Abstract
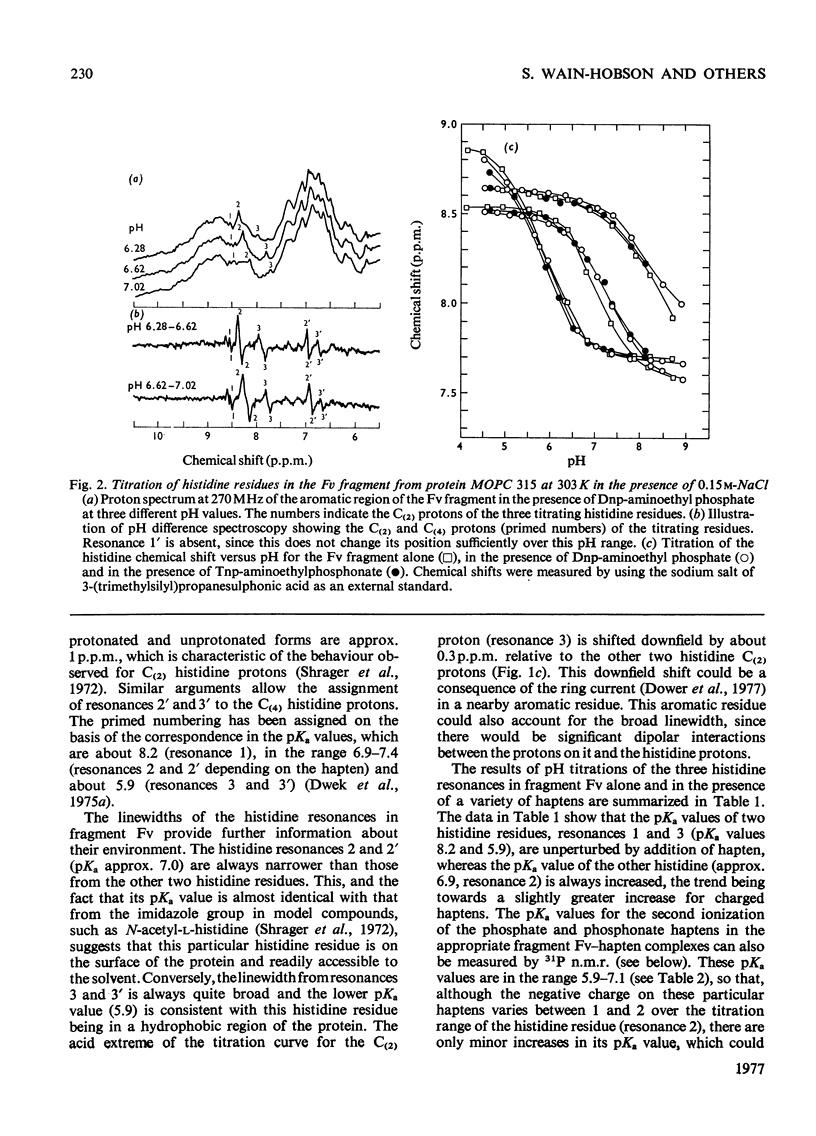
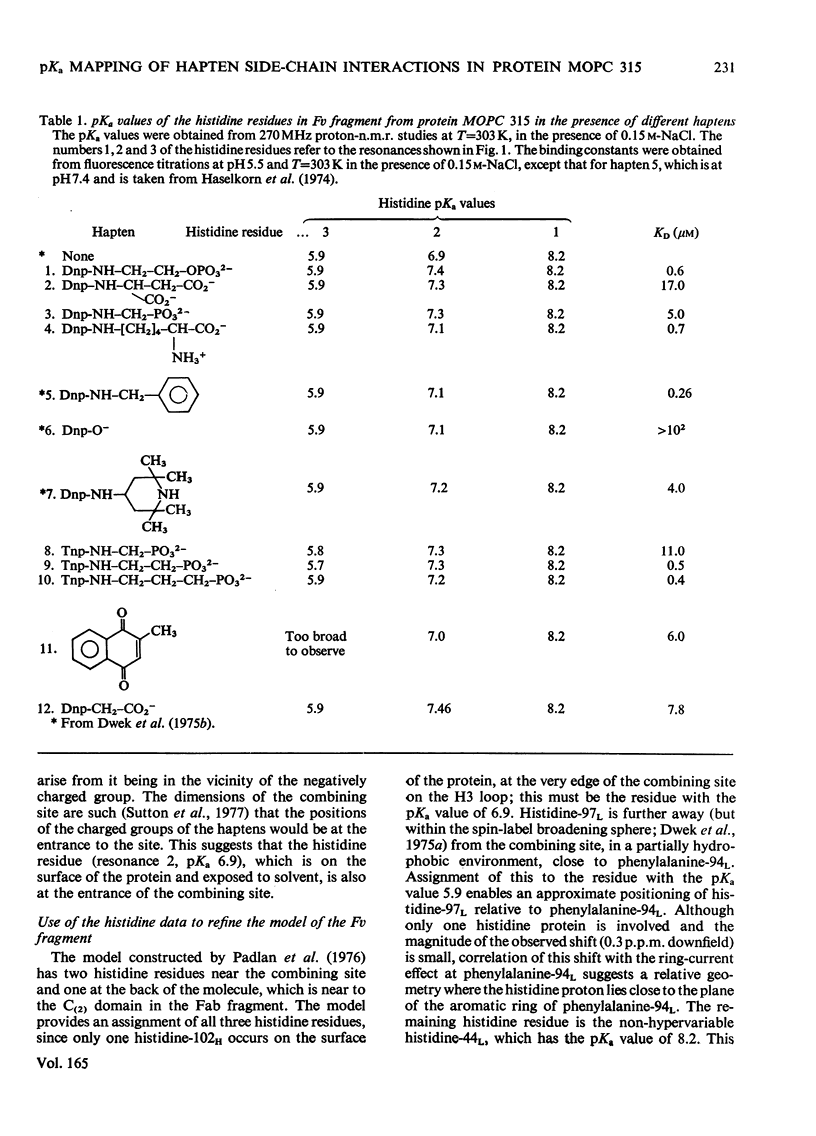
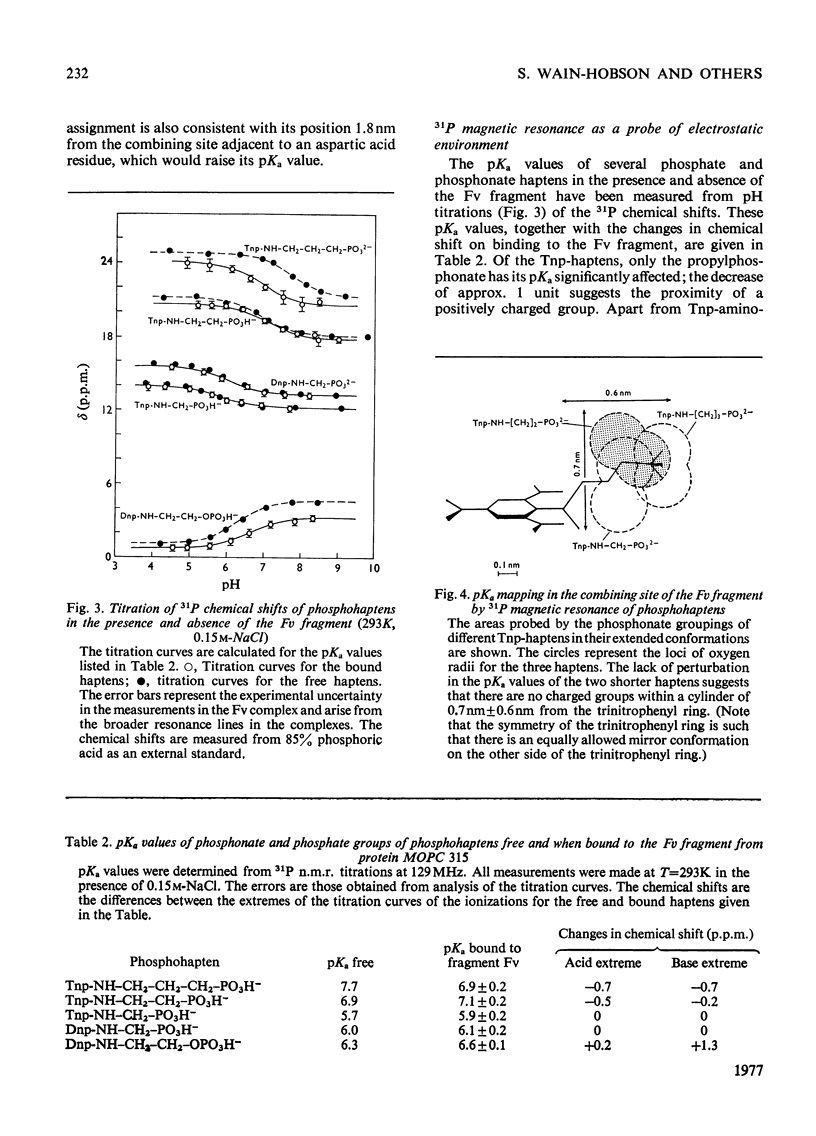
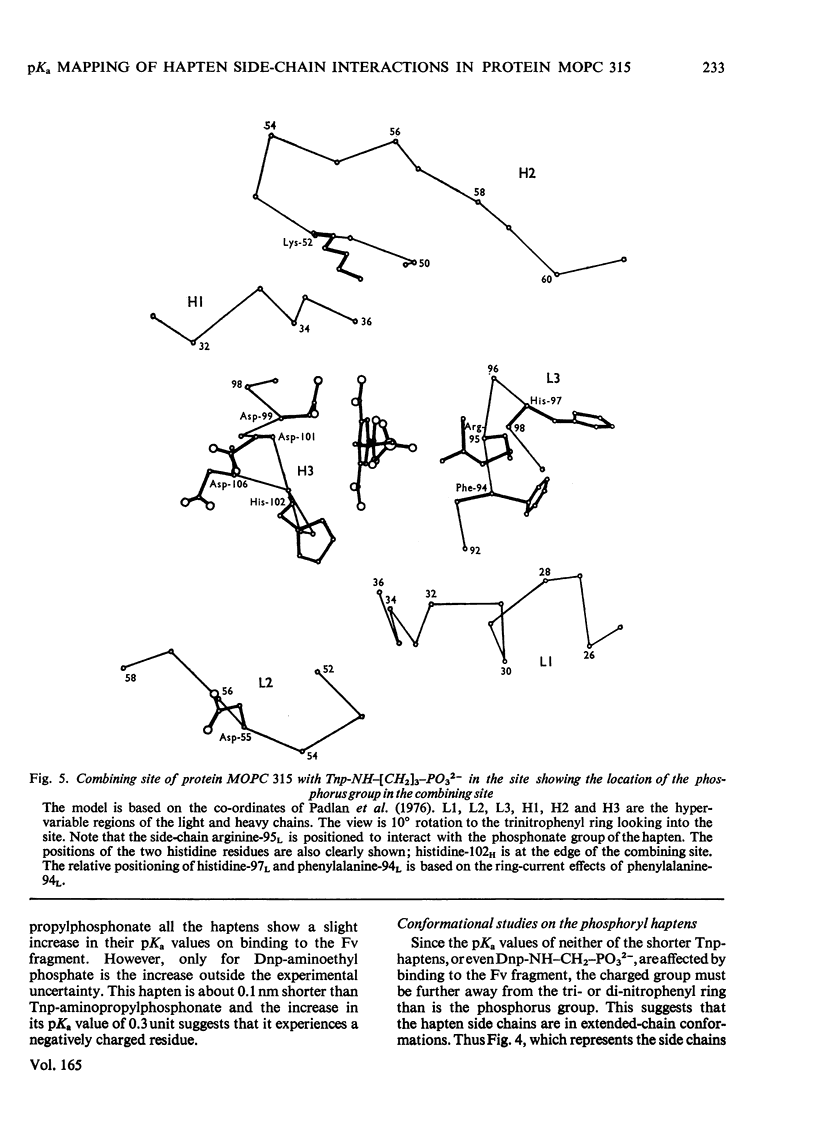
The pKa values of the three histidine residues in the Fv fragment (variable region of the heavy and light chains) of the mouse myeloma protein MOPC 315, measured by high resolution n.m.r. (nuclear magnetic resonance), are 5.9, 6.9 and 8.2. The perturbation of the pKa of one of the histidines (pKa 6.9) on the addition of hapten and the narrow linewidth of its proton resonances suggests that it is at the edge of the combining site. References to the model of the Fv fragment [Padlan, Davies, Pecht, Givol & Wright (1976) Cold Spring Harbor Symp. Quant. Biol. 41, in the press] allows assignment of the three histidine residues, histidine-102H, histidine-97L and histidine-44L. The determination of the pKa of the phosphorus group, by 31P n.m.r., of a homologous series of Dnp- and Tnp- (di- and tri-nitrophenyl) haptens has located a positively charged residue. Molecular-model studies on the conformations of these haptens show that the residue is at the edge of the site. The model suggests that the positively charged residue is either arginine-95L or lysine-52H.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Buehner M., Chandrasekhar K., Ford G. C., Hackert M. L., Liljas A., Rossmann M. G., Smiley I. E., Allison W. S., Everse J. Structure-function relationships in lactate dehydrogenase. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1968–1972. doi: 10.1073/pnas.70.7.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Dower S. K., Wain-Hobson S., Gettins P., Givol D., Jackson W. R., Perkins S. J., Sunderland C. A., Sutton B. J., Wright C. E., Dwek R. A. The combining site of the dinitrophenyl-binding immunoglobulin A myeloma protein MOPC 315. Biochem J. 1977 Aug 1;165(2):207–223. doi: 10.1042/bj1650207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Dwek R. A., Jones R., Marsh D., McLaughlin A. C., Press E. M., Price N. C., White A. I. Antibody--hapten interactions in solution. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):53–74. doi: 10.1098/rstb.1975.0070. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Leslie R. G., Hood L., Eisen H. N. Amino-acid sequence of the variable region of the heavy (alpha) chain of a mouse myeloma protein with anti-hapten activity. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1123–1127. doi: 10.1073/pnas.71.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givol D., Strausbauch P. H., Hurwitz E., Wilchek M., Haimovich J., Eisen H. N. Affinity labeling and cross-linking of the heavy and light chains of a myeloma protein with anti-2,4-dinitrophenyl activity. Biochemistry. 1971 Aug 31;10(18):3461–3466. doi: 10.1021/bi00794a023. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Sequence and position of a labeled tryptic peptide. Biochemistry. 1970 Sep 29;9(20):3862–3871. doi: 10.1021/bi00822a003. [DOI] [PubMed] [Google Scholar]
- Haimovich J., Eisen H. N., Hurwitz E., Givol D. Localization of affinity-labeled residues on the heavy and light chain of two myeloma proteins with anti-hapten activity. Biochemistry. 1972 Jun 20;11(13):2389–2398. doi: 10.1021/bi00763a001. [DOI] [PubMed] [Google Scholar]
- Haselkorn D., Friedman S., Givol D., Pecht I. Kinetic mapping of the antibody combining site by chemical relaxation spectrometry. Biochemistry. 1974 May 7;13(10):2210–2222. doi: 10.1021/bi00707a030. [DOI] [PubMed] [Google Scholar]
- Inbar D., Hochman J., Givol D. Localization of antibody-combining sites within the variable portions of heavy and light chains. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2659–2662. doi: 10.1073/pnas.69.9.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. Attempts to locate complementarity-determining residues in the variable positions of light and heavy chains. Ann N Y Acad Sci. 1971 Dec 31;190:382–393. doi: 10.1111/j.1749-6632.1971.tb13550.x. [DOI] [PubMed] [Google Scholar]
- Klostergaard J., Grossberg A. L., Krausz L. M., Pressman D. Absence of lysine from the DNP-lysine binding site of protein 315; designation of lysine 52 of the heavy chain as a peripheral residue. Immunochemistry. 1977 Jan;14(1):37–44. doi: 10.1016/0019-2791(77)90331-7. [DOI] [PubMed] [Google Scholar]
- Klostergaard J., Krausz L. M., Grossberg A. L., Pressman D. Arginine as a contact residue in the hapten-binding site of protein 315. Immunochemistry. 1977 Feb;14(2):107–110. doi: 10.1016/0019-2791(77)90288-9. [DOI] [PubMed] [Google Scholar]
- Poljak R. J. X-ray diffraction studies of immunoglobulins. Adv Immunol. 1975;21:1–33. [PubMed] [Google Scholar]
- Shrager R. I., Cohen J. S., Heller S. R., Sachs D. H., Schechter A. N. Mathematical models for interacting groups in nuclear magnetic resonance titration curves. Biochemistry. 1972 Feb 15;11(4):541–547. doi: 10.1021/bi00754a010. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Gettins P., Givol D., Marsh D., Wain-Hobson S., Willan K. J., Dwek R. A. The gross architecture of an antibody-combining site as determined by spin-label mapping. Biochem J. 1977 Aug 1;165(2):177–197. doi: 10.1042/bj1650177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


