Abstract
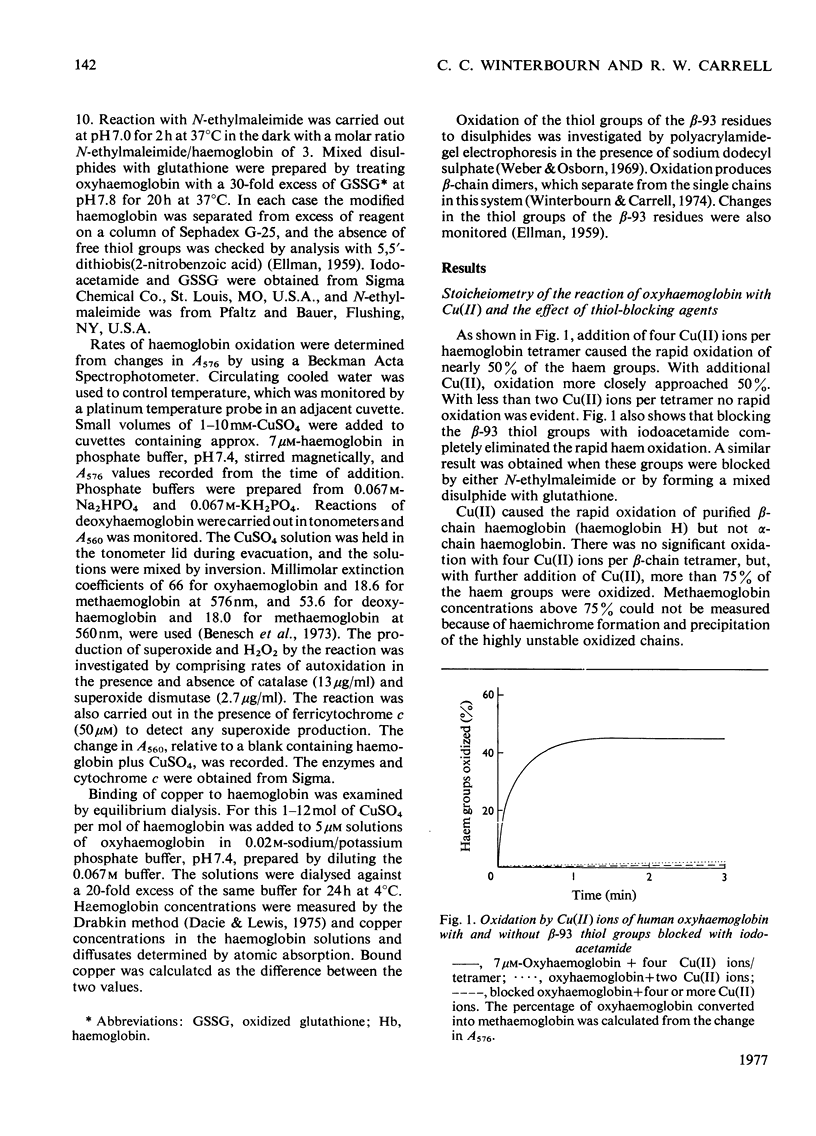
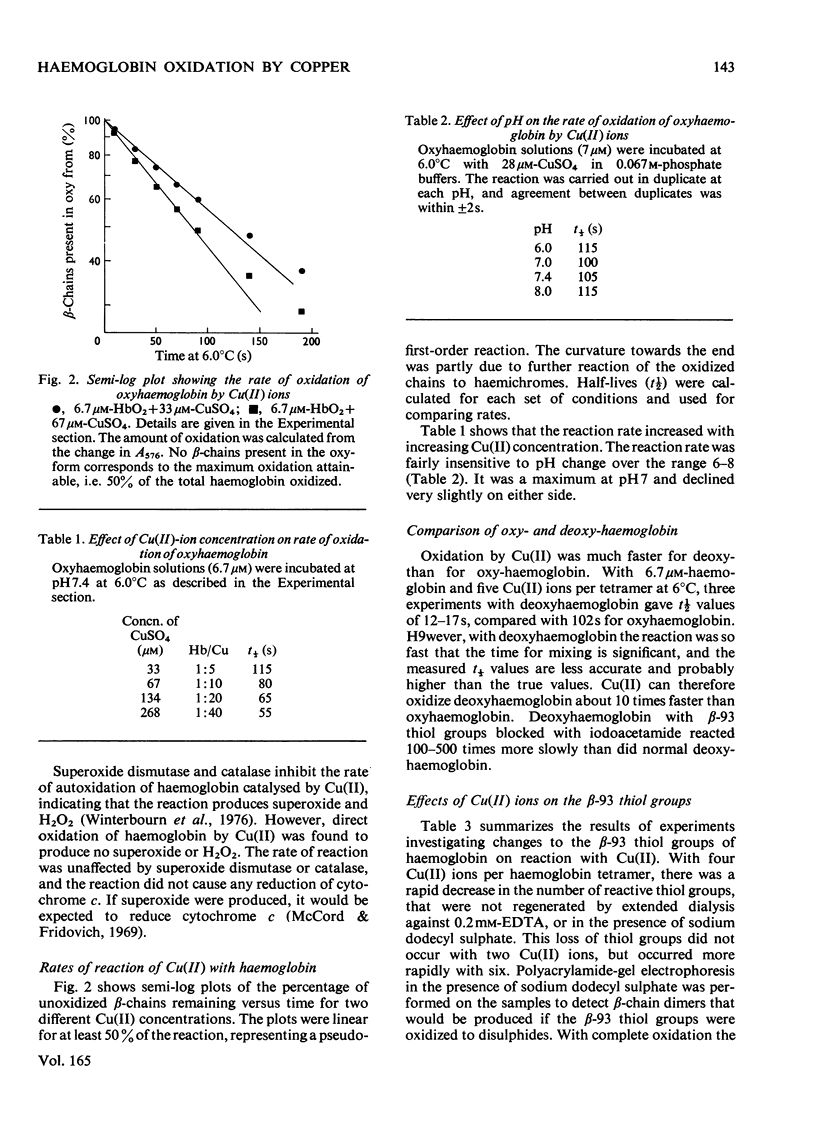
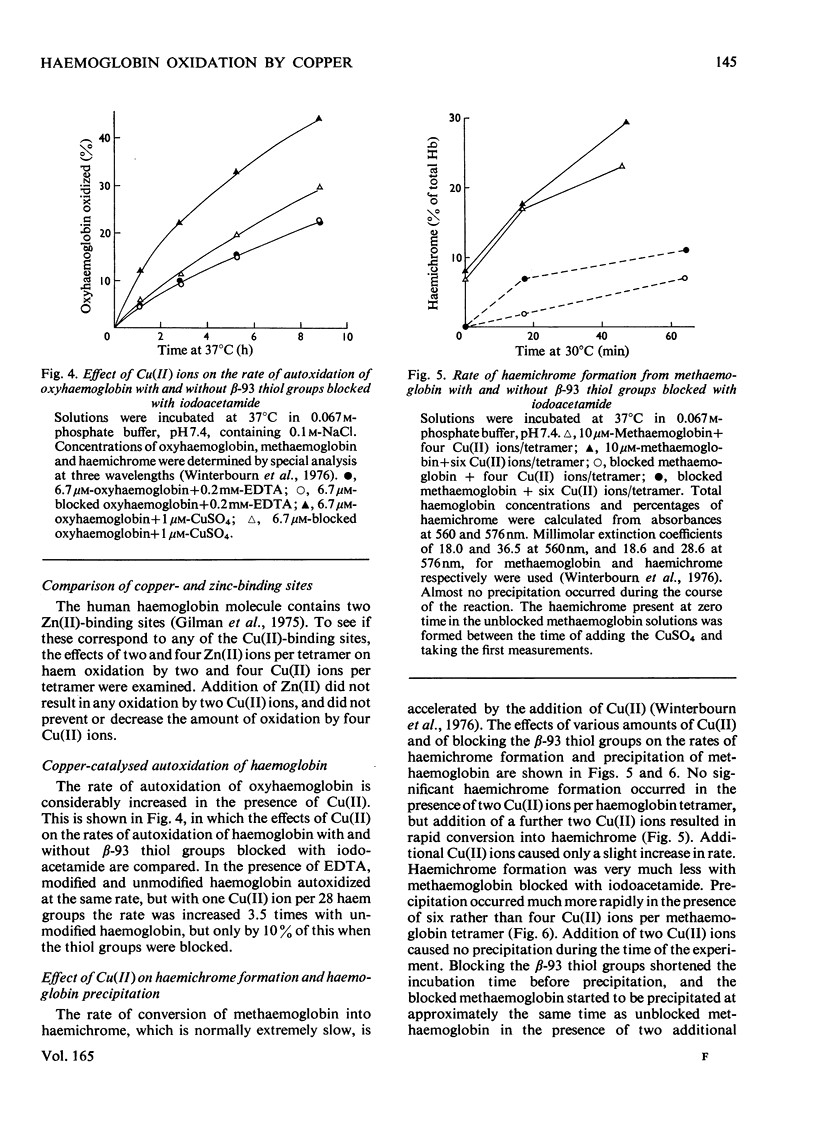
Addition of Cu(II) ions to human oxyhaemoglobin caused the rapid oxidation of the haem groups of the beta-chain. Oxidation required binding of Cu(II) to sites involving the thiol group of beta-93 residues and was prevented when these groups were blocked with iodoacetamide or N-ethylmaleimide. Equilibrium-dialysis studies showed three pairs of binding sites, two pairs with high affinity for Cu(II) and one pair with lower affinity. It was the second pair of high-affinity sites that were blocked with iodoacetamide and were involved in haem oxidation. Cu(II) oxidized deoxyhaemoglobin at least ten times as fast as oxyhaemoglobin, and analysis of rates suggested that binding rather than electron transfer was the rate-determining step. No thiol-group oxidation to disulphides occurred during the period of haem oxidation, although it did occur subsequently in the presence of oxygen, or when Cu(II) was added to methaemoglobin. It is proposed that thiol oxidation did not occur because there exists a pathway of electron transfer between the haem group and copper bound to the beta-93 thiol groups. The route for this electron transfer is discussed, as well as the implications as to the function of the beta-93 cysteine in the haemoglobin molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANASZAK L. J., WATSON H. C., KENDREW J. C. THE BINDING OF CUPRIC AND ZINC IONS TO CRYSTALLINE SPERM WHALE MYOGLOBIN. J Mol Biol. 1965 May;12:130–137. doi: 10.1016/s0022-2836(65)80287-x. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal Biochem. 1973 Sep;55(1):245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- Cavallini D., De Marco C., Duprè S., Rotilio G. The copper catalyzed oxidation of cysteine to cystine. Arch Biochem Biophys. 1969 Mar;130(1):354–361. doi: 10.1016/0003-9861(69)90044-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Pryor W. A. Free radicals in biological systems. Sci Am. 1970 Aug;223(2):70–passim. doi: 10.1038/scientificamerican0870-70. [DOI] [PubMed] [Google Scholar]
- Rifkind J. M. Copper and the autoxidation of hemoglobin. Biochemistry. 1974 Jun 4;13(12):2475–2481. doi: 10.1021/bi00709a003. [DOI] [PubMed] [Google Scholar]
- TAKAGI T., ISEMURA T. ACCELERATING EFFECT OF COPPER ION ON THE REACTIVATION OF REDUCED TAKA-AMYLASE A THROUGH CATALYSIS OF THE OXIDATION OF SULFHYDRYL GROUPS. J Biochem. 1964 Oct;56:344–350. doi: 10.1093/oxfordjournals.jbchem.a127999. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Studies of hemoglobin denaturation and Heinz body formation in the unstable hemoglobins. J Clin Invest. 1974 Sep;54(3):678–689. doi: 10.1172/JCI107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]


