Abstract
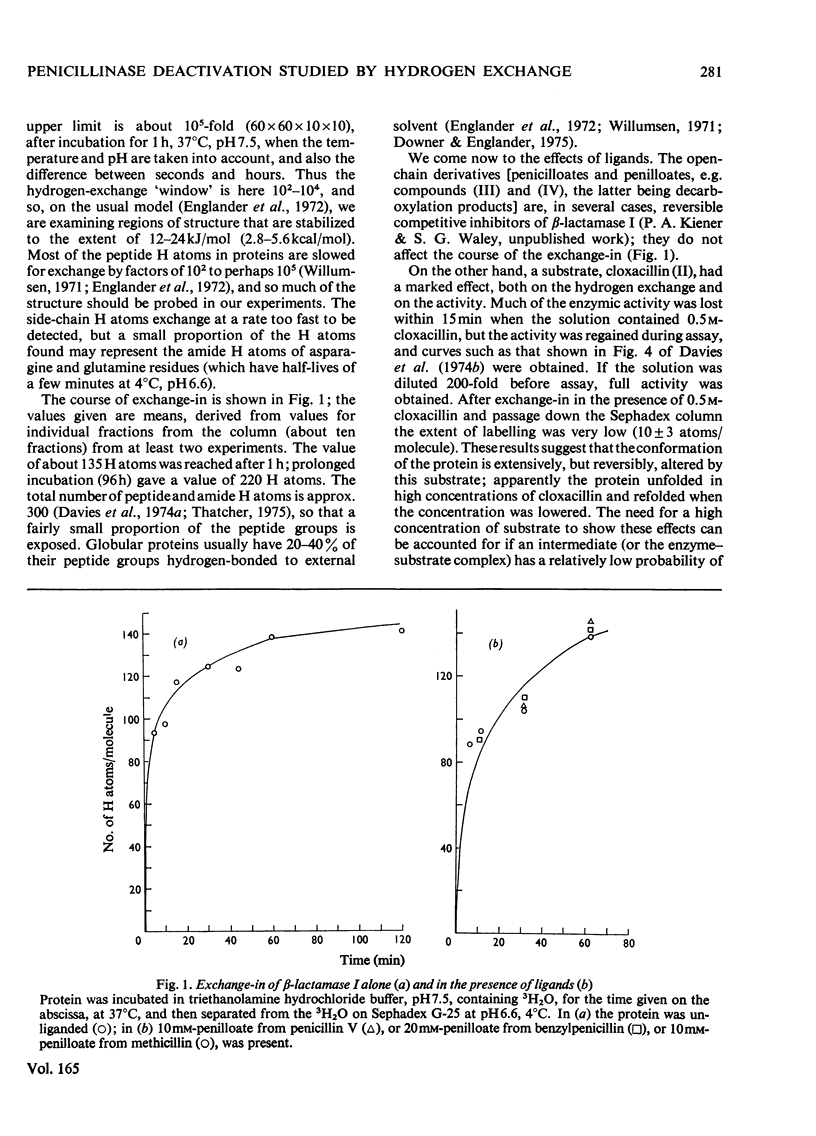
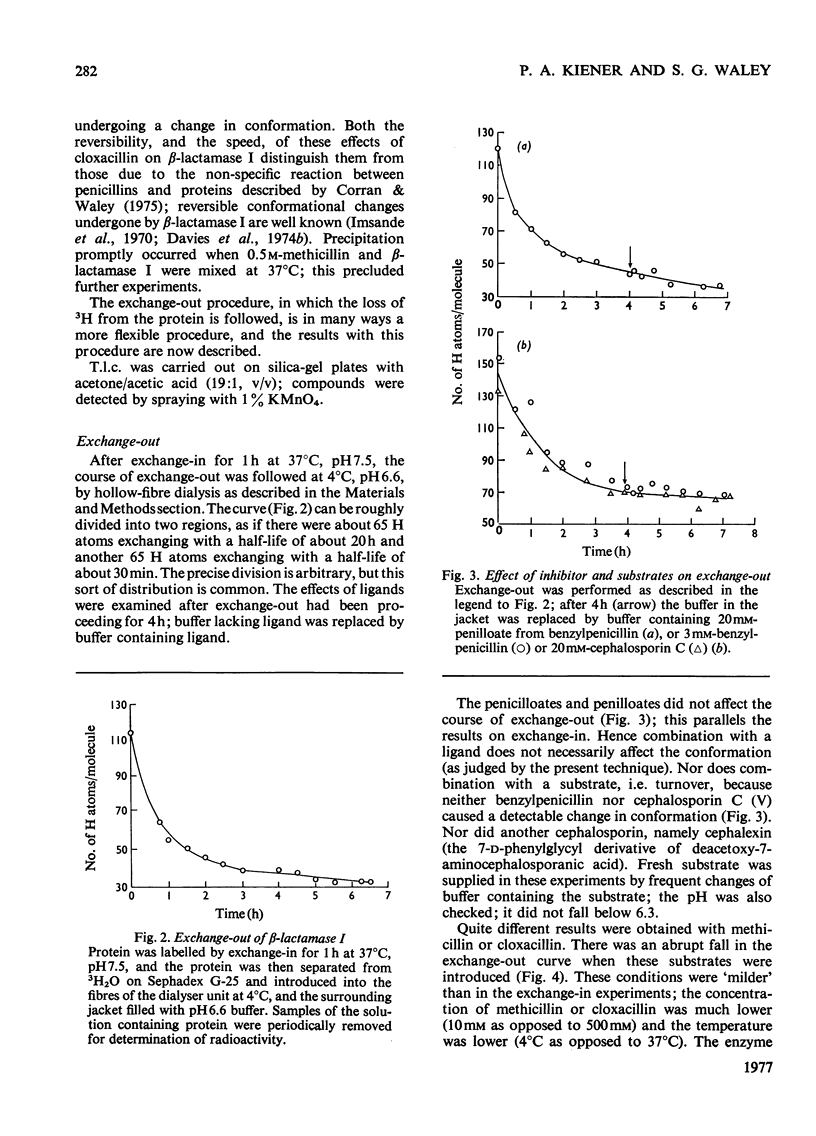
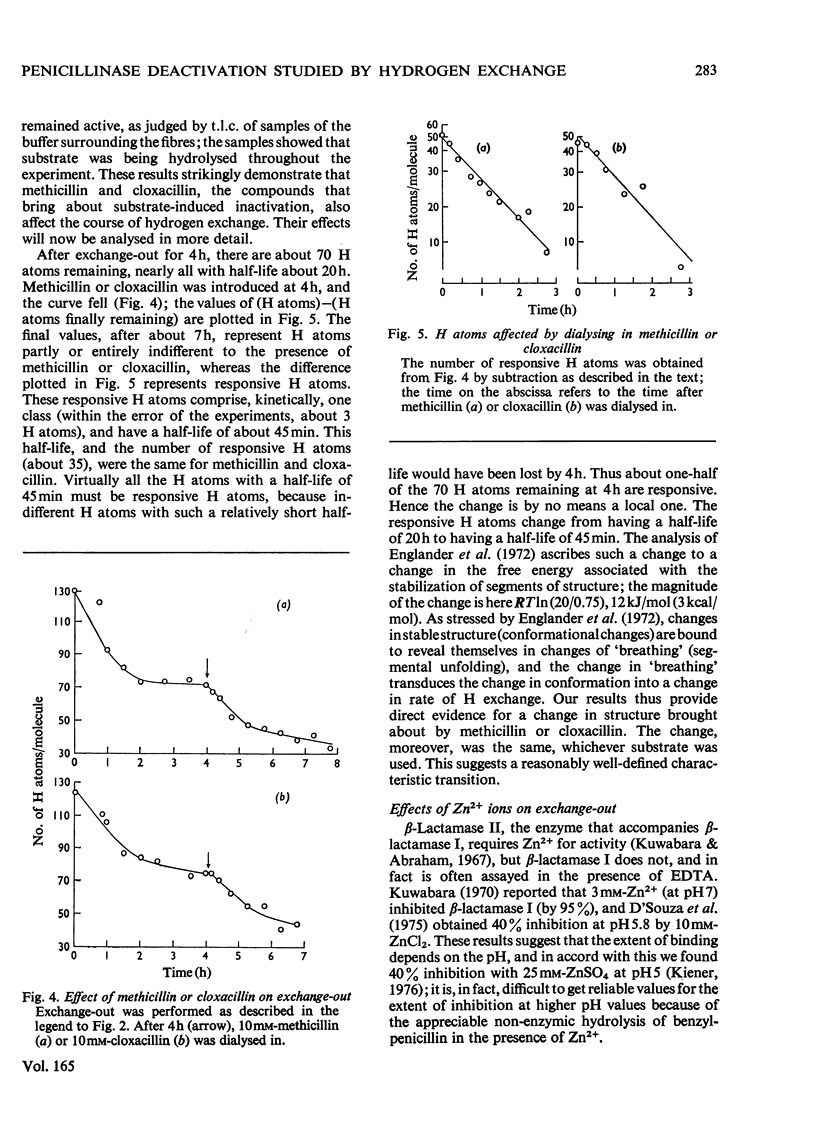
The conformational motility of beta-lactamase I from Bacillus cereus was studied by hydrogen exhange. The time course of the isotopic replacement of peptide hydrogen atoms was followed by 'exchange-in' or 'exchange-out' experiments. Many of the substrates for this enzyme that have o-substituted aromatic or heterocyclic side chains (e.g. methicillin or cloxacillin) are known to effect a decrease in enzymic activity ('substrate-induced deactivation'). There was a marked discontinuity in the exchange-out curve when methicillin or cloxacillin was diffused into the enzyme solution. About one-half of the hydrogen atoms that were probed were affected by the presence of these substrates, and the change in the reactivity of the hydrogen atoms was also large. Substrates that do not bring about deactivation (benzylpenicillin and cephalosporin C) do not affect the hydrogen exchange, nor do reversible competitive inhibitors such as the penicilloic acid or penilloic acid. On the other hand, Zn2+ ions do affect the hydrogen exchange; their effect is similar to that of methicillin or cloxacillin.
Full text
PDF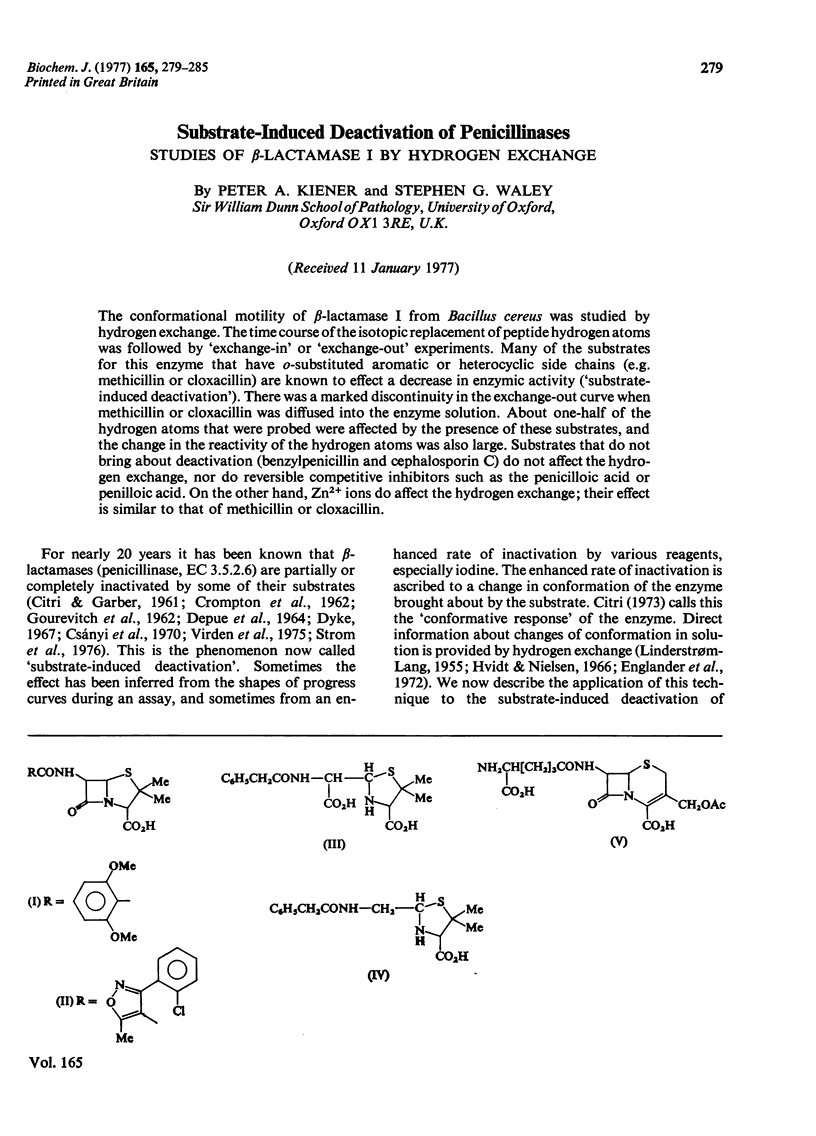


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browne C. A., Waley S. G. Studies of triose phosphate isomerase by hydrogen exchange. Biochem J. 1974 Sep;141(3):753–760. doi: 10.1042/bj1410753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C. A., Waley S. G. The use of hollow-fiber dialysis in tritium-hydrogen exchange. Anal Biochem. 1973 Nov;56(1):289–291. doi: 10.1016/0003-2697(73)90191-7. [DOI] [PubMed] [Google Scholar]
- CITRI N., GARBER N. Evidence for a change in the active site of penicillinase caused by a competitive inhibitor. Biochem Biophys Res Commun. 1961 Feb 24;4:143–146. doi: 10.1016/0006-291x(61)90364-3. [DOI] [PubMed] [Google Scholar]
- CROMPTON B., JAGO M., CRAWFORD K., NEWTON G. G., ABRAHAM E. P. Behaviour of some derivatives of 7-aminocephalosporanic acid and 6-aminopenicillanic acidas substrates, inhibitors and inducers of penicillinases. Biochem J. 1962 Apr;83:52–63. doi: 10.1042/bj0830052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N. Conformational adaptability in enzymes. Adv Enzymol Relat Areas Mol Biol. 1973;37:397–648. doi: 10.1002/9780470122822.ch7. [DOI] [PubMed] [Google Scholar]
- Citri N., Samuni A., Zyk N. Acquisition of substrate-specific parameters during the catalytic reaction of penicillinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1048–1052. doi: 10.1073/pnas.73.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Zyk N. The interaction of penicillinase with penicillins. IV. Structural aspects of catalytic and non-catalytic interactions. Biochim Biophys Acta. 1965 Jun 22;99(3):427–441. doi: 10.1016/s0926-6593(65)80197-7. [DOI] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The reaction of penicillin with proteins. Biochem J. 1975 Aug;149(2):357–364. doi: 10.1042/bj1490357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csányi V., Mile I., Koczka I., Badár E., Horváth I. A particular conformational change of Bacillus cereus penicillinase under the action of a new penicillin analogue pyrazocillin. Biochim Biophys Acta. 1970 Nov 11;220(2):317–325. doi: 10.1016/0005-2744(70)90016-1. [DOI] [PubMed] [Google Scholar]
- D'Souza L., Madaiah M., Day R. A., Nickerson K. W. A Hg (II) induced conformational change in penicillinase. Int J Pept Protein Res. 1975;7(3):251–259. doi: 10.1111/j.1399-3011.1975.tb02440.x. [DOI] [PubMed] [Google Scholar]
- DEPUE R. H., MOAT A. G., BONDI A. THE RELATION BETWEEN PENICILLIN STRUCTURE AND PENICILLINASE ACTIVITY. Arch Biochem Biophys. 1964 Sep;107:374–381. doi: 10.1016/0003-9861(64)90293-0. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Conformational changes in the extracellular beta-lactamase I from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):137–141. doi: 10.1042/bj1430137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Englander S. W. Molecular structure of membrane-bound rhodopsin. Nature. 1975 Apr 17;254(5501):625–627. doi: 10.1038/254625a0. [DOI] [PubMed] [Google Scholar]
- Dyke K. G. Substrate-specific inactivation of staphylococcal penicillinase. Biochem J. 1967 Jun;103(3):641–646. doi: 10.1042/bj1030641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Downer N. W., Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Staley R. Measurement of the free and the H-bonded amides of myoglobin. J Mol Biol. 1969 Oct 28;45(2):277–295. doi: 10.1016/0022-2836(69)90105-3. [DOI] [PubMed] [Google Scholar]
- GOUREVITCH A., PURSIANO T. A., LEIN J. Inactivation of staphylococcal penicillinase by reaction with resistant penicillins. Nature. 1962 Aug 4;195:496–497. doi: 10.1038/195496a0. [DOI] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Imsande J., Gillin F. D., Tanis R. J., Atherly A. G. Properties of penicillinase from Bacillus cereus 569. J Biol Chem. 1970 May 10;245(9):2205–2212. [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of two extracellular beta-lactamases from Bacillus cereus 569-H. Biochem J. 1970 Jul;118(3):457–465. doi: 10.1042/bj1180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik M. B., Imsande J. Interconversion of alpha- and gamma-penicillinase from Bacillus cereus 569. J Biol Chem. 1970 Jul 25;245(14):3556–3560. [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Strom R., Ravagnan G., Salfi V. Inactivation of Bacillus cereus beta-lactamase by halogenated isoxazolylpenicillins. Eur J Biochem. 1976 Feb 2;62(1):95–101. doi: 10.1111/j.1432-1033.1976.tb10101.x. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. The partial amino acid sequence of the extracellular beta-lactamase I of Bacillus cereus 569/H. Biochem J. 1975 May;147(2):313–326. doi: 10.1042/bj1470313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virden R., Bristow A. F., Pain R. H. The active site of penicillinase from Staphylococcus aureus PC1. Isolation of a specific covalent complex with the substrate quinacillin. Biochem J. 1975 Aug;149(2):397–401. doi: 10.1042/bj1490397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen L. Hydrogen isotope exchange in the study of protein conformation. A quantitative test of an exchange mechanism. C R Trav Lab Carlsberg. 1971;38(14):223–295. [PubMed] [Google Scholar]


