medicines control blood glucose in type 2 diabetic patients by one or more of the mechanisms shown in Fig. 1. Those drugs include sulfonylureas, biguanides, α-glucosidase inhibitors, thiazolidinediones, and non-sulfonylureas secretagogues as shown in Fig. 2.
Fig. 1.
Mechanisms of action of currently available antidiabetic drugs.
Fig. 2.
Chemical structure of the different groups of antidiabetic medicine.
Oral sulfonylureas such as glimepiride and glyburide work by increasing insulin secretion from the pancreas. Such drugs bind to sulfonylureas receptors on β-cells while closing adenosine triphosphate-dependent potassium channels. When this happens, the cell membrane depolarizes and insulin is secreted by the calcium influx mechanism [7,8]. The effectiveness of sulfonylureas decreases after 6 years in 44 % of patients [9]. Meglitinide and repaglinide are known as non-sulfonylureas drugs which increase insulin secretion in the same way as sulfonylureas.
Biguanides drugs such as metformin lower blood glucose by reducing hepatic gluconeogenesis and insulin-stimulated uptake; drugs are not so effective in the absence of insulin [6].
Acarbose and miglitol are α-glucosidase inhibitors that inhibit pancreatic α-amylase and α-glucosidase to interfere with the digestion of carbohydrates and promote slower absorption of sugars into the body [7,10].
Pioglitazone and rosiglitazone are thiazolidinediones (TZDs) hypoglycemic agents. These act by increasing tissue sensitivity to insulin and by antagonizing nuclear peroxisome proliferator-activated receptor gamma (PPARγ), which is responsible for the transcription of insulin-responsive genes involved in the regulation of transportation, production, and glucose utilization [11].
Antidiabetic medicines such as acarbose, metformin, and sulfonylureas have common side effects and disadvantages, including gastrointestinal upset, drug resistance, diarrhea and lactic acidosis, hepatic failure, weight gain, tachycardia, and hypothyroidism [12]. More investigations are needed to discover new medicines for all forms of diabetes and natural compounds are relatively unexplored alternatives.
1.1. Antidiabetic medicinal plants
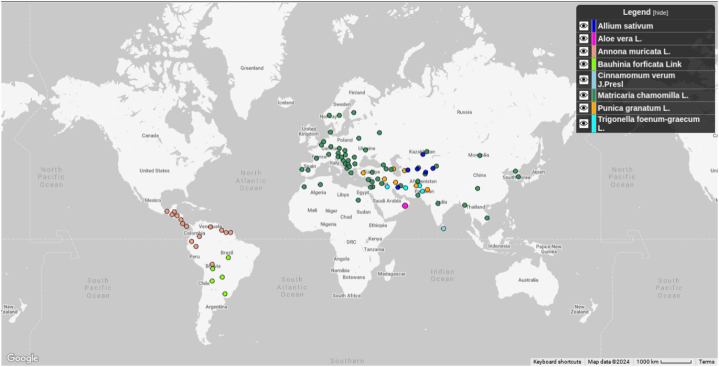
Plant based products can be relatively safe, readily available, have minimal side effects, and cheaper than synthetic drugs [13,14]. In fact, many synthetic drugs are directly or indirectly derived from medicinal plants. Medicinal plants are rich sources of bioactive chemicals with medicinal effects [15,16] due to phytoconstituents such as flavonoids, terpenoids, saponins, carotenoids, alkaloids, and glycosides [17]. In view of this, herbal medicines have been used as alternative treatment for diabetes worldwide [18,19]. These plant extracts could exert hyperglycemia control by enhancing insulin secretion and sensitivity of pancreatic islets, glucose uptake by muscle cells and adipose tissues, inhibition of intestinal glucose absorption, and hepatic glycogenolysis [6,12]. Fig. 3 depicts native origin of the most commonly used medicinal plants which have gone through clinical trials for antidiabetic effect.
Fig. 3.
Map showing native origin of the medicinal plants [20].
4. Conclusion
Diabetes mellitus is one of the most common endocrine disorders, which affects more than 463 million people worldwide. Current drug treatments can have negative side effects, are out of reach for many people, and are expensive. As a result, numerous investigations have been and continue to be undertaken in search of effective and safe herbal treatments. In this review, we compiled information from in vitro, in vivo, and clinical studies on the eight most promising plants in controlling diabetes complications. Research into these plant extracts is abundant, but isolating the active compounds and confirming their efficacy remains a significant challenge. Nevertheless, the potential benefits are substantial, given the current global prevalence of diabetes and its complications. Effective plant-based treatments could offer a natural and complementary approach to managing diabetes and its associated health issues, underscoring the need for continued and focused research in this area.
CRediT authorship contribution statement
Valizadeh Lakeh Mahmoud: Writing – original draft, Visualization, Formal analysis. Ramtin Shayesteh: Writing – original draft, Visualization. Trisha Krishni Foong Yun Loh: Visualization, Investigation. Sook Wah Chan: Writing – review & editing, Project administration, Funding acquisition. Gautam Sethi: Writing – review & editing, Formal analysis. Kevin Burgess: Writing – review & editing, Methodology. Sau Har Lee: Writing – original draft, Investigation. Won Fen Wong: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Chung Yeng Looi: Writing – original draft, Validation, Supervision, Project administration, Conceptualization.
2. Most promising antidiabetic phytochemicals and plant extracts
2.1. Allium sativum L
2.1.1. Native origin
Allium sativum L. (garlic) belongs to the Amaryllidaceae family, an aromatic herbaceous annual spice that has been used as a medicine for a long time [21,22]. The native range of garlic stretches from the rocky valleys and riverbeds of Iran to the stream beds and ravines of Kazakhstan. Allium species have been found to possess antidiabetic properties, prevent cardiovascular disorders, and strengthen the immune system [23].
2.1.2. Major compound and mechanistic action
As shown in Table 1, Garlic bulbs and their products contain sulfur compounds (ajoenes, allicin, vinyldithiins, and sulfides), flavonoids (quercetin), and Alliin (the main precursor of allicin) [24,25]. Garlic ethanolic extract improves the complication of diabetes in STZ/alloxan-induced diabetic rats by increasing insulin secretion from pancreatic β-cells [26]. Allyl propyl disulfide, allicin, cysteine sulfoxide, and S-allyl cysteine sulfoxide, were shown to be effective in reducing blood glucose, and increasing insulin secretion and insulin sensitivity. So far, similar results have been reported between glibenclamide and Aline in reducing the complications of diabetes [27,28]. Garlic oil was able to decrease the serum aspartate aminotransferase, alanine transferases, and alkaline phosphatase in diabetic rats [29]. Moreover, it has been demonstrated that garlic bioactive compounds are able to promote antioxidant synthesis and reduce oxidizer production [30]. It was stated that garlic extract increases the activity of enzymes such as hepatic superoxide dismutase in rats (SOD) [25]. Alliin, the major component isolated from garlic extract, has great antioxidant activity through controlling ROS generation, preventing mitogen-activated protein kinase, and inhibiting NADPH oxidase 1 [31].
Table 1.
In alphabetical orders, plants and their bioactive compounds, mechanism and structure.
| No | Plant | Compound (s) | Plant Part | Category | Mechanism of action | Structure | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Aloe vera L. | Aloe-emodin | Latex | Anthraquinones | ↓ ROS generation ↓ Pro inflammatory cytokine |
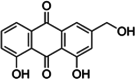 |
[38] |
| Anthraquinones | Latex | Anthraquinones | ↑ IRS1 ↑ PI3Ks |
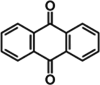 |
|||
| 2 | Annona muricata L. | Rutin | Leaves | Flavonoids | ↓α-amylase activity ↓α-glucosidase activity ↓ Hepatic G6Pase activity ↓ Glycogen phosphorylase ↑Insulin secretion ↑ Expression of PPARγ |
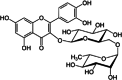 |
[139] |
| 3 | Allium sativum L. | Allicin | Bulb | Organosulfides | ↓ Bcl-2 ↓ Fas ↓ CTGF ↓ TGFβ1 |
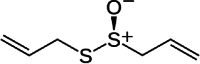 |
[140] |
| 4 | Bauhinia forficata Link | Astragalin | Leaves | Flavonoids | ↓α-amylase activity ↓α-glucosidase activity ↓SERCA activity ↑Release of Ca2+ ↑Ca2+ trough L-VDCC |
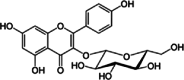 |
[141] |
| Kaempferitrin | Leaves | Flavonoids | ↑Activates PKC ↑Glucose uptake |
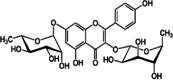 |
|||
| 5 | Cinnamomum verum J.Presl | Cinnamaldehyde | Bark | Flavonoids | ↓ FBG ↑ insulin sensitivity ↑ GLUT 4 ↓ PTP-1B ↓NO |
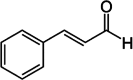 |
[142] |
| 6 | Trigonella foenum-graecum L. | 4-hydroxyisoleucine | Seed | Amino acid | ↑ Insulin secretion ↓ Lipid peroxidation |
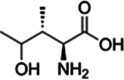 |
[143] |
| 7 | Matricaria chamomilla L. | Apigenin | Flower | Flavonoids | ↓ α-glucosidase ↓ROS ↑SOD ↑CAT ↑GSH ↑ Insulin |
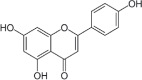 |
[6] |
| Quercetin | Flower | Flavonoids | ↓ G6Pase activity ↓ PEPCK activity ↓ ALT ↓AST ↓ALP ↓GGT ↓CRE ↓BUN |
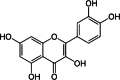 |
[144] | ||
| 8 | Punica granatum L. | Ellagic acid | Peel & Fruit | Ellagitannins | ↓ Serum resistin ↑The association of recombinant PON1 to HDL |
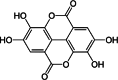 |
[145] |
| Chlorogenic acid | Peel | Quinic acid | ↓ Hepatic G6Pase activity | 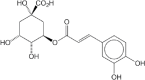 |
[146] |
ROS: Reactive oxygen species, IRS1: Insulin receptor substrate 1, PI3K: Phosphoinositide 3-kinase, Fas: Death receptor, CTGF: Connective tissue growth factor, TGFβ: Transforming growth factor beta 1, PEPCK: Phosphoenolpyruvate carboxykinase, ALT: Alanine transaminase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase, GGT: Gamma-glutamyltransferase, BUN: Blood urea nitrogen, SERCA: Sarcoendoplasmic reticulum calcium ATPase pump, L-VDCC: L-type voltage-dependent calcium channel, PKC: Protein kinase C, SOD: Superoxide dismutases, GLUT: Glucose transporter, FBG: Fasting blood glucose, PTP-1B: Protein-tyrosine phosphatase 1B, NO: Nitric oxide, CAT: Catalase, GSH: Glutathione.
2.1.3. Clinical trial
As summarized in Table 2, a triple-blind randomized clinical trial on the antidiabetic effects of garlic involving 49 prediabetic pregnant women by Faroughi et al. reported a decrease in fasting blood sugar, cholesterol, and serum lipids [27]. The study showed the administration of 400 mg garlic pills per day for eight weeks resulted in significantly greater improvement in prediabetic symptoms than placebo patients.
Table 2.
Summary of clinical trials evaluated metabolic effects of the plants for diabetes.
| Plant | End Product | Dose | Sample Size | Duration | Effect | Reference |
|---|---|---|---|---|---|---|
| Aloe vera L. | Aloe vera gel extract | 250 mL/day | 53 healthy individuals | 14 days | ↑ plasma antioxidant capacity | [51] |
| Aloe emodin treatment | 300 & 500 mg twice a day | – | – | ↓ HbA1C ↓ TC ↓ LDL ↓ TG |
[52] | |
| Allium sativum L. | Garlic pill | 400 mg/day | 49 prediabetic pregnant woman | 8 weeks | ↓ fasting blood sugar ↓ cholesterol ↓ serum lipids |
[27] |
| Annona muricata L. | Annona muricata capsule | 180 mg/day | 60 T2DM patients | 1 month | ↓ blood glucose ↑ nausea ↑ burning pain in abdomen |
[59] |
| Bauhinia forficata Link | Capsule | 300 mg/day | 92 T2DM patients | 4 months | ↓ plasma glucose ↓ glycated hemoglobin |
[70] |
| Cinnamomum verum J.Presl | CZ capsule | 1, 3, 6 g/day | 30 healthy adults | 3 months | ↓ LDL ↓ mean TG ↓ TC |
[93] |
| Gelatin capsule | 3 g | 9 healthy adults | 1 day trials 28 days apart |
∼ TC ∼ LDL |
[94] | |
| Capsule | 1 g/day | 43 T2DM patients | 3 months | ∼ TC ∼ LDL ∼ mean TG |
[95] | |
| Capsule | 1, 3, 6 g/day | 60 T2DM patients | 40 days | ↓ 18–29 % fasting blood glucose | [96] | |
| Tablet | 120 & 360 mg/day | 66 T2DM patients | 3 months | ↓ FBG ↓ HbA1C |
[97] | |
| Tablet or capsule | 2 g/day | 577 diabetic patients | 4–16 weeks | ∼ blood glucose | [98] | |
| Matricaria chamomilla L. | Chamomile tea | 3 g/150 mL tea three times a day | 64 T2DM patients | 8 weeks | ↓ blood glucose ↓ fatty acids ↑ insulin sensitivity |
[107] |
| Punica granatum L. | Pomegranate seed oil capsule | 1 g/day | 52 obese T2DM patients | 8 weeks | ↓ FBS ↓ GLUT-4 gene expression |
[126] |
| Trigonella foenum-graecum L. | Fenugreek seeds | 2.5 & 5 g/day | 60 T2DM patients | 4 weeks | ↓ blood glucose ↓ total lipid content ↓ serum α-amylase |
[134] |
| Fenfuro capsule | 500 mg/day | 108 male T2DM patients 46 female T2DM patients |
90 days | ↓ 83 % fasting plasma glucose ↓ 89 % postprandial glucose ↑ fasting C-peptide ↑ postprandial C-peptide |
[135] | |
| Capsule | 2 g/day | 12 T2DM patients | 12 weeks | ↑ fasting insulin | [136] | |
| Fenugreek seed powder dissolved in water | 15 g/day | 48 T2DM patients | 8 weeks | ↑ SOD activity ↓ high sensitivity CRP ∼ total antioxidant capacity ∼ IL-6 ∼ TNF-α ∼ GPx activity |
[137] | |
| Capsule | 500 mg twice a day | 60 T2DM patients | 12 weeks | ∼ HbA1c ↓ blood sugar |
[138] |
The limited clinical trial data available has been summarized by the end product of the plant used in testing (in capsule, tablet, oil, or other form), the dose and frequency, the medical status (healthy or type 2 diabetic patients), and the number of participants, duration, and main antidiabetic findings of the study. The plants are displayed in the table in alphabetical order.
2.2. Aloe vera L
2.2.1. Native origin
Aloe vera L. is a succulent plant from the family Aloaceae. The plant has been used by natives to treat various diseases. The genus Aloe originated more than 16 million years ago from Southern Africa. To date, there are over 500 species of Aloe where 25 % of these plants were used for various therapeutic purposes [32,33].
2.2.2. Major compound and mechanistic action
Aloe vera L. contains 200 bioactive compounds including anthraquinones, vitamins, phytosterols, polysaccharides, carbohydrates, amino acids, and minerals [34]. A study done by Mohammed et al. showed that anthraquinones found in the latex of the plant could increase insulin sensitivity via upregulation of insulin receptor substrates-1 (IRS-1) and phosphoinositide-3-kinase (PI3Ks) [35]. Besides, oral administration of Aloe vera extract (300 mg/kg) prepared from the plant's gel reduced blood glucose levels, lowered the fasting plasma, and was also able to increase the serum insulin levels from 116.5 pmol/L (1st week) to 146.1 pmol/L (3rd week) in the STZ-induced diabetic rats [36]. Furthermore, it was shown that daily consumption of Aloe vera at a dose of 400 mg/kg in the form of concentrated gel for 8 weeks significantly reduces blood sugar and body weight of diabetic rats [37]. Researchers stated that Aloe vera gel extract is able to increase an insulin secretory function parameter, the homeostasis model assessment of β-cell function (HOMA-β), which indicated a glucose tolerance with slower diabetes development [38]. In another study, the analysis of the volume, number, diameter, and area of the pancreatic islets of diabetic rats showed improvement after treatment with Aloe vera gel extract in diabetic rats, with 84.6 % in total number of islets, 85.1 % in islet diameter, 71.7 % of islet area, and 60.5 % of islet volume [36]. Besides, sterols isolated from Aloe vera gel can also activate peroxisome proliferator-activated receptors (PPAR) which play roles in regulating metabolism of carbohydrates and lipids [39,40]. Saponins are another important ingredient in Aloe vera leaves which regulate nutrient absorption, reduce protein digestibility, lower blood cholesterol, and have antiviral and antidiabetic properties [41,42]. In another experiment, treatment with Aloe vera extract had resulted in a significant decline in triglyceride and very low density lipoprotein (VLDL) levels in the blood [38]. Other than improving diabetic condition, Aloe vera bioactive components could exhibit a positive impact on diabetics' nerve and brain tissue complications. It is shown that Aloe vera possibly reduces apoptosis phenomenon by increasing the expression of nerve growth factor (NGF) and tropomyosin receptor kinase A (TrkA) in the brain. At the same time, it reduces the activity and expression of p75 neurotrophin receptors in diabetic rats which may decline the atrophy of the hippocampus by lowering the activity of this receptor in the hippocampus [37]. Similarly, another study that fed Aloe vera extracts in gel format to the rats showed the ability to protect the hippocampus and have positive effects on behavioral deficits in diabetic rats due to its antioxidative and hypoglycemic properties [43]. Aloe vera extract could also reduce the level of mutagenic base modifications and DNA fragmentation in diabetics significantly through its antioxidant capability [44,45]. One other recent study showed that Aloe vera slowed down the progression of nephropathy by altering its lipids profile and decreasing kidney oxidative stress [46]. However, there are several adverse effects associated with the use of Aloe vera. For instance, the anthraquinone content of the extracts appear to be responsible for most allergic reactions related to Aloe vera [47]. Also, it could have toxic effects on the fetus when Aloe vera is used during pregnancy [48]. Likewise, Boudreau and Beland recommended that patients with a history of kidney or heart problems should avoid using Aloe vera latex [49]. Also, it was reported that Aloe vera juice supplementation may affect glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) enzymes' function in the kidneys of diabetic rats [6]. This was further proven by another research team that demonstrated increased levels of glutathione and antioxidant SOD enzyme activities in diabetic rats treated with Aloe vera extract [50].
2.2.3. Clinical trial
The effect of drinking 250 mL of Aloe vera gel extract daily in a clinical trial with 53 healthy volunteers over 14 days showed increased plasma total antioxidant capacity with no reported clinical side effects [51]. According to a separate double-blind randomized controlled in vitro trial, 300 and 500 mg of Aloe emodin (commercially available leave extracts from Aloe vera) treatments twice a day, had successfully reduced HbA1C level in pre-diabetics, indicating its potential use to control the blood glucose level [52].
2.3. Annona muricata L
2.3.1. Native origin
Annona muricata L., called Graviola, is a plant of the Annonaceae family, which is mainly distributed in tropical and subtropical regions of the world, while it's native to the tropical Americas.
2.3.2. Major compound and mechanistic action
Acetogenins, flavonoids, tannins, alkaloids, and coumarins are the major bioactive components of Annona muricata L. leaf extract [53]. Some identified phenolic compounds of Annona muricata are flavonoids, phenolic acids, and gallotannins [54]. Annona muricata L. leaves have been reported to contain alkaloids such as aporphines, isoquinolines, imino sugars, and protoberberines [55,56]. Annona muricata L. leaf extract contains rutin, kaempferol-3-O-rutinoside, quercetin, kaempferol, muricoreacin, annonacin, and annonacinone. It is reported that its extract can improve metabolism of insulin and lipid, and autophagy pathways which are related to non-alcoholic fatty liver disease (NAFLD) risk in type 2 diabetes [57]. Annona muricata L. extract also, increased glucose uptake, improved hexokinase activity, and reduced pancreatic β-cell apoptosis via the PI3K/Akt gene upregulation. It has been shown that Annona muricata L. extract (100 and 200 mg/kg) reduces blood glucose levels by 31.77 % and 45.77 % respectively in STZ-diabetic rats [53]. Furthermore, a significant increase in protein kinase B and B-cell lymphoma 2 expression in liver and pancreas tissue, as well as a decline in insulin resistance in diabetic rats were observed using Annona muricata L. extract [58]. Son et al. stated that a low dose of Annona muricata L. extract stimulates hepatic insulin signaling-related proteins such as insulin receptor subunit (IRS)-1, glucose transporter (GLUT) 2, and p-Akt and significantly reduces the level of triglyceride in the liver concentration [57].
2.3.3. Clinical trial
A clinical trial study was conducted by Arroyo et al. and 60 patients with type 2 diabetes were given either Annona muricata L. ethanolic extract capsules containing 5 mg of glibenclamide or only 5 mg tablets of glibenclamide for a month [59]. A decline in blood glucose was seen in groups treated with Annona muricata L. and glibenclamide. Having said that, 11 % of them experienced side effects such as burning pain in the epigastrium and nausea. Despite the hypoglycemic effects of Annona muricata L., a lack of adequate clinical trials is felt and the efficacy of Annona muricata L. extract as a single therapy or as an adjunctive therapy should be investigated [60].
2.4. Bauhinia forficata Link
2.4.1. Native origin
Bauhinia forficata Link (B. forficata) is a species of flowering tree that is native to Argentina, Brazil, Uruguay, and Peru. B. forficata is often used in traditional medicine to treat type-2 diabetes [61].
2.4.2. Major compound and mechanistic action
Flavonoids such as kaempferitrin, kaempferol, and quercetin, are introduced as major compounds in Bauhinia forficata leaves [62]. Kaempferol improves insulin sensitivity by phosphorylating IRS, increases glucose uptake and adiponectin secretion [63]. The aqueous extract of B. forficata leaves reduces hyperglycemia, glycosuria, uremia, lipid peroxidation, stimulates the activity of glutathione reductase, and increases hepatic glycogen [64,65]. Also it has been shown that the ethanol extract of B. forficata leaves inhibits α-amylase and reduces protein glycation [66]. Franco et al. showed that B. forficata extract is an excellent inhibitor of α-amylase, α-glucosidase, and lipase. Additionally, it is not toxic to rodents, erythrocytes, and macrophages [67]. Ajebli et al. reported that the natural alkaloids of Bauhinia forficata blocks protein tyrosine phosfatase 1B, deactivates dipeptydil peptidase-IV, increases insulin sensitivity, reduces oxidative stress, and inhibits glucosidase [68]. In a study it was found that B. forficata extract can decrease hepatic oxidative stress, MDA levels and increase catalase activity [69].
2.4.3. Clinical trial
In an investigation conducted by Tonelli et al., it was demonstrated that the use of B. forficata leaf extract capsule (300 mg) decreases plasma glucose levels and glycated hemoglobin in type II diabetics compared with the placebo group [70]. Indeed they concluded that the adjunctive use of capsules containing B. forficata could be beneficial if added to regular oral antidiabetics in type II diabetic patients [70]. However, the study only included 92 type 2 diabetic patients from a single center, and the experts concur that a larger trial is required to validate the findings and fully analyze the undesirable side effects.
2.5. Cinnamomum verum J.Presl
2.5.1. Native origin
Cinnamomum verum J.Presl (Cinnamon), also known as Cinnamomum zeylanicum Blume, is a small tree belonging to the family Lauraceae. Cinnamon has various beneficial effects such as antiemetic, antidiarrheal, and antiflatulent [71,72]. The tree is native to the island of Sri Lanka and grows in wet tropical biomes.
2.5.2. Major compound and mechanistic action
Cinnamic aldehyde and Eugenol are the main constituents found in the leaf oil. Other compounds including camphor, eucalyptol, and safrol are present in cinnamon roots [73]. Eugenol, cinnamaldehyde, camphor, cinnamyl acetate, and copane are the cinnamon major components [74]. The polyphenols present in cinnamon contribute to its antidiabetic activity and mitigate pathological damage to pancreatic β-cells in STZ-diabetic mice [75]. It was shown that water extract from cinnamon stem barks had a blood glucose-lowering effect in STZ-induced rats [76]. Cinnamon extract has been reported to be effective in lowering fasting blood glucose and homeostatic model assessment for insulin resistance (HOMA-IR) levels [77,78]. Studies have reported the neuroprotective, fat-lowering, antioxidant, anti-inflammatory, and hypoglycemic effects of cinnamon so that it can be effective in preventing the onset of diabetes and cardiovascular diseases [79,80]. Cinnamon essential oil is a free radical scavenger and has a dose-dependent anti-proliferative effect on adipose-derived mesenchymal stem cells [81]. Multiple studies have reported that cinnamon extract increases insulin sensitivity and insulin secretion [82]. The usage of Cinnamon cassia and zeylanicum extracts was reported to enhance plasma insulin in rats [83]. Cinnamic acid can improve glucose tolerance and enhance insulin secretion in islet cells for both in vivo and in vitro studies [84]. Besides, cinnamon extract was revealed to have an inhibitory effect against α-glycosidase, and α-amylase, and exhibited reducing glucose manners [85,86]. An in vitro study showed that its extract may inhibit lipase, α-amylase, and α-glucosidase. Cinnamon aqueous extract was also reported to decrease the gene expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), thus declining hepatic gluconeogenesis [87]. Furthermore, the aqueous extract can upregulate mitochondrial uncoupling protein 1 (UCP-1) and GLUT4 in brown adipose tissues and muscles in STZ-induced rats [88]. In another study, it was shown that cinnamaldehyde, found in cinnamon bark, upregulates the IRS1/PI3K/AKT2 signaling pathway in STZ-induced rats [89]. Cinnamaldehyde was also revealed to activate PPARδ, PPARγ and retinoid X receptor (RXR) to enhance insulin sensitivity [90]. Cinnamon Burmanii, Vietnamese, Loureirii, and Ceylon were found to have great potential to suppress the formation of AGEs [91]. Procyanidin-B2 (PCB2), one of its bioactive compounds, also inhibits AGE accumulation and improves AGE-mediated pathogenesis of diabetic nephropathy [92].
2.5.3. Clinical trial
A clinical study involving 30 healthy adults was performed to investigate the side effects of taking water extracts of C. zeylanicum with the dose increased at monthly intervals at doses of 85, 250, and 500 mg/day. No adverse side effects were seen due to taking C. zeylanicum extract. It was reported that daily consumption of 1, 3, 6 g of cinnamon reduces LDL, mean TG, and TC [93]. In contrast, Markey et al. reported that no obvious differences were seen in the TC or LDL levels in a single-blinded randomized crossover study by using 3 g cinnamon and placebo [94]. Also, another trial showed that there is no significant alteration between the mentioned parameters after taking 1 g of cinnamon for 3 months [95]. Cinnamon's improving effects were clarified by multiple clinical trials through its beneficial effects on fasting blood glucose, 2-h postprandial glucose, insulin resistance, and HbA1C. Khan et al. conducted a randomized placebo controlled study, cinnamon was given at doses 1, 3, and 6 g/day to T2DM patients for 40 days [96]. Cinnamon decreased fasting blood glucose by 18–29 %. In another clinical study, cinnamon extract (120 and 360 mg/day) decreased FBG and HbA1C levels [97]. The previous study is in contrast to Leach and Kumar's results, who reported that cinnamon does not control blood glucose and cannot improve or prevent metabolic diseases [98].
2.6. Matricaria chamomilla L
2.6.1. Native origin
Matricaria chamomilla L. is one of the oldest plants with antidiabetic and anti-cancer activities. Matricaria chamomilla is native to most of continental Europe, northern Africa, and northern and western Asia and grows in all kinds of soils [99,100].
2.6.2. Major compound and mechanistic action
Flavonoids such as apigenin, quercetin, patoletin, luteolin, and their glucosides including several phenolic compounds are present in Matricaria chamomilla flowers [101]. The bioactive compounds of Matricaria chamomilla essential oil and extracts include terpenoids such as bisabolol and its oxides A and B, bisabolone oxide A, chamazulene, and farnesene [100]. The findings showed that a 12-week endurance training along with 200 mg/kg/day of Matricaria chamomilla flower extract reduces fasting blood glucose (FBG) and increases serum insulin levels in diabetic rats [99]. Administration of Matricaria chamomilla extracts caused a reduction in glycated hemoglobin and glycated serum protein (GSP) levels in diabetic rats [102]. Besides, the results showed that Matricaria chamomilla bioactive compounds protect pancreatic islet cells and reduces the oxidative stress occurring in hyperglycemia [103]. Hyperglycemia and oxidative stress can lead to brain damage which results in cognitive impairments. The results of crystal vessel staining in a study showed an increase in the number of necrotic cells in the hippocampus of diabetic rats [99]. Matricaria chamomilla seems to be effective in controlling adverse changes in hippocampal neurons and its protective effects against the brain damage are likely due to its antioxidant activity. Asgharzade et al. reported that Matricaria chamomilla extract inhibits the peroxidation of brain tissue lipids and increases antioxidant capacity [104]. Heidarianpour et al. stated that exercise and consumption of Matricaria chamomilla extract together reduce the number of necrotic cells and MDA levels in hippocampal tissue in diabetic rats [99]. Matricaria chamomilla extract bioactive compounds such as α-bisabolol oxide can improve the serum level, and activity of MDA and AST of diabetic rats [99,105]. Quercetin is one of its extract flavonoids with good antioxidant properties that can change cell function. Apigenin is another flavonoid with antioxidant properties that stimulates neuronal differentiation, accelerates neurogenesis in hippocampal tissue, and improves memory and learning in rats [106].
2.6.3. Clinical trial
In a single-blind randomized controlled clinical trial conducted on 64 type 2 diabetic patients, the intervention group consumed 150 mL of chamomile flower tea three times a day after each meal, for 8 weeks. It was found that short-term intake of M. chamomilla tea can decrease blood glucose, and fatty acids, and increase insulin sensitivity in type II diabetes patients [107].
2.7. Punica granatum L
2.7.1. Native origin
Punica granatum L. (pomegranate) is a fruit native to the Middle East and used in Indian medicine to cure disease and disorders like diabetes, cardiovascular diseases, inflammation, and cancer [108,109].
2.7.2. Major compound and mechanistic action
The beneficial effects of pomegranate are related to its bioactive compounds such as flavonoids and phenolic acids including: anthocyanin, ellagitannin, and gallotannin [[110], [111], [112]]. In vitro studies have shown that α-amylase and α-glucosidase are inhibited in the presence of pomegranate hydrolyzable tannins (i.e., ellagitannins and gallotannins) in its peel extract [113]. In another study which was conducted by Çam and İçyer, it was reported that the aqueous extracts of pomegranate peels inhibit the α-glucosidase (IC50 = 5.56 ± 2.23 μg/mL) [114]. The α-glucosidase inhibitory activity of pomegranate peel extracts was attributed to bioactive components such as punicalagins, ellagic acid, and anthocyanins. It was also suggested that the phenolic compounds may inhibit α-amylase and α-glucosidase activities by mechanisms like mixed/non-competitive [115]. Rock et al. reported that the activity of PON1 enzyme is increased by using pomegranate juice [116], also pomegranate flower aqueous extract enhances the activity of SOD and CAT in diabetic rats [117]. Such components exhibit metal chelation activity, so it is recommended that it can reduce ROS accumulation. It has been shown that ferrous ion chelation by pomegranate juice can reduce the production of hydroxyl radical (•OH) from hydrogen peroxide [118]. Pomegranate extract also can decrease diabetes complications due to its ROS scavenging activity [119]. In vitro studies have shown that pomegranate wine can inhibit NF- κB activation. Active compounds such as gallic acid which has been found in the pomegranate extract shows effects like increasing PPAR-γ activity [120]. In another study, it was stated that consumption of pomegranate juice can reduce serum resistin levels in mice [121]. Insulin-stimulated glucose uptake has been found to increase by resisting neutralization [122,123]. It was revealed that the administration of aqueous pomegranate peel extract (200 mg/kg) reduces blood glucose and LPO in cardiac, hepatic, and renal tissues [124]. Jafri et al. reported that the use of the aqueous-ethanolic (50 %, v/v) pomegranate flower extract (400 mg/kg) lowers blood glucose [125].
2.7.3. Clinical trial
A randomized clinical trial was conducted to find pomegranate seed oil effects on the GLUT4 gene expression in 52 obese type II diabetic patients for 8 weeks. Results showed that pomegranate seed oil decreases FBS and increases the GLUT-4 gene expression in diabetic patients without any adverse effects [126]. However, further studies, with a larger sample size and extending beyond 8 weeks, are needed to confirm the results.
2.8. Trigonella foenum-graecum L
2.8.1. Native origin
Trigonella foenum-graecum L. (Fenugreek) is an annual plant, native to the Middle East region, in the family Fabaceae and used as a dietary supplement which shows its hypoglycemic effects both in insulin-dependent and non-insulin-dependent diabetes [127].
2.8.2. Major compound and mechanistic action
Various antidiabetic properties of Fenugreek are attributed to its bioactive compounds including steroid, saponins, trigoneosides, glycoside D, trigofoenoside A, and steroidal sapogenins such as diosgenin and yamogenin [128]. A novel amino acid, 4-hydroxyisoleucine, is shown to be present in fenugreek seed extract which facilitates insulin secretion [129]. It was also reported that fenugreek seed fibers reduce postprandial blood glucose levels and have hepatoprotective, anti-microbial, cardioprotective, and neuroprotective effects [127,130]. A study showed that blood glucose was reduced by fenugreek seed extract administration in diabetic rats [131]. Fenugreek seed is also rich in antioxidants and shows free radical scavenging activity [127]. Galactomannan, saponin, trigonelline, and 4-hydroxyisoleucine are found in fenugreek seeds and have antidiabetic and anti-cancer effects [132]. Fenugreek bioactive compounds play a role in the prevention of inflammation, controlling the cyclooxygenase enzyme inhibitory activities, and lipid peroxidation [127]. It also can reduce the glucose tolerance curve and improve glucose-induced insulin response. Such effects were attributed to its stimulatory impact on pancreatic β-cells [133]. Bafadam et al. reported that fenugreek extract at doses 50 and 100 mg/kg showed similar results to metformin in terms of catalase enzyme activity [131]. Additionally, SOD activity in groups including 200 mg/kg fenugreek extract and metformin, was higher than others.
2.8.3. Clinical trial
A reduction in blood glucose level, total lipid content, and serum α-amylase were seen following the use of 2.5 and 5 g/day of fenugreek seed powder for 4 weeks in 60 patients with type II diabetes [134]. A multi-site, randomized, double-blind, placebo-controlled trial was performed on 108 male and 46 female type II diabetes patients to assess fenugreek seed extract hypoglycemic activities. The fasting plasma glucose and postprandial glucose declined by 83 % and 89 %, respectively, while an increase in the level of fasting and postprandial C-peptide was observed [135]. Najdi et al. performed a randomized control study on 12 type II diabetes patients using 2 g of fenugreek powder per day [136]. A rise in the fasting insulin level was seen after 12 weeks. Besides, the anti-inflammatory and antioxidative effects of fenugreek seed compounds were investigated in a clinical trial study on 48 type II diabetes patients for 8 weeks [137]. A dose of 15 g/day of fenugreek seed powder enhanced the activity of SOD and reduced high-sensitivity C-reactive protein. However, no significant effects were seen in the total antioxidant capacity, IL-6, TNF-α, and GPx activity [137]. Singh et al. designed a randomized, open-labeled, parallel-group comparative trial to evaluate the effect of fenugreek seed extract (500 mg two times a day) with glipizide (5 mg/day) [138]. It was reported that the extract was not as effective as glipizide in reducing HbA1c but significantly improved blood sugar levels.
3. Solving diabetic complications, the challenges and limitations
Complications of diabetes add another layer of complexity to the research. Diabetes is associated with a range of severe complications, including cardiovascular disease, neuropathy, nephropathy, retinopathy, and an increased risk of infections. These complications result from chronic hyperglycemia, which damages blood vessels and nerves over time. The interplay between these complications means that effective antidiabetic compounds must not only regulate blood glucose levels but also mitigate or prevent the onset of these associated conditions.
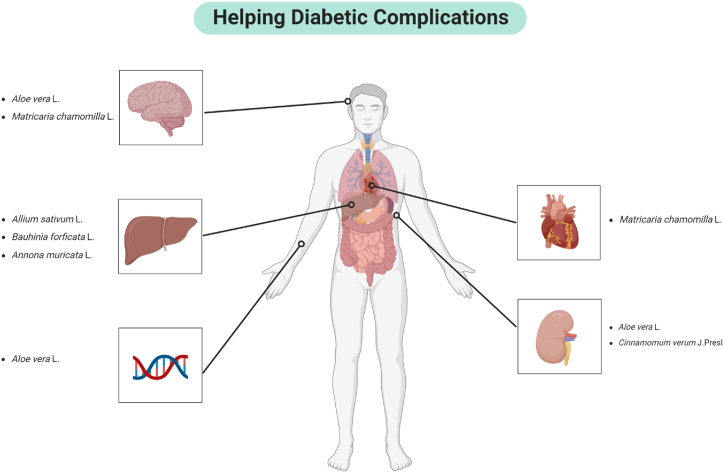
Cardiovascular complications, such as heart disease and stroke, are common in diabetic patients due to the accelerated atherosclerosis caused by prolonged high blood sugar levels. Certain flavonoids and polyphenols found in plants have been shown to possess cardioprotective properties. For example, quercetin, a flavonoid present in many fruits and vegetables, has antioxidant and anti-inflammatory effects that can help reduce the risk of atherosclerosis and improve endothelial function, thereby protecting against cardiovascular complications (Fig. 4). Diabetic neuropathy, resulting in nerve damage, can lead to severe pain and loss of sensation, particularly in the extremities, increasing the risk of injury and infection. Plant extracts of aloe vera and chamomile have demonstrated neuroprotective effects. These compounds help reduce oxidative stress and inflammation, which are key contributors to diabetic neuropathy, thereby alleviating pain and preventing nerve damage.
Fig. 4.
Medicinal plants beneficial for diabetic complications [147].
Diabetic nephropathy, or kidney damage, can progress to end-stage renal disease, necessitating dialysis or transplantation. Extracts from multiple plants such as aloe vera and cinnamon have been found to protect kidney function through alleviating oxidative stress.
More detailed studies must be performed before rigorous long-term clinical studies can take place. Specifically, of the 118 articles cited in this review and found on PubMed, none of the summarized studies isolate the active compounds that are primarily responsible for the antidiabetic effect, and subsequently test them in clinical trials. This could be due to multiple factors, such as the lengthy process of isolating the active compounds from the plant extracts, and the high cost of conducting clinical trials [148]. However, studies structured in this way can feature quantifiable doses of those active compounds, whereas this is impractical with plant extracts. Dose-response studies in vitro, in cells, and in animals are then possible, but until then they will be impractical. Further, the identification of active compounds based on data, rather than hypotheses, facilitates studies to prove mechanisms, rather than speculate on what they might be. Determining the active compounds in the extract is generally a time-consuming process and is particularly challenging for antidiabetic compounds compared to, for example, anti-cancer leads, where cytotoxicity studies can be done in vitro using cell lines. For antidiabetic research, the complexity is heightened due to the multifactorial nature of diabetes, involving multiple biological pathways and systems. Isolating and identifying active compounds requires extensive bioassay-guided fractionation, advanced analytical techniques, and comprehensive in vivo testing to assess efficacy and safety [149]. Furthermore, the lack of straightforward in vitro models for diabetes makes it difficult to screen for active compounds quickly, increasing the reliance on time-consuming in vivo models [150,151]. This intricate process demands significant resources, expertise, and time, making it a particularly daunting task for researchers in the field of diabetes.
Ethics declaration
Review and/or approval by an ethics committee was not needed for this study because it does not involve any animal or human experimental research.
Data availability statement
No data were used for the research described in the article.
Additional information
No additional information is available for this paper.
Funding statement
This project was supported by the Malaysian Ministry of Higher Education Fundamental Research Grant Scheme, grant number FRGS/1/2022/SKK12/UM/02/34. We also acknowledge research grant from Malaysian Ministry of Higher Education, FRGS/1/2024/SKK15/TAYLOR/02/1. This project was provided by NIH R21NS130471, NIH 1R21NS13834-01A1, The Welch Foundation AU-2182-20240404, and Texas A&M University T3-Grants Program (246292-00000).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We acknowledge Dr Ang Kuan Ping for his helpful advice and comments.
Contributor Information
Won Fen Wong, Email: wonfen@um.edu.my.
Chung Yeng Looi, Email: chungyeng.looi@taylors.edu.my.
References
- 1.Soumya D., Srilatha B. Late stage complications of diabetes and insulin resistance. J. Diabetes Metabol. 2011;2(9) doi: 10.4172/2155-6156.1000167. [DOI] [Google Scholar]
- 2.Yvonne O. Diabetes mellitus in developing countries and case series, diabetes mellitus - insights and perspectives. 2013. [DOI]
- 3.Murea M., Ma L., Freedman B.I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. 2012;9(1):6–22. doi: 10.1900/RDS.2012.9.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling M. Pediatr. Endocrinol. fourth ed. 2014. Pediatric endocrinology. Fourth Ed. [Google Scholar]
- 5.Salsali A., Nathan M. A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am. J. Therapeut. 2006;13(4) doi: 10.1097/00045391-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Salehi A.B., Obat T., Ata A., Kumar N.V.A., Sharopov F., Ramírez-alarcón K., Ruiz-ortega A., Abdulmajid S., Zakaria Z.A., Iriti M., Taheri Y., Martorell M., Sureda A., Setzer W.N., Durazzo A., Lucarini M., Ostrander E.A., Rahman A., Choudhary M.I., Cho W.C., Sharifi-Rad J. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10):551. doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFronzo R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999;131(4) doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi S.E. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287(3):360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 9.Dey L., Attele A.S., Yuan C.S. Alternative therapies for type 2 diabetes. Alternative Med. Rev. 2002;7:45–58. [PubMed] [Google Scholar]
- 10.Lebovitz H.E. Alpha-glucosidase inhibitors. Endocrinol Metab. Clin. N. Am. 1997;26:539–551. doi: 10.1016/s0889-8529(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 11.Koski R.R. Oral antidiabetic agents: a comparative review. J. Pharm. Pract. 2004;17(1) doi: 10.1177/0897190003261307. [DOI] [Google Scholar]
- 12.Li G.Q., Kam A., Wong K.H., Zhou X., Omar E.A., Alqahtani A., Li K.M., Razmovski-Naumovski V., Chan K. Herbal medicines for the management of diabetes. Adv. Exp. Med. Biol. 2013;771:396–413. doi: 10.1007/978-1-4614-5441-0_28. [DOI] [PubMed] [Google Scholar]
- 13.Alam S., Sarker M.M.R., Sultana T.N., Chowdhury M.N.R., Rashid M.A., Chaity N.I., Zhao C., Xiao J., Hafez E.E., Khan S.A., Mohamed I.N. Antidiabetic phytochemicals from medicinal plants: prospective candidates for new drug discovery and development. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R., Arya V. A review on fruits having anti-diabetic potential. J. Chem. Pharmaceut. Res. 2011;3(2) [Google Scholar]
- 15.Nasser Singab A., Youssef F.S. Medicinal plants with potential antidiabetic activity and their assessment, med. Aromat. Plants. 2014;3(1) [Google Scholar]
- 16.Sharifi-Rad M., Roberts T.H., Matthews K.R., Bezerra C.F., Morais-Braga M.F.B., Coutinho H.D.M., Sharopov F., Salehi B., Yousaf Z., Sharifi-Rad M., del Mar Contreras M., Varoni E.M., Verma D.R., Iriti M., Sharifi-Rad J. Ethnobotany of the genus Taraxacum—phytochemicals and antimicrobial activity. Phytother Res. 2018;32(11) doi: 10.1002/ptr.6157. [DOI] [PubMed] [Google Scholar]
- 17.Afrisham R., Aberomand M., Ghaffari M.A., Siahpoosh A., Jamalan M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on activity of alpha-amylase. J. Bot., Le. 2015;2015 doi: 10.1155/2015/824683. [DOI] [Google Scholar]
- 18.Arumugam G., Manjula P., Paari N. A review: anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013;2(3):196–200. doi: 10.1016/s2221-6189(13)60126-2. [DOI] [Google Scholar]
- 19.Jacob B., Narendhirakannan R.T. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. 2019;9(1):4. doi: 10.1007/s13205-018-1528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Map Maker, (n.d.) https://maps.co/gis/(accessed January 26, 2024).
- 21.Ayaz E., Alpsoy H.C. Garlic (Allium sativum) and traditional medicine. Turk. Parazitoloji Derg. 2007;31(2) [PubMed] [Google Scholar]
- 22.Badal D.S., Azad C.S., Kumar V., Singh S., Prakash A., Patel S.V., Verma S., Kumar J., Dipankar C., Badal S., Dwivedi A.K. Effect of organic manures and inorganic fertilizers on growth, yield and its attributing traits in garlic (Allium sativum L.) J. Pharmacogn. Phytochem. 2019;8(3) [Google Scholar]
- 23.Rahman K. Historical perspective on garlic and cardiovascular disease. J. Nutr. 2001;131(3):977S–979S. doi: 10.1093/jn/131.3.977s. [DOI] [PubMed] [Google Scholar]
- 24.Al-Snafi A. Pharmacological effects of allium species grown in Iraq. An overview. Int. J. Pharm. Health Care Res. 2013;1:132–147. [Google Scholar]
- 25.Jang H.J., Lee H.J., Yoon D.K., Ji D.S., Kim J.H., Lee C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018;27(1):219–225. doi: 10.1007/s10068-017-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel D.K., Prasad S.K., Kumar R., Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faroughi F., Mohammad-Alizadeh S., Javadzadeh Y., Mirghafourvand M. Effects of garlic pill on blood glucose level in borderline gestational diabetes mellitus: a randomized controlled trial. Iran. Red Crescent Med. J. 2018;20(7) doi: 10.5812/ircmj.60675. [DOI] [Google Scholar]
- 28.Zhai B., Zhang C., Sheng Y., Zhao C., Xiaoyun H., Xu W.-T., Huang K., Luo Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-21421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batiha G.E.S., Beshbishy A.M., Wasef L.G., Elewa Y.H.A., Al-Sagan A.A., El-Hack M.E.A., Taha A.E., Abd-Elhakim Y.M., Devkota H.P. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrition. 2020;12(3):872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asdaq S.M.B., Inamdar M.N. Pharmacodynamic and pharmacokinetic interactions of propranolol with garlic (Allium sativum) in rats. J. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1093/ecam/neq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Sun J., Dou C., Li N., Kang F., Wang Y., Cao Z., Yang X., Dong S. Alliin attenuated RANKL-induced osteoclastogenesis by scavenging reactive oxygen species through inhibiting Nox1. Int. J. Mol. Sci. 2016;17(9):1516. doi: 10.3390/ijms17091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maan A.A., Nazir A., Khan M.K.I., Ahmad T., Zia R., Murid M., Abrar M. The therapeutic properties and applications of Aloe vera: a review. J. Herb. Med. 2018;12:1–10. doi: 10.1016/j.hermed.2018.01.002. [DOI] [Google Scholar]
- 33.Grace O.M., Buerki S., Symonds M.R., Forest F., van Wyk A.E., Smith G.F., Klopper R.R., Bjora C.S., Neale S., Demissew S., Simmonds M.S.J., Ronsted N. Evolutionary history and leaf succulence as explanations for medicinal use in aloes and the global popularity of Aloe vera. BMC Evol. Biol. 2015;15(29) doi: 10.1186/s12862-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misir J., Brishti F.H., Hoque M.M. Aloe vera gel as a novel edible coating for fresh fruits: a review. Adv. J. Food Sci. Technol. 2014;2(3) doi: 10.12691/ajfst-2-3-3. [DOI] [Google Scholar]
- 35.Mohammed A., Ibrahim M.A., Tajuddeen N., Aliyu A.B., Isah M.B. Antidiabetic potential of anthraquinones: a review. Phytother Res. 2019;2019:1–19. doi: 10.1002/ptr.6544. [DOI] [PubMed] [Google Scholar]
- 36.Noor A., Gunasekaran S., Vijayalakshmi M.A. Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with aloe vera extract. Pharmacogn. Res. 2017;9(5):s99–s104. doi: 10.4103/pr.pr_75_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahabady M.K., Tapebur M.B., Mazaheri Y., Tabandeh M.R., Tabatabaei S.R.F. Effect of Aloe vera on the expression of nerve factors, p75 and TrkA receptors in the Hippocampus of diabetic rats. Int. J. Morphol. 2021;39(2) doi: 10.4067/S0717-95022021000200577. [DOI] [Google Scholar]
- 38.Deora N., Sunitha M.M., Satyavani M., Harishankar N., Vijayalakshmi M.A., Venkataraman K., Venkateshan V. Alleviation of diabetes mellitus through the restoration of β-cell function and lipid metabolism by Aloe vera (L.) Burm. f. extract in obesogenic WNIN/GR-Ob rats. J. Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113921. [DOI] [PubMed] [Google Scholar]
- 39.Nomaguchi K., Tanaka M., Misawa E., Yamada M., Toida T., Iwatsuki K., Goto T., Kawada T. Aloe vera phytosterols act as ligands for PPAR and improve the expression levels of PPAR target genes in the livers of mice with diet-induced obesity. Obes. Res. Clin. Pract. 2011;5(3) doi: 10.1016/j.orcp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Bragt M.C.E., Popeijus H.E. Peroxisome proliferator-activated receptors and the metabolic syndrome. Physiol. Behav. 2008;94(2):187–197. doi: 10.1016/j.physbeh.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 41.Choudhri P., Rani M., Sangwan R.S., Kumar R., Kumar A., Chhokar V. De novo sequencing, assembly and characterisation of Aloe vera transcriptome and analysis of expression profiles of genes related to saponin and anthraquinone metabolism. BMC Genom. 2018;19(1) doi: 10.1186/s12864-018-4819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinarova L., Vinarov Z., Atanasov V., Pantcheva I., Tcholakova S., Denkov N., Stoyanov S. Lowering of cholesterol bioaccessibility and serum concentrations by saponins: In vitro and in vivo studies. Food Funct. 2015;6(2) doi: 10.1039/c4fo00785a. [DOI] [PubMed] [Google Scholar]
- 43.Tabatabaei S.R.F., Ghaderi S., Bahrami-Tapehebur M., Farbood Y., Rashno M. Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017;96:279–290. doi: 10.1016/j.biopha.2017.09.146. [DOI] [PubMed] [Google Scholar]
- 44.Christijanti W., Marianti A., Isnaeni W. Aloe vera extract reduces 8-oxo-2’-deoxyguanosine levels and improves total antioxidants in streptozotocin-induced diabetic rats. Univ. Med. 2017;36(1) doi: 10.18051/univmed.2017.v36.34-41. [DOI] [Google Scholar]
- 45.Christijanti W., Juniarto A.Z., Suromo L.B. Sperm DNA fragmentation index and malondialdehyde of diabetic rats treated by aloe vera peel extract, pak. J. Med. Health Sci. 2019;13(4) [Google Scholar]
- 46.Arora M.K., Sarup Y., Tomar R., Singh M., Kumar P. Amelioration of diabetes-induced diabetic nephropathy by Aloe vera: implication of oxidative stress and hyperlipidemia. J. Diet. Suppl. 2019;16(2):227–244. doi: 10.1080/19390211.2018.1449159. [DOI] [PubMed] [Google Scholar]
- 47.Bottenberg M.M., Wall G.C., Harvey R.L., Habib S. Oral aloe vera-induced hepatitis. Ann. Pharmacother. 2007;41(10) doi: 10.1345/aph.1K132. [DOI] [PubMed] [Google Scholar]
- 48.Ulbricht C., Armstrong J., Basch E., Basch S., Bent S., Dacey C., Dalton S., Foppa I., Giese N., Hammerness P., Kirkwood C., Sollars D., Tanguay-Colucci S., Weissner W. An evidence-based systematic review of Aloe vera by the natural standard research collaboration. J. Herb. Pharmacother. 2008;7(3–4):279–323. doi: 10.1080/15228940802153339. [DOI] [PubMed] [Google Scholar]
- 49.Boudreau M.D., Beland F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), aloe vera. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2006;24(1):103–154. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- 50.Christijanti W., Juniarto A.Z., Suromo L.B. Aloe vera peel extract administration increased antioxidant enzyme levels of serum and seminal plasma in type 2 diabetic rats. Pharmacogn. J. 2019;11(5):962–967. doi: 10.5530/pj.2019.11.152. [DOI] [Google Scholar]
- 51.Prueksrisakul T., Chantarangsu S., Thunyakitpisal P. Effect of daily drinking of Aloe vera gel extract on plasma total antioxidant capacity and oral pathogenic bacteria in healthy volunteer: a short-term study, J. Altern. Complement. Integr. Med. 2015;12 doi: 10.1515/jcim-2014-0060. [DOI] [PubMed] [Google Scholar]
- 52.Alshatwi A., babu Pandurangan S. Aloe-emodin protects RIN-5F (pancreatic β-cell) cell from glucotoxicity via regulation of pro-inflammatory cytokine and downregulation of bax and caspase 3. Biomol. Ther. 2016;24:49–56. doi: 10.4062/biomolther.2015.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Florence N.T., Benoit M.Z., Jonas K., Alexandra T., Désiré D.D.P., Pierre K., Théophile D. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2014;151(2):784–790. doi: 10.1016/j.jep.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Justino A.B., Miranda N.C., Franco R.R., Martins M.M., da Silva N.M., Espindola F.S. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 2018;100:83–92. doi: 10.1016/j.biopha.2018.01.172. [DOI] [PubMed] [Google Scholar]
- 55.Fofana S., Keita A., Balde S., Ziyaev R., Aripova S.F. Alkaloids from leaves of Annona muricata. Chem. Nat. Compd. 2012;48(4):714. doi: 10.1007/s10600-012-0363-5. [DOI] [Google Scholar]
- 56.Hasrat J.A., De Bruyne T., De Backer J.P., Vauquelin G., Vlietinck A.J. Isoquinoline derivatives isolated from the fruit of Annona muricata as 5-HTergic 5-HT1A receptor agonists in rats: unexploited antidepressive (lead) products. J. Pharm. Pharmacol. 1997;49(11):1145–1149. doi: 10.1111/j.2042-7158.1997.tb06058.x. [DOI] [PubMed] [Google Scholar]
- 57.Son Y., Lee H., Son S.Y., Lee C.H., Kim S.Y., Lim Y. Ameliorative effect of Annona muricata (Graviola) extract on hyperglycemia induced hepatic damage in type 2 diabetic mice. Antioxidants. 2021;10(10):1546. doi: 10.3390/antiox10101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ojo O.A., Grant S., Amanze J.C., Oni A.I., Ojo A.B., Elebiyo T.C., Obafemi T.O., Ayokunle D.I., Ogunlakin A.D. Annona muricata L. peel extract inhibits carbohydrate metabolizing enzymes and reduces pancreatic β-cells, inflammation, and apoptosis via upregulation of PI3K/AKT genes. PLoS One. 2022;17(10) doi: 10.1371/journal.pone.0276984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arroyo J., Jaime M., Gerardo, Ronceros Robert P., Aníbal V., Pablo B., Christian P.M.Q. 1. Hypoglycemic Effect adjuvant extract ethanolic leaf Annona muricata L (guanábana), in patients with diabetes type 2 in treatment of glibenclamide. Ann. Fac. Med. 2009;70(3):163–167. [Google Scholar]
- 60.Alwan I., Lim V., Samad N., Widyawati T., Nor Adlin Y. Effect of Annona muricata L. on metabolic parameters in diabetes mellitus: a systematic review. Curr. Res. Nutr. Food Sci. 2020;8:1–11. doi: 10.12944/CRNFSJ.8.1.01. [DOI] [Google Scholar]
- 61.Pio Corrêa M., Penna L. de A. Diccionário das plantas úteis do Brasil e das exóticas cultivadas. 1984 [Google Scholar]
- 62.Pinheiro T.S.D.B., Johansson L.A.P., Pizzolatti M.G., Biavatti M.W. Comparative assessment of kaempferitrin from medicinal extracts of Bauhinia forficata Link. J. Pharm. Biomed. Anal. 2006;41(2):431–436. doi: 10.1016/j.jpba.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Ren J., Lu Y., Qian Y., Chen B., Wu T., Ji G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019:2759–2776. doi: 10.3892/etm.2019.7886. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Damasceno D.C., Volpato G.T., De Mattos Paranhos Calderon I., Aguilar R., Rudge M.V.C. Effect of Bauhinia forficata extract in diabetic pregnant rats: maternal repercussions. Phytomedicine. 2004;11(2–3):196–201. doi: 10.1078/0944-7113-00348. [DOI] [PubMed] [Google Scholar]
- 65.Pepato M.T., Keller E.H., Baviera A.M., Kettelhut I.C., Vendramini R.C., Brunetti I.L. Anti-diabetic activity of Bauhinia forficata decoction in streptozotocin-diabetic rats. J. Ethnopharmacol. 2002;81(2):191–197. doi: 10.1016/S0378-8741(02)00075-2. [DOI] [PubMed] [Google Scholar]
- 66.Franco R.R., da Silva Carvalho D., de Moura F.B.R., Justino A.B., Silva H.C.G., Peixoto L.G., Espindola F.S. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J. Ethnopharmacol. 2018;215:140–146. doi: 10.1016/j.jep.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 67.Franco R.R., Mota Alves V.H., Ribeiro Zabisky L.F., Justino A.B., Martins M.M., Saraiva A.L., Goulart L.R., Espindola F.S. Antidiabetic potential of Bauhinia forficata Link leaves: a non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomed. Pharmacother. 2020;123 doi: 10.1016/j.biopha.2019.109798. [DOI] [PubMed] [Google Scholar]
- 68.Ajebli M., Khan H., Eddouks M. Natural alkaloids and diabetes mellitus: a review. Endocr. Metab. Immune Disord. - Drug Targets. 2020;21(1):111–130. doi: 10.2174/1871530320666200821124817. [DOI] [PubMed] [Google Scholar]
- 69.Pinafo M.S., Benedetti P.R., Gaiotte L.B., Costa F.G., Schoffen J.P.F., Fernandes G.S.A., Chuffa L.G.A., Seiva F.R.F. Effects of Bauhinia forficata on glycaemia, lipid profile, hepatic glycogen content and oxidative stress in rats exposed to Bisphenol A. Toxicol Rep. 2019;6:244–252. doi: 10.1016/j.toxrep.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tonelli C.A., de Oliveira S.Q., da Silva Vieira A.A., Biavatti M.W., Ritter C., Reginatto F.H., de Campos A.M., Dal-Pizzol F. Clinical efficacy of capsules containing standardized extract of Bauhinia forficata Link (pata-de-vaca) as adjuvant treatment in type 2 diabetes patients: a randomized, double blind clinical trial. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114616. [DOI] [PubMed] [Google Scholar]
- 71.Arambewela L.S., Arawwawala L.D. Antioxidant activities of ethanolic and hot aqueous extracts of Alpinia calcarata rhizomes. Aust. J. Med. Herb. 2005;17(3):91. https://search.informit.org/doi/10.3316/informit.407174355738341 [Google Scholar]
- 72.Rao P.V., Gan S.H. Cinnamon: a multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jayaweera D.M.A. Medicinal Plants (Indigenous and exotic) Used in Ceylon, Medicinal Plants (Indigenous and Exotic) Used in Ceylon Part I. 1981 [Google Scholar]
- 74.Shang C., Lin H., Fang X., Wang Y., Jiang Z., Qu Y., Xiang M., Shen Z., Xin L., Lu Y., Gao J., Cui X. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021;12(24) doi: 10.1039/d1fo01935j. [DOI] [PubMed] [Google Scholar]
- 75.Filho J., Van-Koiij A., Mancini D., Cozzolino F., Torres R. Antioxidant activity of cinnamon (Cinnamomum zeylanicum, breyne) extracts. Boll. Chim. Farm. 1999;137:443–447. [PubMed] [Google Scholar]
- 76.Ranasinghe P., Perera S., Gunatilake M., Abeywardene E., Gunapala N., Premakumara S., Perera K., Lokuhetty D., Katulanda P. Effects of Cinnamomum zeylanicum (Ceylon cinnamon) on blood glucose and lipids in a diabetic and healthy rat model. Pharmacogn. Res. 2012;4(2):73–79. doi: 10.4103/0974-8490.94719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choudhury H., Pandey M., Hua C.K., Mun C.S., Jing J.K., Kong L., Ern L.Y., Ashraf N.A., Kit S.W., Yee T.S., Pichika M.R., Gorain B., Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J. Tradit. Complement. Med. 2018;8(3):361–376. doi: 10.1016/j.jtcme.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prakash O., Kumar R., Srivastava R., Tripathi P., Mishra S. Ajeet, plants explored with anti-diabetic properties: a review. Am. J. Pharmacol. Sci. 2015;(3) [Google Scholar]
- 79.Kwon H.K., Jeon W.K., Hwang J.S., Lee C.G., So J.S., Park J.A., Ko B.S., Im S.H. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009;278(2):174–182. doi: 10.1016/j.canlet.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 80.Nabavi S.F., Di Lorenzo A., Izadi M., Sobarzo-Sánchez E., Daglia M., Nabavi S.M. Antibacterial effects of cinnamon: from farm to food, cosmetic and pharmaceutical industries. Nutrients. 2015;7(9):7729–7748. doi: 10.3390/nu7095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Behbahani B.A., Falah F., Arab F.L., Vasiee M., Yazdi F.T. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. J. Evid. Based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/5190603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jitomir J., Willoughby D.S. Cassia cinnamon for the attenuation of glucose intolerance and insulin resistance resulting from sleep loss. J. Med. Food. 2009;12(3) doi: 10.1089/jmf.2008.0128. [DOI] [PubMed] [Google Scholar]
- 83.Verspohl E.J., Bauer K., Neddermann E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum In vivo and In vitro. Phytother Res. 2005;19(3) doi: 10.1002/ptr.1643. [DOI] [PubMed] [Google Scholar]
- 84.Hafizur R.M., Hameed A., Shukrana M., Raza S.A., Chishti S., Kabir N., Siddiqui R.A. Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro. Phytomedicine. 2015;22(2):297–300. doi: 10.1016/j.phymed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Adisakwattana S., Lerdsuwankij O., Poputtachai U., Minipun A., Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum. Nutr. 2011;66:143–148. doi: 10.1007/s11130-011-0226-4. [DOI] [PubMed] [Google Scholar]
- 86.Kim S.H., Hyun S.H., Choung S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006;104(1–2):119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 87.Cheng D.M., Kuhn P., Poulev A., Rojo L.E., Lila M.A., Raskin I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012;135(4):2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen Y., Fukushima M., Ito Y., Muraki E., Hosono T., Seki T., Ariga T. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci. Biotechnol. Biochem. 2010;74(12):2418–2425. doi: 10.1271/bbb.100453. [DOI] [PubMed] [Google Scholar]
- 89.Abdelmageed M., Shehatou G., Abdelsalam R., Suddek G., Salem H. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019;392:243–258. doi: 10.1007/s00210-018-1583-4. [DOI] [PubMed] [Google Scholar]
- 90.Li J.-E., Futawaka K., Yamamoto H., Kasahara M., Tagami T., Liu T.-H., Moriyama K. Cinnamaldehyde contributes to insulin sensitivity by activating PPARδ, PPARγ, and RXR. Am. J. Chin. Med. 2015;43:1–14. doi: 10.1142/S0192415X15500512. [DOI] [PubMed] [Google Scholar]
- 91.Hayward N.J., McDougall G.J., Farag S., Allwood J.W., Austin C., Campbell F., Horgan G., Ranawana V. Cinnamon shows antidiabetic properties that are species-specific: effects on enzyme activity inhibition and starch digestion. Plant Foods Hum. Nutr. 2019;74(4):544–552. doi: 10.1007/s11130-019-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muthenna P., Raghu G., Kumar P.A., Surekha M.V., Reddy G.B. Effect of cinnamon and its procyanidin-B2 enriched fraction on diabetic nephropathy in rats. Chem. Biol. Interact. 2014;222:68–76. doi: 10.1016/j.cbi.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Ranasinghe P., Jayawardena R., Pigera S., Wathurapatha W.S., Weeratunga H.D., Premakumara G.A.S., Katulanda P., Constantine G.R., Galappaththy P. Evaluation of pharmacodynamic properties and safety of Cinnamomum zeylanicum (Ceylon cinnamon) in healthy adults: a phase I clinical trial. BMC Complement. Med. Ther. 2017;17:550. doi: 10.1186/s12906-017-2067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markey O., McClean C.M., Medlow P., Davison G.W., Trinick T.R., Duly E., Shafat A. Effect of cinnamon on gastric emptying, arterial stiffness, postprandial lipemia, glycemia, and appetite responses to high-fat breakfast. Cardiovasc. Diabetol. 2011;10 doi: 10.1186/1475-2840-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blevins S.M., Leyva M.J., Brown J., Wright J., Scofield R.H., Aston C.E. Effect of cinnamon on glucose and lipid levels in non–insulin-dependent type 2 diabetes. Diabetes Care. 2007;30(9):2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 96.Khan A., Safdar M., Ali Khan M.M., Khattak K.N., Anderson R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 97.Lu T., Sheng H., Wu J., Cheng Y., Zhu J., Chen Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr. Res. 2012;32(6):408–412. doi: 10.1016/j.nutres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Leach M.J., Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst. Rev. 2012;2017(12) doi: 10.1002/14651858.CD007170.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heidarianpour A., Mohammadi F., Keshvari M., Mirazi N. Ameliorative effects of endurance training and Matricaria chamomilla flowers hydroethanolic extract on cognitive deficit in type 2 diabetes rats. Biomed. Pharmacother. 2021;135 doi: 10.1016/j.biopha.2021.111230. [DOI] [PubMed] [Google Scholar]
- 100.El Mihyaoui A., Esteves Da Silva J.C.G., Charfi S., Castillo M.E.C., Lamarti A., Arnao M.B. Chamomile (Matricaria chamomilla L.): a review of ethnomedicinal use, phytochemistry and pharmacological uses. Life. 2022;12(4):479. doi: 10.3390/life12040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stanojevic L.P., Marjanovic-Balaban Z.R., Kalaba V.D., Stanojevic J.S., Cvetkovic D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.) J. Essent. Oil-Bear. Plants. 2016;19(8):2017–2028. doi: 10.1080/0972060X.2016.1224689. [DOI] [Google Scholar]
- 102.Yang Y.L., Wang Y., Ma H.M., Lan W. Hypoglycemic effect of German chamomile total flavonoids on diabetic mice. J. Food Saf. Qual. 2020;11:1524–1528. [Google Scholar]
- 103.Cemek M., Kaǧa S., Şimşek N., Büyükokuroǧlu M.E., Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 2008;62(3):284–293. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 104.Asgharzade S., Rabiei Z., Rafieian-Kopaei M. Effects of Matricaria chamomilla extract on motor coordination impairment induced by scopolamine in rats. Asian Pac. J. Trop. Biomed. 2015;5(10):829–833. doi: 10.1016/j.apjtb.2015.06.006. [DOI] [Google Scholar]
- 105.Kamatou G.P.P., Viljoen A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010;87(1) doi: 10.1007/s11746-009-1483-3. [DOI] [Google Scholar]
- 106.Abdanipour A. Effect of hydro-ethanolic extract of Chamaemelum nobile on cell proliferation and apoptosis of rat hippocampal neural stem cells in the oxidative stress condition. J. Gorgan Univ. Med. Sci. 2015;16(4):14–20. [Google Scholar]
- 107.Rafraf M., Zemestani M., Asghari-Jafarabadi M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J. Endocrinol. Invest. 2015;38(2):163–170. doi: 10.1007/s40618-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 108.Banihani S., Swedan S., Alguraan Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013;33(5):341–348. doi: 10.1016/j.nutres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Loizzo M.R., Aiello F., Tenuta M.C., Leporini M., Falco T., Tundis R. In: Nonvitamin and Nonmineral Nutr. Suppl. Nabavi S.M., Silva A.S., editors. Academic Press; 2019. Chapter 3.46 - pomegranate (punica granatum L.) pp. 467–472. [DOI] [Google Scholar]
- 110.Chaudhari S.M., Patel K.Y., Badole S.L. In: Treatment Cancer, Watson R.R., Preedy V.R., Zibadi S., editors. vol. 2. Academic Press; 2014. Chapter 106 - Punica granatum (pomegranate fruit) pp. 1393–1400. (Polyphenols in Human Health and Dis). [DOI] [Google Scholar]
- 111.Šavikin K., Živković J., Alimpić A., Zdunić G., Janković T., Duletić-Laušević S., Menković N. Activity guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind. Crops Prod. 2018;113:142–149. doi: 10.1016/j.indcrop.2018.01.031. [DOI] [Google Scholar]
- 112.Arun K.B., Jayamurthy P., Anusha C.V., Mahesh S.K., Nisha P. Studies on activity guided fractionation of pomegranate peel extracts and its effect on antidiabetic and cardiovascular protection properties. J. Food Process. Preserv. 2017;41(1) doi: 10.1111/jfpp.13108. [DOI] [Google Scholar]
- 113.Sotto A., Locatelli M., Macone A., Toniolo C., Cesa S., Carradori S., Eufemi M., Mazzanti G., Di Giacomo S. Hypoglycemic, antiglycation and cytoprotective properties of A phenol rich extract from waste peel of Punica granatum L. Var. Dente Di Cavallo DC2. Molecules. 2019;24 doi: 10.20944/preprints201908.0028.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Çam M., İçyer N.C. Phenolics of pomegranate peels: extraction optimization by central composite design and alpha glucosidase inhibition potentials. J. Food Sci. Technol. 2015;52(3):1489–1497. doi: 10.1007/s13197-013-1148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Papoutsis K., Zhang J., Bowyer M.C., Brunton N., Gibney E.R., Lyng J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: a review. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128119. [DOI] [PubMed] [Google Scholar]
- 116.Rock W., Rosenblat M., Miller-Lotan R., Levy A.P., Elias M., Aviram M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008;56(18) doi: 10.1021/jf801756x. [DOI] [PubMed] [Google Scholar]
- 117.Mohan M., Waghulde H., Kasture S. Effect of pomegranate juice on angiotensin II-induced hypertension in diabetic wistar rats. Phytother Res. 2010;24(SUPPL. 2):S196–S203. doi: 10.1002/ptr.3090. [DOI] [PubMed] [Google Scholar]
- 118.Rozenberg O., Howell A., Aviram M. Pomegranate juice sugar fraction reduces macrophage oxidative state, whereas white grape juice sugar fraction increases it. Atherosclerosis. 2006;188(1):68–76. doi: 10.1016/j.atherosclerosis.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 119.Sun C., Liu Y., Zhan L., Rayat G.R., Xiao J., Jiang H., Li X., Chen K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2021;117:3–14. doi: 10.1016/j.tifs.2020.07.024. [DOI] [Google Scholar]
- 120.Huang T., Peng G., Kota B., Li G., Yamahara J., Roufogalis B., Yuhao L. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-γ and identification of an active component. Toxicol. Appl. Pharmacol. 2005;207:160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Makino-Wakagi Y., Yoshimura Y., Uzawa Y., Zaima N., Moriyama T., Kawamura Y. Ellagic acid in pomegranate suppresses resistin secretion by a novel regulatory mechanism involving the degradation of intracellular resistin protein in adipocytes. Biochem. Biophys. Res. Commun. 2011;417:880–885. doi: 10.1016/j.bbrc.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 122.McTernan P.G., Fisher F.M., Valsamakis G., Chetty R., Harte A., McTernan C.L., Clark P.M.S., Smith S.A., Barnett A.H., Kumar S. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J. Clin. Endocrinol. Metab. 2003;88(12):6098–6106. doi: 10.1210/jc.2003-030898. [DOI] [PubMed] [Google Scholar]
- 123.Steppan C.M., Bailey S.T., Bhat S., Brown E.J., Banerjee R.R., Wright C.M., Patel H.R., Ahima R.S., Lazar M.A. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 124.Parmar H.S., Kar A. Medicinal values of fruit peels from citrus sinensis, medicinal values of fruit peels from Citrus sinensis, Punica granatum, and Musa paradisiaca with respect to alterations in tissue lipid peroxidation and serum concentration of glucose, insulin, and thyroid hormones. J. Med. Food. 2008;11(2) doi: 10.1089/jmf.2006.010. [DOI] [PubMed] [Google Scholar]
- 125.Jafri M.A., Aslam M., Javed K., Singh S. Effect of Punica granatum Linn. (flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2000;70(3):309–314. doi: 10.1016/S0378-8741(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 126.Khajebishak Y., Payahoo L., Alivand M., Hamishekar H., Mobasseri M., Ebrahimzadeh V., Alipour M., Alipour B. Effect of pomegranate seed oil supplementation on the GLUT‐4 gene expression and glycemic control in obese people with type 2 diabetes: a randomized controlled clinical trial. J. Cell. Physiol. 2019;234(11) doi: 10.1002/jcp.28561. [DOI] [PubMed] [Google Scholar]
- 127.Almatroodi S.A., Almatroudi A., Alsahli M.A., Rahmani A.H. Fenugreek (trigonella foenum-graecum) and its active compounds: a review of its effects on human health through modulating biological activities. Pharmacogn. J. 2021;13(3):813–821. doi: 10.5530/pj.2021.13.103. [DOI] [Google Scholar]
- 128.Sauvaire Y., Baissac Y., Leconte O., Petit P., Ribes G. Steroid saponins from fenugreek and some of their biological properties. Adv. Exp. Med. Biol. 1996;405:37–46. doi: 10.1007/978-1-4613-0413-5_4. [DOI] [PubMed] [Google Scholar]
- 129.Sridevi P., Ga L., Vunutha K., Akshitha K., MaheshKBhagavanRaju M. Dipeptide synthesis and evaluation of antidiabetic activity of 4-hydroxyisoleucine from fenugreek seeds. Pharm. Bioprocess. 2017;5:44–53. [Google Scholar]
- 130.Srinivasan K. Plant foods in the management of diabetes mellitus: spices as beneficial antidiabetic food adjuncts. Int. J. Food Sci. Nutr. 2005;56:399–414. doi: 10.1080/09637480500512872. [DOI] [PubMed] [Google Scholar]
- 131.Bafadam S., Mahmoudabady M., Niazmand S., Rezaee S.A., Soukhtanloo M. Cardioprotective effects of Fenugreek (Trigonella foenum-graceum) seed extract in streptozotocin induced diabetic rats. J. Cardiovasc. Thorac. Res. 2021;13(1):28–36. doi: 10.34172/jcvtr.2021.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rizvi S.I., Mishra N. Traditional Indian medicines used for the management of diabetes mellitus. J. Diabetes Res. 2013;2013 doi: 10.1155/2013/712092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Puri D., Prabhu K.M., Murthy P.S. Mechanism of action of a hypoglycemic principle isolated from fenugreek seeds. Indian J. Physiol. Pharmacol. 2002;46(4) [PubMed] [Google Scholar]
- 134.Sarra K., Jemaa H.B., Hmad H., Hajer A., Inchirah K., Abid A., Benzarti A., Elati J., Aouidet A. Antioxidant, antidiabetic and antihyperlipidemic effects of Trigonella foenum-graecum seeds. Int. J. Pharmacol. 2016;12:394–400. doi: 10.3923/ijp.2016.394.400. [DOI] [Google Scholar]
- 135.Verma N., Usman K., Patel N., Jain A., Dhakre S., Swaroop A., Bagchi M., Kumar P., Preuss H.G., Bagchi D. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (FenfuroTM) in patients with type 2 diabetes. Food Nutr. Res. 2016;60(1) doi: 10.3402/fnr.v60.32382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Najdi R.A., Hagras M.M., Kamel F.O., Magadmi R.M. A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr. Health Sci. 2019 doi: 10.4314/ahs.v19i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tavakoly R., Maracy M.R., Karimifar M., Entezari M.H. Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur. J. Integr. Med. 2018;18:13–17. doi: 10.1016/j.eujim.2018.01.005. [DOI] [Google Scholar]
- 138.Singh A., Rai J., Mahajan D.S. Comparative evaluation of glipizide and fenugreek (Trigonella foenum-graecum) seeds as monotherapy and combination therapy on glycaemic control and lipid profile in patients with type 2 diabetes mellitus. Int. J. Basic Clin. Pharmacol. 2016;5(3):942–950. doi: 10.18203/2319-2003.ijbcp20161549. [DOI] [Google Scholar]
- 139.Zubaidi S.N., Mohd Nani H., Ahmad Kamal M.S., Abdul Qayyum T., Maarof S., Afzan A., Mohmad Misnan N., Hamezah H.S., Baharum S.N., Mediani A. Annona muricata: comprehensive review on the ethnomedicinal, phytochemistry, and pharmacological aspects focusing on antidiabetic properties. Life. 2023;13(2):353. doi: 10.3390/life13020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tresina P.S., Selvam M.S., Doss A., Mohan V.R. Chapter 3 - antidiabetic bioactive natural products from medicinal plants. Stud. Nat. Prod. Chem. 2022;75:75–118. doi: 10.1016/B978-0-323-91250-1.00004-5. [DOI] [Google Scholar]
- 141.Rey D., Sulis P.M., Fernandes T.A., Gonçalves R., Frederico M.J.S., Costa G.M., Aragon M., Ospina L.F., Silva F.R.M.B. Astragalin augments basal calcium influx and insulin secretion in rat pancreatic islets. Cell Calcium. 2019;80:56–62. doi: 10.1016/j.ceca.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 142.Zhu R., Liu H., Liu C., Wang L., Ma R., Chen B., Li L., Niu J., Fu M., Zhang D., Gao S. Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017;122:78–89. doi: 10.1016/j.phrs.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 143.Rangari V.D., Shukla P., Badole S.L. vol. 2. Academic Press; 2015. Chapter 15 - 4-hydroxyisoleucine: a potential antidiabetic agent from Trigonella foenum-graecum, glucose intake and utilization in pre-diabet; pp. 191–198. (Diabet). [DOI] [Google Scholar]
- 144.Ansari P., Choudhury S.T., Seidel V., Rahman A.B., Aziz M.A., Richi A.E., Rahman A., Jafrin U.H., Hannan J.M.A., Abdel-Wahab Y.H.A. Therapeutic potential of quercetin in the management of type-2 diabetes mellitus. Life. 2022;12(8):1146. doi: 10.3390/life12081146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fuhrman B., Volkova N., Aviram M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: studies in vitro and in diabetic patients. Nutr. 2010;26(4):359–366. doi: 10.1016/j.nut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 146.Arion W.J., Canfield W.K., Ramos F.C., Schindler P.W., Burger H.J., Hemmerle H., Schubert G., Below P., Herling A.W. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997;339(2):315–322. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 147.BioRender Scientific image and illustration software. https://www.biorender.com/
- 148.Sertkaya A., Wong H.H., Jessup A., Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States, Clin. Trials. 2016;13(2):117–126. doi: 10.1177/1740774515625964. [DOI] [PubMed] [Google Scholar]
- 149.Phukhatmuen P., Raksat A., Laphookhieo S., Charoensup R., Duangyod T., Maneerat W. Bioassay-guided isolation and identification of antidiabetic compounds from Garcinia cowa leaf extract. Heliyon. 2020;6(4) doi: 10.1016/j.heliyon.2020.e03625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vhora N., Naskar U., Hiray A., Kate A.S., Jain A. Recent advances in in-vitro assays for type 2 diabetes mellitus: an overview. Rev. Diabet. Stud. 2020;16(1):13–23. doi: 10.1900/RDS.2020.16.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh R., Farooq S.A., Mannan A., Singh T.G., Najda A., Grażyna Z., Albadrani G.M., Sayed A.A., Abdel-Daim M.M. Animal models of diabetic microvascular complications: relevance to clinical features. Biomed. Pharmacother. 2022;145 doi: 10.1016/j.biopha.2021.112305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.