Abstract
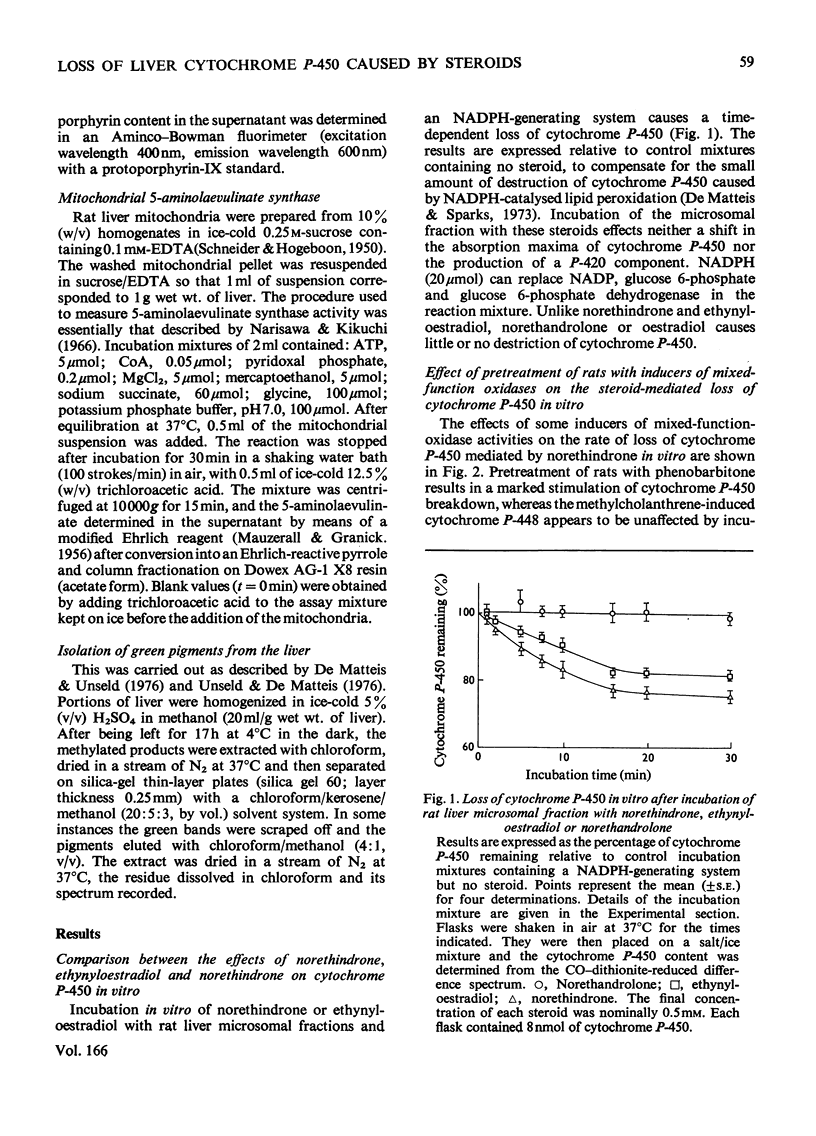
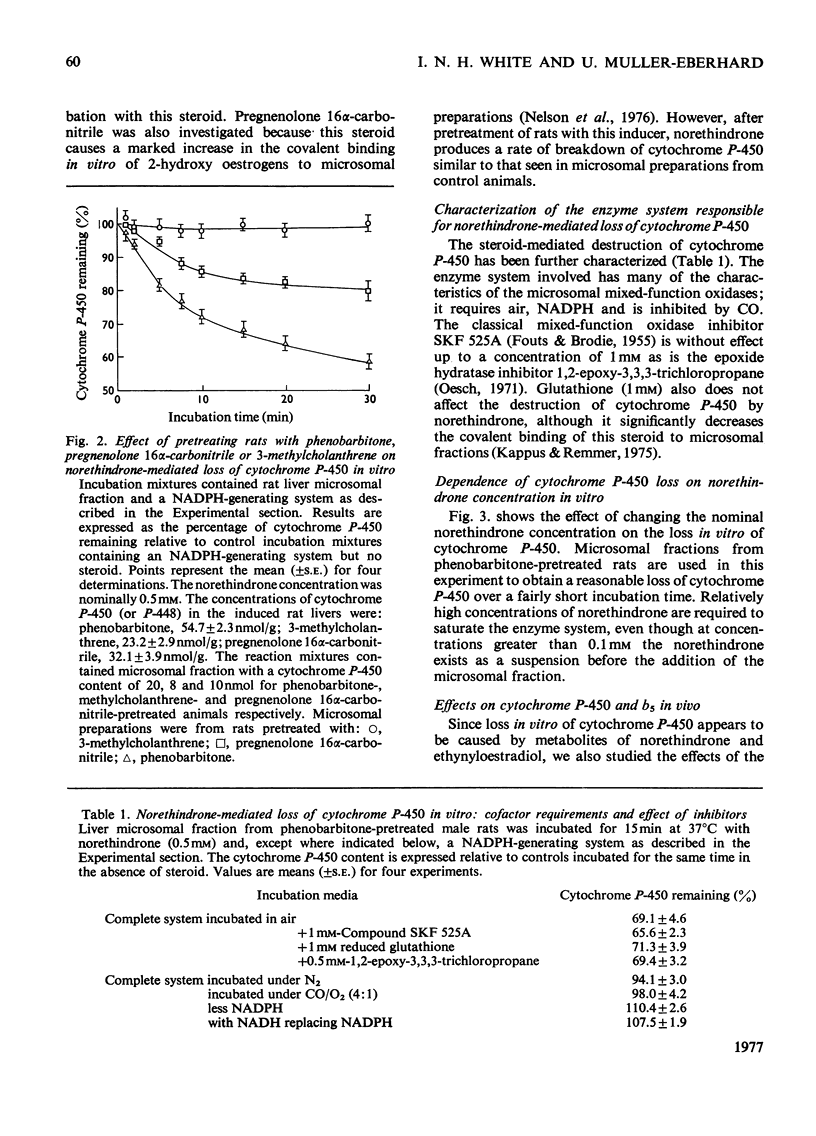
1. 19-Nor-17alpha-pregna-1,3,5(10)-trien-20-yne-3,17-diol (ethynyloestradiol) or 17beta-hydroxy-19-nor-17alpha-pregn-4-en-20-yn-3-one (norethindrone) but not 17alpha-ethyl-17beta-hydroxy-19-norandrost-4-en-3-one (norethandrolone) caused a time-dependent loss of cytochrome P-450 when incubated in vitro with rat liver microsomal fractions and NADPH-generating systems. 2. The enzyme system catalysing the norethindrone-mediated loss of cytochrome P-450 had many characteristics of the microsomal mixed-function oxidases. It required NADPH and air, and was inhibited by Co. However, it was unaffected by 1 mM-compound SKF 525A. 3. In microsomal fractions from phenobarbitone-pretreated rats the norethindrone-mediated loss of cytochrome P-450 was increased relative to controls. The norethindrone-mediated cytochrome P-450 loss was less pronounced when the animals were pretreated with 3beta-hydroxy-pregn-5-en-2-one 16alpha-carbonitrile (pregnenolone 16alpha-carbonitrile). Pretreatment with 3-methylcholanthrene rendered the animals resistant to the norethindrone effect. 4. Administration in vivo [100mg/kg, intraperitoneally] of norethindrone or ethinyl oestradiol also produced a time-dependent loss of liver cytochrome P-450. Norethandrolone had a similar, though much less-marked, effect. All three steroids lead to an induction of 5-aminolaevulinate synthase and an accumulation of porphyrins in the liver. 5. The loss of cytochrome P-450 and the accumulation of porphyrins in the liver 2 h after the administration of norethindrone to female rats was similar to that seen in males. 6. Rats pretreated with phenobarbitone and given norethindrone or ethynyloestradiol (100mg/kg, intraperitoneally) formed green pigments in their livers. These had characteristics similar to the green pigments produced in the livers of rats after the administration of 2-allyl-2-isopropylacetamide. No green pigments could be extracted from the livers of control rats or those given norethandrolone, oestradiol or progesterone.
Full text
PDF

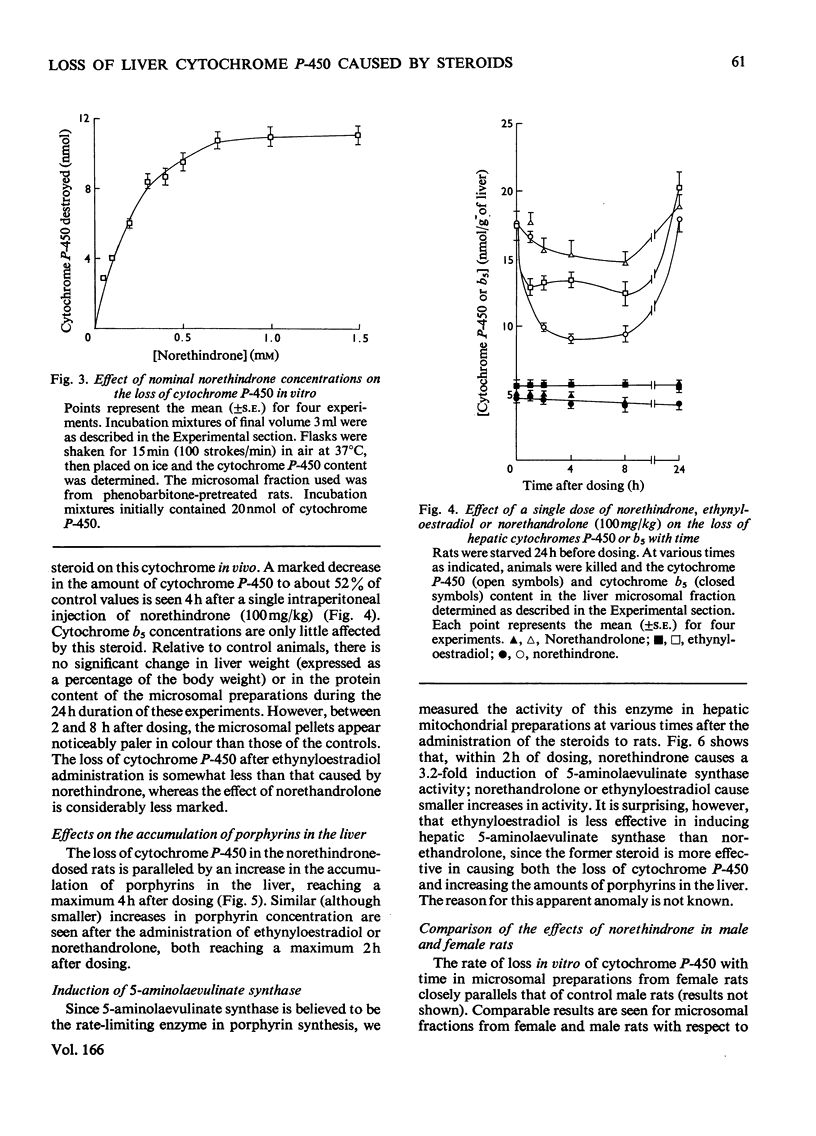
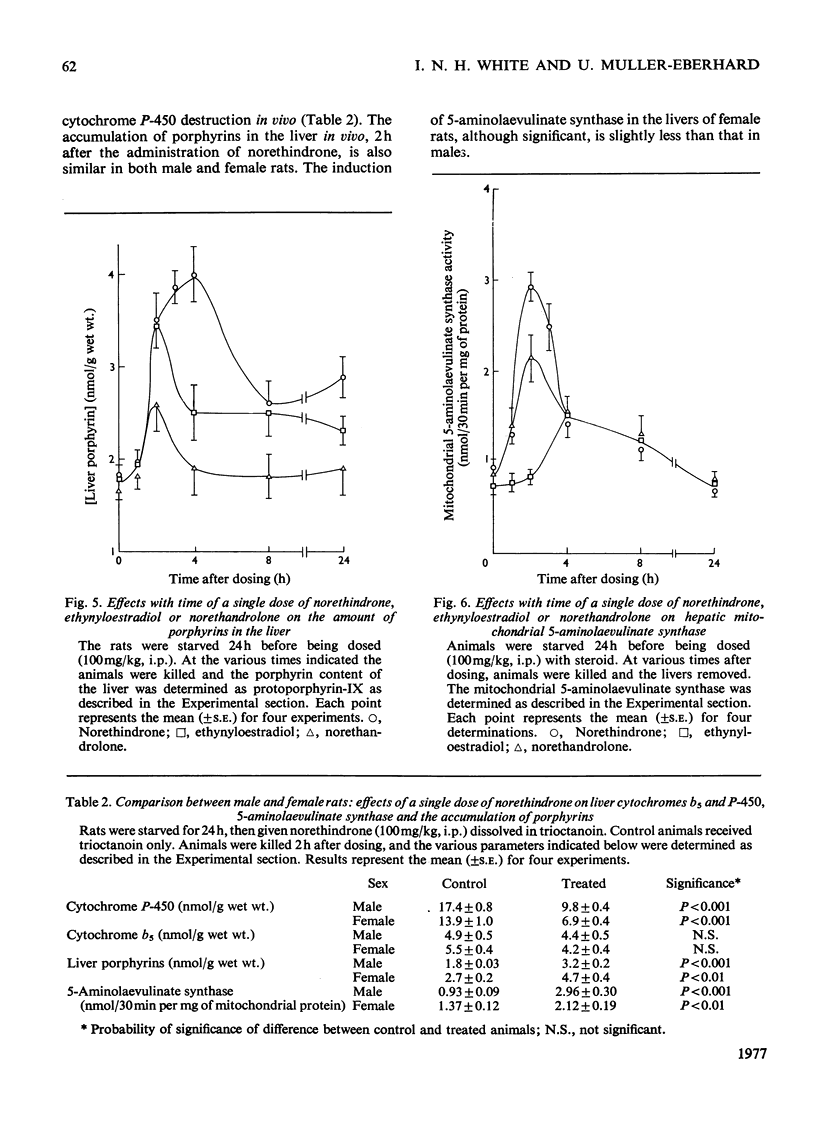


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbritti G., De Matteis F. Decreased levels of cytochrome P-450 and catalase in hepatic porphyria caused by substituted acetamides and barbiturates. Importance of the allyl group in the molecule of the active drugs. Chem Biol Interact. 1972 Mar;4(4):281–286. doi: 10.1016/0009-2797(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Behm A. R., Unger W. P. Oral contraceptives and porphyria cutanea tarda. Can Med Assoc J. 1974 May 4;110(9):1052–1054. [PMC free article] [PubMed] [Google Scholar]
- Bolt H. M., Kappus H. Irreversible binding of ethynyl-estradiol metabolites to protein and nucleic acids as catalyzed by rat liver microsomes and mushroom tyrosinase. J Steroid Biochem. 1974 Apr;5(2):179–184. doi: 10.1016/0022-4731(74)90126-5. [DOI] [PubMed] [Google Scholar]
- Brewster D., Jones R. S., Symons A. M. Biliary excretion of mestranol in the female rat: effect of neomycin pretreatment on enterohepatic recirculation. Biochem Soc Trans. 1976;4(3):516–518. doi: 10.1042/bst0040516. [DOI] [PubMed] [Google Scholar]
- Cole R., Cole J. Correlations between disturbed haem synthesis and fetal malformation. Lancet. 1976 Sep 18;2(7986):640–640. doi: 10.1016/s0140-6736(76)90717-0. [DOI] [PubMed] [Google Scholar]
- Congote L. F., Stern M. D., Solomon S. Hormone control of heme synthesis in cultures of human fetal liver cells. Biochemistry. 1974 Oct 8;13(21):4255–4263. doi: 10.1021/bi00718a002. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Cook C. E., Dickey M. C., Christensen H. D. Oxygenated norethindrone derivatives from incubation with beagle liver. Structure, synthesis, and biological activity. Drug Metab Dispos. 1974 Jan-Feb;2(1):58–64. [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Sparks R. G. Iron-dependent loss of liver cytochrome P-450 haem in vivo and in vitro. FEBS Lett. 1973 Jan 15;29(2):141–144. doi: 10.1016/0014-5793(73)80545-9. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Unseld A. Increased liver haem degradation caused by foreign chemicals: a comparison of the effects of 2-allyl-2-isopropylacetamide and cobaltous chloride. Biochem Soc Trans. 1976;4(2):205–209. doi: 10.1042/bst0040205. [DOI] [PubMed] [Google Scholar]
- FOUTS J. R., BRODIE B. B. Inhibition of drug metabolic pathways by the potentiating agent, 2, 4-dichloro-6-phenyl-phenoxyethyl diethylamine. J Pharmacol Exp Ther. 1955 Sep;115(1):68–73. [PubMed] [Google Scholar]
- Gerhards E., Hecker W., Hitze H., Nieuweboer B., Bellmann O. Zum Stoffwechsel von Norethisteron (17 -äthinyl-4-östren-17 -ol-3-on) und DL- sowie D-Norgestrel (18-methyl-17 -äthinyl-4-östren-17 -ol-3-on) beim Menschen. (Alkyl-substituierte Steroide. 8. Acta Endocrinol (Copenh) 1971 Oct;68(2):219–248. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Haberman H. F., Rosenberg F., Menon I. A. Porphyria cutanea tarda: comparison of cases precipitated by alcohol and estrogens. Can Med Assoc J. 1975 Oct 4;113(7):653–655. [PMC free article] [PubMed] [Google Scholar]
- Hanasono G. K., Fischer L. J. The excretion of tritium-labeled chlormadinone acetate, mestranol, norethindrone, and norethynodrel in rats and the enterohepatic circulation of metabolites. Drug Metab Dispos. 1974 Mar-Apr;2(2):159–168. [PubMed] [Google Scholar]
- Kamath S. A., Rubin E. Interaction of calcium with microsomes: a modified method for the rapid isolation of rat liver microsomes. Biochem Biophys Res Commun. 1972 Oct 6;49(1):52–59. doi: 10.1016/0006-291x(72)90008-3. [DOI] [PubMed] [Google Scholar]
- Kappus H., Remmer H. Metabolic activation of norethisterone (norethindrone) to an irreversibly protein-bound derivative by rat liver microsomes. Drug Metab Dispos. 1975 Sep-Oct;3(5):338–344. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Jacobson M., Sernatinger E., Kuntzman R. Breakdown of cytochrome P-450 heme by secobarbital and other allyl-containing barbiturates. Drug Metab Dispos. 1973 Jan-Feb;1(1):275–285. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Mackinnon M., Simon F. Pharmacological reversal of cholestasis-associated decrease in hepatic cytochrome P-450. Biochem Pharmacol. 1975 Mar 15;24(6):748–749. doi: 10.1016/0006-2952(75)90256-7. [DOI] [PubMed] [Google Scholar]
- Marks F., Hecker E. Metabolism and mechanism of action of oestrogens. XII. Structure and mechanism of formation of water-soluble and protein-bound metabolites of oestrone in rat-liver microsomes in vitro and in vivo. Biochim Biophys Acta. 1969;187(2):250–265. doi: 10.1016/0005-2760(69)90035-6. [DOI] [PubMed] [Google Scholar]
- Narisawa K., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in rat-liver mitochondria. Biochim Biophys Acta. 1966 Sep;123(3):596–605. doi: 10.1016/0005-2787(66)90226-7. [DOI] [PubMed] [Google Scholar]
- Nelson S. D., Mitchell J. R., Dybing E., Sasame H. A. Cytochrome P-450-mediated oxidation of 2-hydroxyestrogens to reactive intermediates. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1157–1165. doi: 10.1016/0006-291x(76)91024-x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Oesch F., Kaubisch N., Jerina D. M., Daly J. W. Hepatic epoxide hydrase. Structure-activity relationships for substrates and inhibitors. Biochemistry. 1971 Dec 21;10(26):4858–4866. doi: 10.1021/bi00802a005. [DOI] [PubMed] [Google Scholar]
- Palmer K. H., Feierabend J. F., Baggett B., Wall M. E. Metabolic removal of a 17-alpha-ethynyl group from the antifertility steroid, norethindrone. J Pharmacol Exp Ther. 1969 Jun;167(2):217–222. [PubMed] [Google Scholar]
- SHUSTER L. METABOLISM OF DRUGS AND TOXIC SUBSTANCES. Annu Rev Biochem. 1964;33:571–596. doi: 10.1146/annurev.bi.33.070164.003035. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B., Wilson B. J., Cinti D. L. Dimethylaminoethyl 2,2-diphenylvalerate HCl (SKF 525-A)--in vivo and in vitro effects of metabolism by rat liver microsomes--formation of an oxygenated complex. Biochem Pharmacol. 1972 Sep 1;21(17):2373–2383. doi: 10.1016/0006-2952(72)90389-9. [DOI] [PubMed] [Google Scholar]
- Soyka L. F., Deckert F. W. Further studies on the inhibition of drug metabolism by pregnanolone and related steroids. Biochem Pharmacol. 1974 May 1;23(11):1629–1639. doi: 10.1016/0006-2952(74)90375-x. [DOI] [PubMed] [Google Scholar]


