Abstract
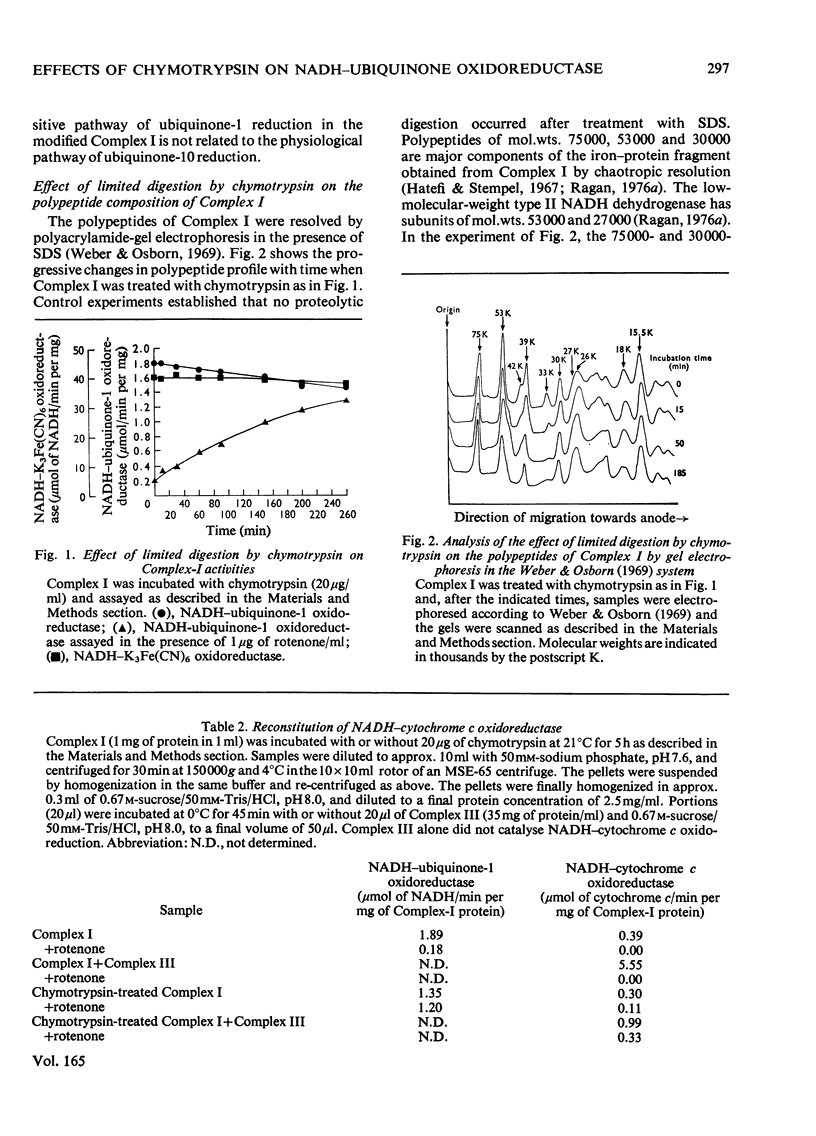
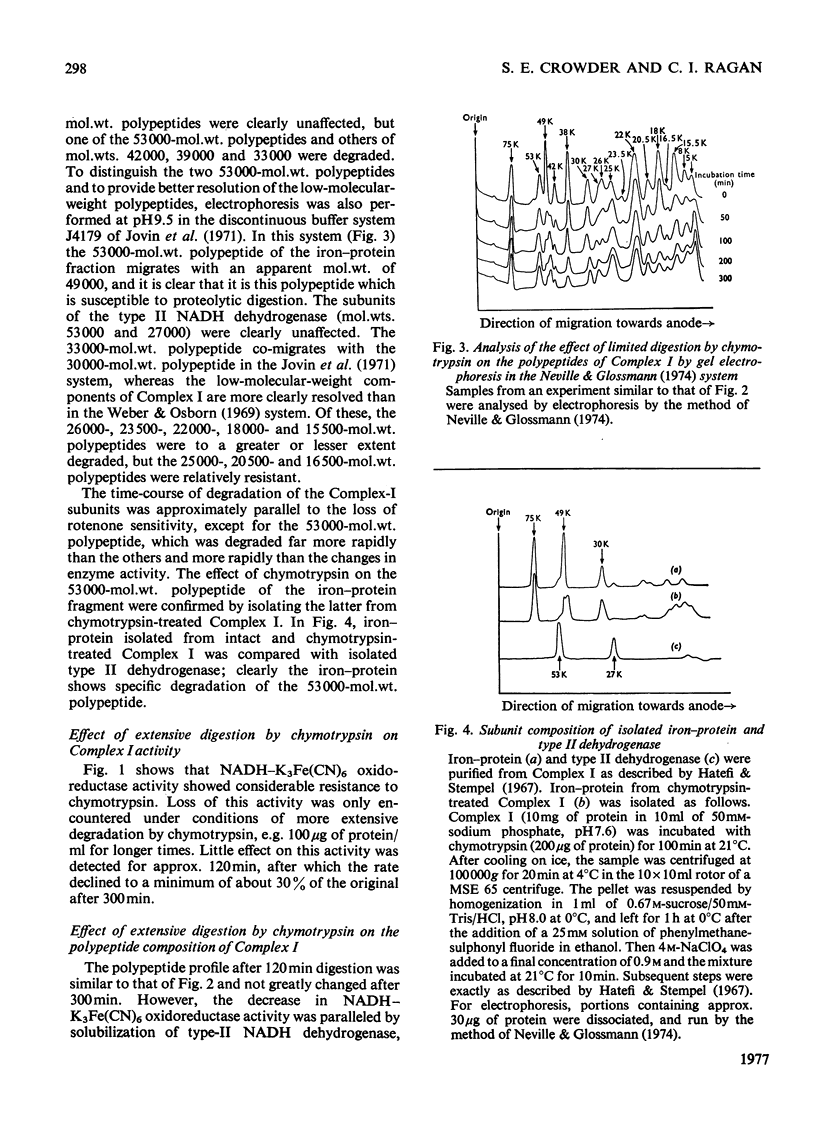
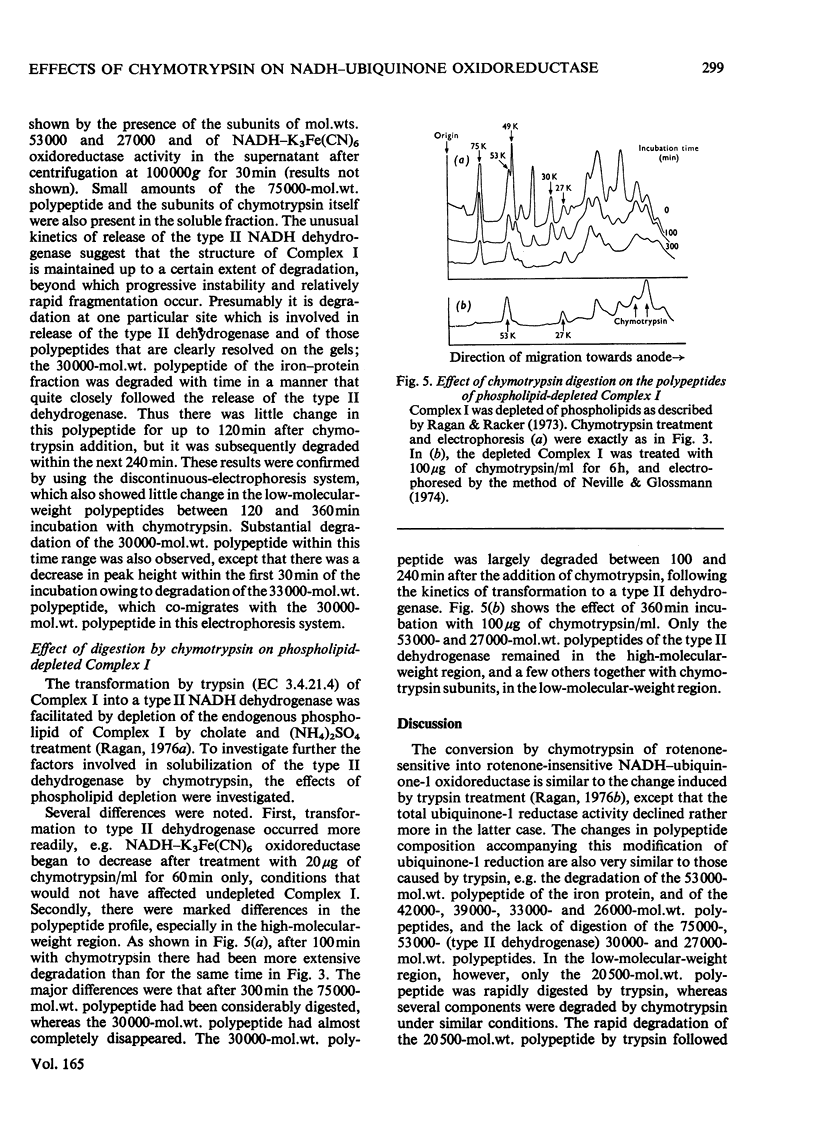
1. Incubation of NADH–ubiquinone oxidoreductase (Complex I) with chymotrypsin caused loss of rotenone-sensitive ubiquinone-1 reduction and an increase in rotenone-insensitive ubiquinone reduction. 2. Within the same time-course, NADH–K3Fe(CN)6 oxidoreductase activity was unaffected. 3. Mixing of chymotrypsin-treated Complex I with Complex III did not give rise to NADH–cytochrome c oxidoreductase activity. 4. Gel electrophoresis in the presence of sodium dodecyl sulphate revealed selective degradation of several constituent polypeptides by chymotrypsin. 5. With higher chymotrypsin concentrations and longer incubation times, a decrease in NADH–K3Fe(CN)6 oxidoreductase was observed. The kinetics of this decrease correlated with solubilization of the low-molecular-weight type-II NADH dehydrogenase (subunit mol.wts. 53000 and 27000) and with degradation of a polypeptide of mol.wt. 30000. 6. Phospholipid-depleted Complex I was more rapidly degraded by chymotrypsin. Specifically, a subunit of mol.wt. 75000, resistant to chymotrypsin in untreated Complex I, was degraded in phospholipid-depleted Complex I. In addition, the 30000-mol.wt. polypeptide was also more rapidly digested, correlating with an increased rate of transformation to type II NADH dehydrogenase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CREMONA T., KEARNEY E. B., VILLAVICENCIO M., SINGER T. P. STUDIES ON THE RESPIRATORY CHAIN-LINKED DPNH DEHYDROGENASE. V. TRANSFORMATION OF DPNH DEHYDROGENASE TO DPNH-CYTOCHROME REDUCTASE AND DIAPHORASE UNDER THE INFLUENCE OF HEAT, PROTEOLYTIC ENZYMES, AND UREA. Biochem Z. 1963;338:407–442. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Resolution of complex I (DPNH-coenzyme Q reductase) of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1967 Feb 8;26(3):301–308. doi: 10.1016/0006-291x(67)90122-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- RINGLER R. L., MINAKAMI S., SINGER T. P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. II. Isolation and molecular properties of the enzyme from beef heart. J Biol Chem. 1963 Feb;238:801–810. [PubMed] [Google Scholar]
- Ragan C. I. NADH-ubiquinone oxidoreductase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):249–290. doi: 10.1016/0304-4173(76)90001-x. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. IV. The reconstitution of rotenone-sensitive reduced nicotinamide adenine dinucleotide-ubiquinone reductase from reduced nicotinamide adenine dinucleotide dehydrogenase and phospholipids. J Biol Chem. 1973 Oct 10;248(19):6876–6884. [PubMed] [Google Scholar]
- Ragan C. I. The effects of proteolytic digestion by trypsin on the structure and catalytic properties of reduced nicotinamide-adenine dinucleotide dehydrogenase from bovine heart mitochondria. Biochem J. 1976 May 15;156(2):367–374. doi: 10.1042/bj1560367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I. The structure and subunit composition of the particulate NADH-ubiquinone reductase of bovine heart mitochondria. Biochem J. 1976 Feb 15;154(2):295–305. doi: 10.1042/bj1540295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


