Abstract
Plants acclimate to temperature by adjusting their photosynthetic capacity over weeks to months. However, most evidence for photosynthetic acclimation derives from leaf-scale experiments. Here we address the scarcity of evidence for canopy-scale photosynthetic acclimation by examining the correlation between maximum photosynthetic rates (Amax,2,000) and growth temperature () across a range of concurrent temperatures and canopy foliage quantity, using data from >200 eddy covariance sites. We detect widespread thermal acclimation of canopy-scale photosynthesis, demonstrated by enhanced Amax,2,000 under higher , across flux sites with adequate water availability. A 14-day period is identified as the most relevant timescale for acclimation across all sites, with a range of 12–25 days for different plant functional types. The mean apparent thermal acclimation rate across all ecosystems is 0.41 (−0.38–1.04 for 5th–95th percentile range) µmol m−2 s−1 °C−1, with croplands showing the largest acclimation rates and grasslands the lowest. Incorporating an optimality-based prediction of leaf photosynthetic capacities into a biochemical photosynthesis model is shown to improve the representation of thermal acclimation. Our results underscore the critical need for enhanced understanding and modelling of canopy-scale photosynthetic capacity to accurately predict plant responses to warmer growing seasons.
Subject terms: Ecosystem ecology, Plant ecology, Ecophysiology, C3 photosynthesis
Analysis of the FLUXNET2015 dataset provides observational evidence for widespread thermal acclimation of canopy-scale photosynthesis and its timescales across diverse biomes, improving its representation in land surface models.
Main
The carbon uptake capacity of terrestrial ecosystem photosynthesis shows large spatiotemporal variation1. Air temperature (Tair) is one of the key factors determining this variation2. Given recent warming of 0.1–0.3 °C per decade3, a better understanding of ecosystem responses to Tair is needed. While the instantaneous temperature dependence of photosynthesis has been a major focus of research4–6 and is represented in vegetation and land surface models7–9, the slower process known as thermal acclimation, through which plants maintain or enhance their photosynthetic efficiency in response to warmer growth temperatures10–14, is less well understood15,16. Several studies have indicated that leaves acclimate to thermal growing conditions within weeks to months, although the relevant timescales for different plant types remain uncertain17–20. The potential mechanisms of this (non-genetic) acclimation include changes in key biochemical parameters (electron-transport potential and carboxylation capacity)12,14,21, the sensitivity of stomatal conductance to atmospheric vapour pressure deficit (VPD)22–24 and enzymatic heat tolerance10,14.
Widespread evidence of thermal acclimation at the leaf and canopy scales indicates that the optimal temperature (Topt) of photosynthesis adjusts in accordance with the prevailing Tair averaged over the time frame most relevant for acclimation ()12,14,21,25,26. Yet the extent to which the maximum carbon assimilation rate under high light (Amax) acclimates to under natural conditions is less clear, particularly since most experiments are conducted on seedlings under highly controlled growth conditions13,27. Given that Topt is well-documented to increase with rising , it is crucial to understand whether Amax can also acclimate to , since only their simultaneous enhancement can lead to consistent increases in photosynthesis28,29. While some process-based photosynthetic models have incorporated Topt acclimation, Amax acclimation has not been adequately represented in models30,31. Demonstrating the presence of thermal acclimation at the canopy scale, quantifying its relevant timescales and rates across ecosystems and assessing the accuracy of photosynthetic models in representing these acclimation processes are essential for understanding how thermal acclimation can mitigate the potentially detrimental effects of warming on the future terrestrial carbon sink16.
In this study, we define evidence for thermal acclimation of canopy photosynthesis as a positive adjustment in canopy-scale Amax in response to elevated . Following the definition used in leaf-scale studies32, canopy-scale Amax is defined as the photosynthetic assimilation rate of the canopy measured under high light, ample water and ambient CO2. We derive Amax from light response curves of half-hourly or hourly eddy covariance carbon fluxes obtained from >200 FLUXNET2015 flux sites (Methods). While canopy-scale Topt has been shown to acclimate to elevated in several previous studies25,26,33, our focus here is solely on thermal acclimation of canopy-scale Amax. To facilitate consistent analysis across different light conditions, we standardize Amax to photosynthetic photon flux density (PPFD) equivalent to 2,000 μmol m−2 s−1 (denoted as Amax,2,000; Methods). Given the limited number of Amax,2,000 samples for individual flux sites, we infer the thermal acclimation of Amax,2,000 across spatial gradients by leveraging the large range of climates sampled by the FLUXNET2015 sites. We examine the correlation between Amax,2,000 and when averaged over different time windows to identify the most relevant timescale (τ) for thermal acclimation, as indicated by peak correlation. Finally, we evaluate a biochemical model of canopy-scale C3 photosynthesis4,31, incorporating recent advances in parameterizing temperature dependence acclimation12 and modelled optimality-based leaf photosynthetic capacity34, to assess its ability to reproduce the observed thermal acclimation rates.
Results and discussion
Evidence for thermal acclimation of canopy photosynthesis
By binning Tair and the fraction of absorbed photosynthetically active radiation (fAPAR) to control for the confounding effects of concurrent temperature and seasonal changes in canopy foliage quantity and the development of the photosynthetic system on Amax,2,000, our analysis reveals a pervasive positive correlation between Amax,2,000 and (see Methods for the derivations of τ for each plant functional type (PFT)) under conditions of adequate water availability as indicated by a high ratio of actual to potential evapotranspiration (ET/PET) (Fig. 1). This correlation is observed both spatially across multiple sites (Fig. 1a) and temporally within individual sites (Fig. 1c). We use linear mixed-effect models (LMMs) to obtain the regression coefficients of when estimating Amax,2,000 (Amax,2,000 ∼ + (1∣site)), which we define as the apparent thermal acclimation rate (γT, μmol CO2 m−2 s−1 °C−1). The concept of apparent rates is used here as the Amax,2,000 response rate to may be influenced by other covarying environmental conditions19, including the growth PPFD () and VPD35 (Supplementary Fig. 1). To account for the potential impact of adaptation12—the modification of Amax,2,000– relationships across different species and populations within a species growing at different sites—sites are treated as random intercepts within the LLMs (see Extended Data Fig. 1a for an example). Cropland sites are included in the PFT-based analyses but excluded from cross-site analyses.
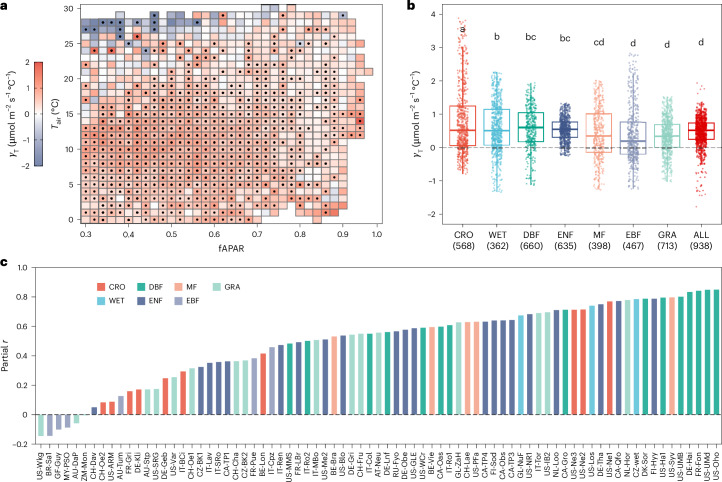
Fig. 1. Relationships between Amax,2,000 and .
a, γT values over fAPAR and Tair bins across flux sites. Black dots indicate significant (two-sided, P < 0.05) correlations between Amax,2,000 and in the LMM (Amax,2,000 ≈ + (1∣site)). b, PFT-specific γT values. PFTs are arranged in descending order on the basis of their mean γT values. In the box plots, the central lines represent the median γT values, the upper and lower box limits represent the 75th and 25th percentiles, and the upper and lower whiskers extend to 1.5 times the interquartile range, respectively. Letters represent statistically significant differences in the average γT values as determined by Tukey’s honestly significant difference test (two-sided, P < 0.05), which adjusts for multiple comparisons. The numbers in parentheses represent the sample size for each PFT. c, Partial correlation coefficients (partial r) between Amax,2,000 and , when controlling for , Tair and fAPAR, across individual longer-term (>5 yr) flux sites. Colours in b and c indicate different PFTs, including CRO, DBF, EBF, ENF, GRA, MF, WET and all natural biomes combined (ALL).
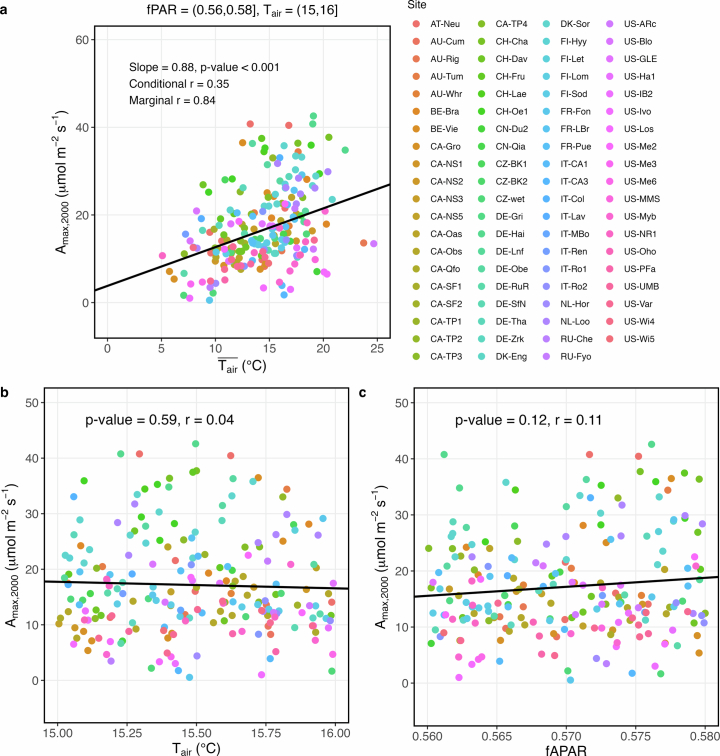
Extended Data Fig. 1. An example of the response of Amax,2000 to , Tair, and fAPAR under a fAPAR-Tair bin pair.
Cross-site Amax,2000, , Tair, and fAPAR samples are collected when 0.56 < fAPAR ≤ 0.58 and 15 °C < Tair ≤ 16 °C. a–c, Relationships between Amax,2000 and (a), Amax,2000 and Tair (b), and Amax,2000 and fAPAR (c). The black lines represent the best fits between Amax,2000 and as a linear mixed-effect function (Amax,2000 ∼ + (1∣Site), two-sided test, P < 0.001) (a), Amax,2000 and Tair as a linear function (Amax,2000 ∼ Tair, two-sided test, P > 0.05) (b), and Amax,2000 and fAPAR as a linear function (Amax,2000 ∼ fAPAR, two-sided test, P > 0.05) (c).
Detectability of thermal acclimation in canopy photosynthesis is quantified as the percentage of Tair–fAPAR bins showing a positive γT. Our cross-site analysis for natural ecosystems finds positive γT values in 87% of the Tair–fAPAR bins (938 in total) (Fig. 1a), with 65% of these positive relationships being statistically significant (P < 0.05), indicating that thermal acclimation is widespread across biomes. Averaged over all Tair–fAPAR bins, γT is 0.41 ± 0.62 (mean ± s.d.) μmol CO2 m−2 s−1 °C−1, with a 5th to 95th percentile range of −0.38–1.04 μmol CO2 m−2 s−1 °C−1. The average of positive γT values is 0.57 ± 0.30 m−2 s−1 °C−1. The PFT-based analysis also shows strong evidence of thermal acclimation, with mean γT values decreasing as follows: croplands (CRO, 0.81) > deciduous broadleaf forests (DBF, 0.58) > wetlands (WET, 0.57) > evergreen needle-leaf forests (ENF, 0.54) > mixed forests (MF, 0.42) > evergreen broadleaf forests (EBF, 0.39) > grasslands (GRA, 0.34) (Fig. 1b and Extended Data Fig. 3). Furthermore, 92% of FLUXNET2015 sites with observations spanning 6 years or more show positive partial correlations between Amax,2,000 and after controlling for potential confounding factors of , Tair and fAPAR (Fig. 1c), indicating widespread acclimation to seasonal temperature variations at individual flux sites. Sites showing a negative correlation are mainly located in the tropics (Extended Data Fig. 4a).
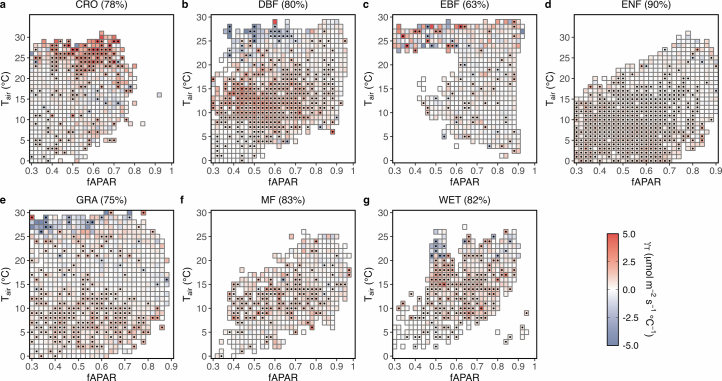
Extended Data Fig. 3. The PFT-specific thermal acclimation rates ().
a–g, PFT-specific for croplands (CRO) (a), deciduous broadleaf forests (DBF) (b), evergreen broadleaf forests (EBF) (c), evergreen needle-leaf forests (ENF) (d), grasslands (GRA) (e), mixed forests (MF) (f), wetlands (WET) (g). Numbers (%) in parentheses represent the detectability of positive values, which is defined as the percentage of the number of bins displaying a positive over the total number of bins. Black dots indicate significant (P < 0.05) correlations between Amax,2000 and .
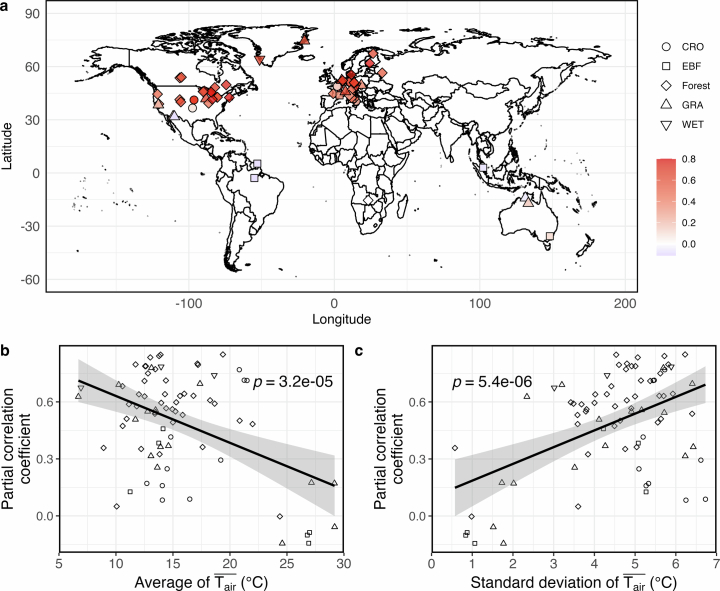
Extended Data Fig. 4. Analyses of the partial correlation coefficients between Amax,2000 and derived from long-term flux sites and their relationships with the site-level average and variability of .
a, Geographic distribution of partial correlation coefficients between Amax,2000 and controlling for , fAPAR and Tair across sites with observations spanning over five years. b, Relationship between partial correlation coefficients and the site-level averages of . c, Relationship between partial correlation coefficients and the site-level standard deviation of . The black lines in b and c represent the predicted mean values from linear regression models, and the grey shaded areas indicate their 95% confidence intervals. P-values are determined through two-sided Pearson’s correlation significance tests. The “Forest” biome category includes evergreen needle-leaf forests, deciduous broadleaf forests, and mixed forests. Other PFTs are croplands (CRO), evergreen broadleaf forests (EBF), grasslands (GRA), and wetlands (WET).
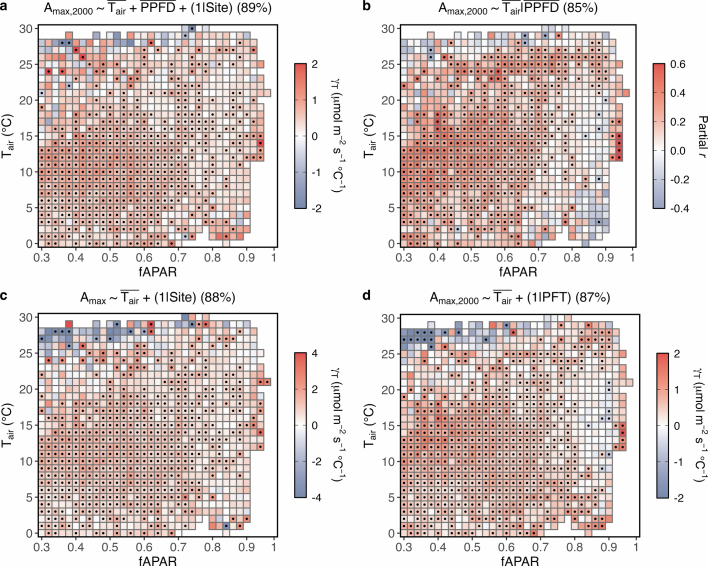
The potential confounding effect of factors other than on Amax,2,000 appears to be minimal as the detectability of thermal acclimation remains high across diverse conditions. The binning approach has proved effective in previous studies for analysing relationships between variables of interest while controlling for confounding factors35–37. The effects of concurrent Tair and seasonal changes in fAPAR on Amax,2,000 under Tair–fAPAR bin pairs are shown to be very weak (Extended Data Fig. 1b,c). To ensure our findings are not skewed by light acclimation35, we consider the detectability of thermal acclimation when incorporating into LLMs (89%; Extended Data Fig. 2a) and controlling for through partial correlation (85%; Extended Data Fig. 2b). The impact of VPD is probably limited, as its negative effect on Amax has been accounted for during the derivation of Amax (equation (3) in Methods) and has been further mitigated by ET/PET filtering. After filtering, there is a positive relationship between Amax,2,000 and VPD (Supplementary Fig. 1c). Any negative VPD impact on Amax,2,000 is expected to reinforce, not diminish, the observed widespread thermal acclimation. Diffuse radiation is expected to increase Amax by penetrating into deep canopy layers where light is limited38,39. However, this effect does not confound the relationship between Amax,2,000 and (Supplementary Fig. 2) since the conditions of diffuse radiation on the days of Amax measurements do not necessarily show a strong positive correlation with (Supplementary Table 1). Additionally, our findings remain robust with respect to the metric choice; detectability is 88% when Amax is unstandardized to a specific PPFD level and 87% when PFTs are treated as random effects within LLMs (Extended Data Fig. 2c,d).
Extended Data Fig. 2. The relationships between canopy photosynthetic capacities and over fAPAR and Tair bins.
a, The partial effect of on Amax,2000 when is also incorporated in the modelling (Amax,2000 ∼ + + (1∣Site)). b, Partial correlation coefficients (partial r) between Amax,2000 and when controlling for (Amax,2000 ∼ ∣). c, The cross-site thermal acclimation rate () is calculated based on Amax (Amax ∼ + (1∣Site)). d, The cross-site is calculated using plant function types (PFTs) as random intercepts (Amax,2000 ∼ + (1∣PFT)). Numbers (%) in parentheses represent the detectability of positive values, which is defined as the percentage of the number of bins displaying a positive over the total number of bins. Black dots indicate significant (P < 0.05) correlations.
Thermal acclimation capability can be influenced by the level and variability of , as well as by species and PFTs27,40–42. We observe negative effects of on Amax,2,000 when fAPAR falls below 0.7 and Tair exceeds 25 °C (Fig. 1a). Limited transpiration, due to a low amount of leaves, may not cool the canopy sufficiently under elevated Tair, making ribulose-1,5-bisphosphate (RuBP) regeneration a limiting process for canopy photosynthesis at high canopy temperature13. The reduction in Amax,2,000 with may be attributed to reduced stomatal conductance under high VPD23 (Supplementary Fig. 3f) and/or decreased maximum quantum yield of photosystem II in response to elevated temperature5,34,43. Additionally, under these conditions, the range of (the difference between the 90th and 10th percentiles; 3.8 °C) is significantly narrower than among the rest (8.4 °C) (two-tailed t-test, P < 0.01) (Supplementary Fig. 3b). Our site-level analyses also show that the correlation between Amax,2,000 and is positively associated with variability and negatively with (Extended Data Fig. 4b,c), which aligns with previous studies indicating that plants grown under low variability and/or high show reduced acclimation potential27,40,44. Conversely, leaf-scale experiments indicate that the acclimation rates of light-saturated net assimilation rates (Anet) under different measurement temperatures are similar41, suggesting a limited impact of Tair on Amax,2,000. Moreover, EBF is the dominant PFT for the bin pairs with high Tair (Supplementary Fig. 4b). There is some evidence that tropical evergreen forests have a limited capability for physiological acclimation because these forests are adapted to relatively stable thermal conditions and/or thrive under high that is beyond the range limit for acclimation33,45,46. The under-representation of EBF in the FLUXNET2015 database47 may also lead to uncertainties in the estimation of γT for this biome.
The observed widespread thermal acclimation of Amax,2,000 (Fig. 1) contrasts with the varying sign of the response of leaf Anet to , which can be positive, negative or neutral27,40,41,48,49. This discrepancy may stem from the fact that, unlike Amax, Anet is not necessarily measured under ample water conditions27,32 and water stress is known to affect the capacities of plant thermal acclimation22. In water-limited situations, plants typically reduce water loss through transpiration by decreasing stomatal conductance50, resulting in decreased Anet.
Timescale of thermal acclimation of canopy photosynthesis
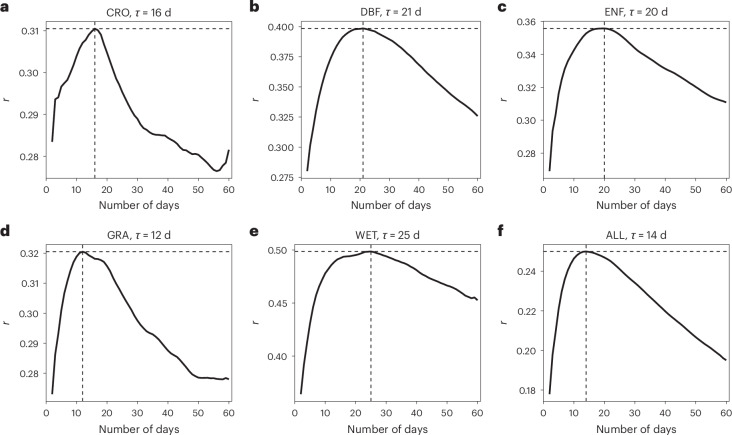
The timescale for canopy photosynthetic acclimation, as measured by the correlation coefficient (r) between Amax,2,000 and over different periods within concurrent Tair and fAPAR bins, varies across PFTs (Fig. 2 and Supplementary Fig. 5), increasing from GRA (12 d) to CRO (16 d), ENF (20 d), DBF (21 d) and finally WET (25 d). The τ value obtained across all sites is 14 d (Fig. 2f). For EBF, an optimal τ cannot be determined using Amax,2,000, even over an extended period of 180 d (Supplementary Fig. 5a). The enhanced vegetation index (EVI) that is derived from reflectance data in the near-infrared, red and blue spectral bands can characterize canopy structure, which closely relates with the canopy photosynthetic capacity51. We use a τ value of 13 d for EBF as identified by remote-sensing EVI for subsequent analysis (Methods and Supplementary Fig. 5b).
Fig. 2. Timescales for thermal acclimation of canopy photosynthesis.
a–f, The timescale for CRO (a), DBF (b), ENF (c), GRA (d), WET (e) and ALL (f). The x axes represent the number of days over which Tair is averaged to derive . The y axes represent the 5-day moving average of positive Pearson correlation coefficients (r) between Amax,2,000 and over fAPAR and Tair bins. The τ value is the length of time frame for which r peaks.
Our estimate of an average of 14 d as τ for thermal acclimation of canopy photosynthesis falls within the range of leaf-scale τ, which varies from days to months depending on species and growth conditions10,18,20,52. Studies that identify τ for photosynthetic acclimation using observational data across a spectrum of time frames are rare. A modelling study reports that a 15 day timescale for acclimation optimally predicts hourly eddy covariance flux measurements53. It is important to note that Amax,2,000 can show positive correlations with over both the optimal τ value and other time frames close to the optimal, due to the potentially high correlation among calculated over different short-term periods.
The timescale τ for photosynthetic acclimation to a changing environment reflects a trade-off between potential benefits (for example, carbon assimilation) and costs (for example, resource re-allocation)48. A rapid adjustment in photosynthetic capacities is expected to enhance photosynthetic performance but is accompanied by higher costs in energy and resources15. The shorter τ observed in GRA and CRO are in line with the expectation that fast-growing plants with a high generation rate of new leaves might show shorter τ than slow-growing species due to their greater physiological plasticity54. Conversely, we found larger τ values in forests and WET, indicating that these ecosystems require more time for acclimation; however, this longer acclimation period is potentially compensated for by a higher acclimation rate (Fig. 1b). The PFT-specific and cross-site τ values for the canopy photosynthetic capacity provide a credible basis for explicitly incorporating the timescale of thermal acclimation into vegetation and land surface models.
Representing acclimation in photosynthesis models
We further explore the representation of Amax,2,000 thermal acclimation in a biochemical model for C3 canopy photosynthesis incorporated in the Breathing Earth System Simulator (BESS)55, based on the Farquhar–von Caemmerer–Berry (FvCB) model4 (Methods). We test three alternative approaches, each under different resource-use allocation assumptions, to estimate maximum carboxylation rates (Vcmax, μmol m−2 s−1) standardized to 25 °C (). These approaches are: (1) assuming a temporally constant and PFT-specific (), where plants do not actively regulate through the growing seasons; (2) scaling leaf by canopy phenology, as indicated by leaf area index (LAI) (LAI-scaled , ); and (3) modelling acclimation to prevailing environments based on the eco-evolutionary optimality (EEO) theory34,56 () (Methods and Supplementary Texts 1 and 2). The FvCB model as applied here incorporates recent advances in parameterizing the temperature dependence of leaf photosynthetic capacities to represent Topt acclimation12 (Supplementary Text 1). We run the model using the site-level forcings from the FLUXNET2015 database and derive Amax,2,000 by setting PPFD equivalent to 2,000 μmol m−2 s−1. Canopy temperature is a key uncertainty in modelling canopy-scale photosynthesis30,57. We evaluate model performance using three temperature approximations, including Tair, aerodynamic surface temperature and radiometric surface temperature58. We finally use Tair to represent canopy temperature because it has comparable performance to the other two approximations and greater data availability (Supplementary Text 1 and Supplementary Fig. 8). For further analysis, we select estimated Amax,2,000 values from 65 C3 sites excluding CRO and water-limited sites, where all three model variants show acceptable accuracy in estimating Amax,2,000 (coefficient of determination (R2) > 0.5) (Supplementary Table 2).
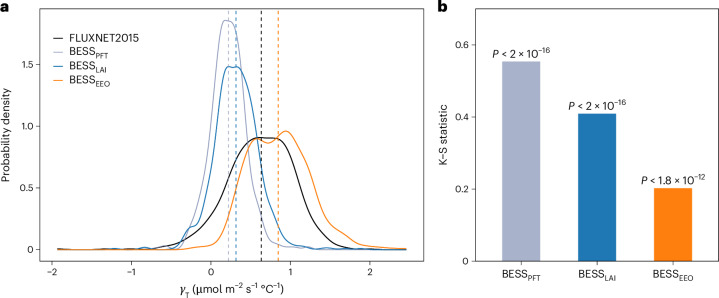
The BESS model variant incorporating optimality-based more closely approximates the observed γT compared to the other two variants, (BESSPFT) and (BESSLAI) (Fig. 3). The Kolmogorov–Smirnov (K–S) test indicates that the cumulative distribution functions of γT between BESSEEO and FLUXNET2015 observations are more closely aligned, despite significant differences between all three BESS model distributions and observations (P < 0.05) (Fig. 3b). BESSPFT and BESSLAI underestimate the median observed γT by 65% and 50%, respectively, while BESSEEO overestimates it by 34% (Fig. 3a).
Fig. 3. Impact of leaf photosynthetic capacities on γT estimation.
a, Probability densities of γT values derived from FLUXNET2015 and three variants of the BESS model (BESSPFT, BESSLAI and BESSEEO). The vertical lines represent the median γT values. b, The statistics of the two-sided K–S tests between FLUXNET2015 observations and three model variants.
The considerable underestimation of γT by BESSPFT and BESSLAI highlights the limitation in process-based photosynthetic models that incorporate only Topt acclimation. To capture γT accurately, process-based models must also integrate seasonal variations in photosynthetic capacities resulting from thermal acclimation. The overestimation by BESSEEO can be attributed to its higher predicted detectability (99%) of thermal acclimation than observed (92%) (Fig. 3a). When calculating , we assume that plants are not water-stressed following ET/PET filtering; a water-stress factor is not applied to scale as described in ref. 43 (Supplementary Text 2). Consequently, in this study, the EEO theory represents an idealized condition where carbon assimilation is optimized under the assumption of sufficient water availability. While plant light use efficiency can be reduced by physiological stress due to water scarcity59, the absence of such water-stress constraints can lead to an overestimation of . Although ET/PET is an effective indicator of soil moisture, it may not fully correspond to plant physiological stress. Bridging the gap between existing water availability metrics and actual plant stress responses remains a challenge60.
Conclusion
Photosynthesis can benefit from future warming through thermal acclimation, resulting in increased carbon uptake under conditions where water is not limiting. While leaf-scale acclimation is widely recognized, our study shows that the positive acclimation of canopy-scale photosynthetic capacity to growth temperature is a widespread phenomenon across various terrestrial biomes. We have shown that, on average, the canopy photosynthetic capacity acclimates to the growth thermal conditions of the preceding 14 days. Incorporating seasonal acclimation of photosynthetic capacities (the maximum carboxylation rate and the maximum electron-transport rate) is critical for achieving accurate simulations of photosynthesis in response to variations in temperature at timescales of weeks to months. Despite warmer growing seasons, water availability is increasingly constrained in many regions, potentially forcing plants to reduce photosynthetic capacity as a water conservation strategy. Improving the understanding of canopy-scale photosynthetic thermal acclimation in response to future conditions characterized by warming and variable water availability is therefore important.
Methods
Global database of ecosystem-scale carbon fluxes
We derive Amax from >200 eddy covariance sites from the global database FLUXNET2015, which covers a wide range of geospatial locations and PFTs47,61 (Supplementary Table 2). FLUXNET2015 is an openly accessible database containing data on the net exchange of carbon (NEE), water and energy between the atmosphere and the biosphere and meteorological observations. Uniform processing approaches are implemented for the flux calculation and quality control across the sites47. We use half-hourly or hourly NEE (NEE_VUT_USTAR50), its corresponding estimation of the uncertainty caused by friction velocity filtering (NEE_VUT_USTAR50_ RANDUNC) and gap-filled meteorological observations, including incoming radiation (SW_IN_F), air temperature (TA_F) and VPD (VPD_F) to derive Amax (refs. 47,62) (described below). Sites are excluded if data are unavailable during the MODIS period from 2002 onwards (for example, US-LWW and US-Me4) or if the uncertainty estimation is missing (for example, CA-Man).
Derivation of ecosystem-scale Amax
We derive Amax from light response curves across the FLUXNET2015 sites according to the daytime flux partitioning methods detailed in refs. 35,63. We fit NEE using the following hyperbolic equation:
| 1 |
where β (μmol CO2 m−2 s−1) is the target variable of interest. Variables α, Rg and γ represent the ecosystem-scale quantum yield (μmol C J−1), global radiation (W m−2) and ecosystem respiration (μmol CO2 m−2 s−1), respectively.
To account for the potential influence of high VPD (hPa), β is scaled using an exponential function only when VPD exceeds 10 hPa. Thus, we obtain Amax as follows:
| 2 |
where β and k are fit parameters to the flux data. The ecosystem respiration term in equation (1), γ, is estimated using an Arrhenius-type function describing the temperature dependence of γ (ref. 64), which is applied to night-time data by assuming that night-time NEE is equivalent to ecosystem respiration:
| 3 |
where Rref and E0 are the basal respiration rate (μmol CO2 m−2 s−1) at a reference temperature (Tref = 15 °C) and temperature sensitivity (°C), respectively. T0 is a constant equal to −46.02 °C (ref. 65).
In practice, E0 is first estimated according to equation (3). With a fixed E0, the remaining parameters of equations (2) and (3) (α, β, k and Rref) are derived using a time window of 2–14 d. The specific time window depends on data availability and the Amax value is assumed invariant within the same fitting window. On average, 25% of estimated Amax values are flagged as medium or low quality because the parameter ranges are unreasonable and/or the curve fitting is unconstrained (Supplementary Fig. 6b) and are subsequently discarded35. Additionally, Amax values that are constant for 14 consecutive days or more are excluded. More than 88% of the Amax values in the remaining dataset are fitted within a 2 d window (Supplementary Fig. 6a), indicating a sufficient sample size for most fitting. Here we derive Amax using the REddyProc R package (https://github.com/bgctw/REddyProc)66, as Amax is not provided in the FLUXNET2015 database. We convert PPFD to Rg using a constant of 2.1 μmol J−1 (ref. 67). We standardize Amax to PPFD = 2,000 μmol m−2 s−1 (Amax,2,000) by setting Rg = 952 W m−2 in equation (1) and calculating the corresponding assimilation rate. This approach can avoid any Amax values obtained from potentially unsaturated light conditions and ensure consistent levels of absorbed PAR35.
Timescale for thermal acclimation of Amax,2,000
We hypothesize that the most relevant timescale for thermal acclimation (τ) ranges between 2 and 60 d, according to the coordination hypothesis and observations18,20,68. We conduct linear regressions between Amax,2,000 derived from the FLUXNET2015 sites and the daytime averaged over the 2–60 d before the time of Amax,2,000 measurements with a time interval of 1 d. On the basis of a previous study35, savanna and shrubland sites are excluded from the analysis because they are frequently subject to water stress. Croplands are excluded from the cross-site analysis. Furthermore, we exclude the Amax,2,000– pairs collected during water-limited conditions, as indicated by the ratio of prevailing actual evapotranspiration to Priestley–Taylor potential evapotranspiration (ET/PET) < 0.7 (ref. 69) and VPD > 20 hPa. Additionally, we only focus on growing seasons, characterized by fAPAR > 0.3 and Tair and > 0 °C. Daily fAPAR and LAI for each site were derived by interpolating the 8 d MODIS MOD15A2H products following ref. 35. Low-quality data affected by cloud contamination are removed31. A total of 149,403 Amax,2,000 records are used for further analyses.
To remove the potential effects of concurrent Tair and fAPAR on Amax,2,000, we group Amax,2,000– pairs into different bins of Tair with 1 °C intervals and fAPAR with 0.02 intervals. This approach allows the analysis of changes in Amax,2,000 along gradients to be made while controlling for the instantaneous temperature dependence of photosynthesis and seasonal changes in leaf quantity and the development of the photosynthetic system. Pearson r between Amax,2,000 and that is averaged over different time frames (that is, 2–60 d with 1 d interval) is calculated for Tair and fAPAR bins. A positive r indicates the thermal acclimation potential of Amax,2,000. Only bins with sampling numbers larger than 10 and 20 for PFT-based and cross-site analyses, respectively, are retained. We examine the relationship between the average of the positive r values obtained from Tair and fAPAR bins and the time frames used to calculate for each PFT and cross sites (Fig. 2). Parameter τ is defined as the corresponding time frame when the 5 d moving average of the positive r reaches its peak. EVI, derived from MODIS reflectance data (MCD43A4) in the near-infrared, red and blue spectral bands51, is used to estimate τ for EBF for subsequent analysis, as an optimal τ cannot be identified for this PFT using Amax,2,000 (Supplementary Fig. 5).
Evidence for thermal acclimation of Amax,2,000
We use PFT-specific τ values for aggregating prevailing Tair to obtain (Fig. 1). We run LMMs, which include a random effect of different sites for removing the site-level adaptation effect, to explore the relationship between Amax,2,000 and PFT-specific (that is, Amax,2,000 ∼ + (1∣site)) (Extended Data Fig. 1a). The same data selection procedure and Tair and fAPAR binning scheme are used for the cross-site analysis (Fig. 1a and see earlier). The coefficient of estimated from LMMs is defined as thermal acclimation rate (γT). The sampling number, conditional and marginal correlation coefficients for the cross-site analysis are shown in Supplementary Fig. 6. The LMM is conducted with the R package lme4 (ref. 70). For each site, the sampling number of Amax,2,000– pairs is insufficient to support the correlation analysis under the binning scheme35. Instead, a partial correlation analysis is run between Amax,2,000 and controlling for , Tair and fAPAR on flux sites with observation lengths longer than 5 yr (Fig. 1c).
The prevailing conditions of Tair and PPFD often show a high correlation (Supplementary Fig. 1a). Therefore, we also include as an additional predictor in the LMM (Extended Data Fig. 2a) and we analyse partial correlations between Amax,2,000 and controlling for (Extended Data Fig. 2b) to eliminate the confounding effect of light acclimation35. Additionally, we repeat LMMs with a different target variable (Amax) and random effect (PFT) to examine the robustness of the detectability of thermal acclimation (Extended Data Fig. 2c,d).
Modelling canopy photosynthesis of C3 plants
We apply the photosynthesis module of the BESS model57 to estimate canopy photosynthesis (A) and subsequently Amax,2,000, for each flux site. This allows a direct comparison to be made of the impacts of different empirical formulations of leaf photosynthetic capacities on thermal acclimation. The photosynthesis module is based on the FvCB model4, where A is determined as the lower CO2 assimilation rate between the maximum rate of ribulose-1,5-bisphosphate carboxylase/oxygenase activity when light is saturated (Ac) and the electron-transport rate for RuBP regeneration when light is limited (Aj). For this study, the two-big-leaf scheme implemented in the BESS model is simplified to a one-big-leaf scheme. We have updated the parameters of temperature dependence of the maximum carboxylation rate (Vcmax, μmol m−2 s−1), the maximum electron-transport rate (Jmax, μmol m−2 s−1), as well as the ratio of their values at 25 °C following ref. 12. A detailed description of the canopy photosynthesis model can be found in Supplementary Text 1 (also see refs. 31,55,57).
Leaf photosynthetic capacities
Vcmax is a key parameter in the FvCB model, particularly under light-saturated conditions4. Previous studies have shown that leaf biochemical components can acclimate to (refs. 11,12,21). In this study, we compare three empirically derived variants of Vcmax at 25 °C () within the FvCB model to evaluate their effectiveness in simulating the observed γT:
: this variant assumes a constant value over the growing season, an assumption that is still widely used in vegetation models16. The prescribed top leaf values are adopted from a look-up table based on PFTs and climatic zones compiled from the TRY trait database31,71.
- : leaf varies seasonally, with its seasonality following LAI. This scheme, implemented in the previous version of the BESS model31, follows equation (4).
where LAImin and LAImax are the 5th and 95th percentile values of LAI over a growing season, respectively, and a is an empirical parameter set to 0.3 (ref. 57).4 : the calculation is based on EEO theory19,34,56, specifically the coordination hypothesis17,72 and the least-cost hypothesis50,73. The coordination hypothesis proposes that plants actively coordinate resource allocation so that Ac tends to equal Aj on weekly to monthly timescales. The least-cost hypothesis proposes that plants minimize the combined costs (per unit assimilation) of maintaining the biochemical capacity for photosynthesis and the water transport capacity required to support it, through stomatal regulation. Combining the two hypotheses results in an optimal intercellular CO2 concentration under representative conditions74. Here we assume that acclimates to prevailing conditions following the same timescale as Amax,2,000 (Fig. 2). The calculation is detailed in Supplementary Text 2 and ref. 34.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Texts 1 and 2, Tables 1–3, Figs. 1–9, and References.
Acknowledgements
This research is a contribution to the LEMONTREE (land ecosystem models based on new theory, observation and experiments) project, funded through the generosity of E. and W. Schmidt by recommendation of the Schmidt Futures programme. It is also a contribution to USMILE European Research Council grant. Y.R. was supported by the Ministry of Environment of Korea (202300218237). B.D.S. was funded by the Swiss National Science Foundation grant no. PCEFP2_181115. B.D. was supported by sDiv, the Synthesis Centre of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG FZT 118, 202548816). T.F.K. acknowledges additional support from a NASA Carbon Cycle Science Award 80NSSC21K1705, a US Department of Energy Early Career Research Program award no. DE-SC0021023 and the RUBISCO SFA, which is sponsored by the Regional and Global Model Analysis Program in the Climate and Environmental Sciences Division of the Office of Biological and Environmental Research in the US DOE Office of Science. X. Luo was supported by National University of Singapore Presidential Young Professorship (A-0003625-01-00). I.C.P. acknowledges funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 787203 REALM). We especially thank the researchers and contributors of the FLUXNET community and the MODIS products. We also acknowledge X. Lian and J. Fang for their helpful reviews and comments on this paper before its submission.
Extended data
Author contributions
J.L. designed the study, performed the analysis and drafted the initial paper. J.L., Y.R., X. Luo, T.F.K. and P.G. participated in the early-stage discussion. I.C.P. substantially revised the paper. All co-authors commented on the results and contributed to the writing of the paper.
Peer review
Peer review information
Nature Plants thanks Mirindi Eric Dusenge, Marc Peaucelle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The dataset of FLUXNET2015 flux sites under the CC-BY-4.0 policy is publicly available for download at http://fluxnet.fluxdata.org. Remote-sensing canopy structure data from the MODIS MCD43A and MOD15A2H products are freely accessible at https://lpdaac.usgs.gov/products/mcd43a3v006/ and https://lpdaac.usgs.gov/products/mod15a2hv006/. BESS flux products are publicly available at https://www.environment.snu.ac.kr/data/.
Code availability
The corresponding R code scripts used in this study are available via Zenodo at 10.5281/zenodo.13854273 (ref. 75). The code for the deviation of Amax from the FLUXNET2015 database is available via GitHub at https://github.com/trevorkeenan/inhibitionPaperCode. The code for modelling optimality-based Vcmax is available via GitHub at https://github.com/chongya/SVOM.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiangong Liu, Email: jl6314@columbia.edu.
Youngryel Ryu, Email: yryu@snu.ac.kr.
Extended data
is available for this paper at 10.1038/s41477-024-01846-1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41477-024-01846-1.
References
- 1.Anav, A. et al. Spatiotemporal patterns of terrestrial gross primary production: a review. Rev. Geophys.53, 785–818 (2015). [Google Scholar]
- 2.Beer, C. et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science329, 834–838 (2010). [DOI] [PubMed] [Google Scholar]
- 3.IPCC Special Report on Impacts of Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) (Cambridge Univ. Press, 2022).
- 4.Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta149, 78–90 (1980). [DOI] [PubMed] [Google Scholar]
- 5.Bernacchi, C. J., Singsaas, E. L., Pimentel, C., Portis, A. R. & Long, S. P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ.24, 253–259 (2001). [Google Scholar]
- 6.Sage, R. F. & Kubien, D. S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ.30, 1086–1106 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Bernacchi, C. J. et al. Modelling C3 photosynthesis from the chloroplast to the ecosystem. Plant Cell Environ.36, 1641–1657 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Mercado, L. M. et al. Large sensitivity in land carbon storage due to geographical and temporal variation in the thermal response of photosynthetic capacity. New Phytol.218, 1462–1477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver, R. J. et al. Improved representation of plant physiology in the JULES-vn5.6 land surface model: photosynthesis, stomatal conductance and thermal acclimation. Geosci. Model. Dev.15, 5567–5592 (2022). [Google Scholar]
- 10.Berry, J. & Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol.31, 491–543 (1980). [Google Scholar]
- 11.Medlyn, B. E. et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ.25, 1167–1179 (2002). [Google Scholar]
- 12.Kumarathunge, D. P. et al. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol.222, 768–784 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Crous, K. Y., Uddling, J. & De Kauwe, M. G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol.234, 353–374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamori, W., Hikosaka, K. & Way, D. A. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth. Res.119, 101–117 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Dietze, M. C. Gaps in knowledge and data driving uncertainty in models of photosynthesis. Photosynth. Res.119, 3–14 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Rogers, A. et al. A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol.213, 22–42 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Maire, V. et al. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE7, e38345 (2012). [DOI] [PMC free article] [PubMed]
- 18.Smith, N. G. & Dukes, J. S. Drivers of leaf carbon exchange capacity across biomes at the continental scale. Ecology99, 1610–1620 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Smith, N. G. et al. Global photosynthetic capacity is optimized to the environment. Ecol. Lett.22, 506–517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, N. G. & Dukes, J. S. Short-term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Glob. Change Biol.23, 4840–4853 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Kattge, J. & Knorr, W. Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ.30, 1176–1190 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Lin, Y. S., Medlyn, B. E. & Ellsworth, D. S. Temperature responses of leaf net photosynthesis: the role of component processes. Tree Physiol.32, 219–231 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Grossiord, C. et al. Plant responses to rising vapor pressure deficit. New Phytol.226, 1550–1566 (2020). [DOI] [PubMed] [Google Scholar]
- 24.López, J., Way, D. A. & Sadok, W. Systemic effects of rising atmospheric vapor pressure deficit on plant physiology and productivity. Glob. Change Biol.27, 1704–1720 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu, S. et al. Thermal optimality of net ecosystem exchange of carbon dioxide and underlying mechanisms. New Phytol.194, 775–783 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Baldocchi, D. et al. FLUXNET: a new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Meteorol. Soc.82, 2415–2434 (2001). [Google Scholar]
- 27.Vico, G., Way, D. A., Hurry, V. & Manzoni, S. Can leaf net photosynthesis acclimate to rising and more variable temperatures? Plant Cell Environ.42, 1913–1928 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Way, D. A. & Yamori, W. Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res.119, 89–100 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Dusenge, M. E. et al. Boreal conifers maintain carbon uptake with warming despite failure to track optimal temperatures. Nat. Commun.14, 4667 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knauer, J. et al. Higher global gross primary productivity under future climate with more advanced representations of photosynthesis. Sci. Adv.9, eadh9444 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, C. & Ryu, Y. Multi-scale evaluation of global gross primary productivity and evapotranspiration products derived from Breathing Earth System Simulator (BESS). Remote Sens. Environ.186, 528–547 (2016). [Google Scholar]
- 32.Wright, I. J. et al. The worldwide leaf economics spectrum. Nature428, 821–827 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Huang, M. et al. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol.3, 772–779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang, C., Ryu, Y., Wang, H. & Keenan, T. F. An optimality-based model explains seasonal variation in C3 plant photosynthetic capacity. Glob. Change Biol.26, 6493–6510 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Luo, X. & Keenan, T. F. Global evidence for the acclimation of ecosystem photosynthesis to light. Nat. Ecol. Evol.4, 1351–1357 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Liu, L. et al. Soil moisture dominates dryness stress on ecosystem production globally. Nat. Commun.11, 4892 (2020). [DOI] [PMC free article] [PubMed]
- 37.Novick, K. A. et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Change6, 1023–1027 (2016). [Google Scholar]
- 38.Zhou, H. et al. Responses of gross primary productivity to diffuse radiation at global FLUXNET sites. Atmos. Environ.244, 117905 (2021). [Google Scholar]
- 39.Mercado, L. M. et al. Impact of changes in diffuse radiation on the global land carbon sink. Nature458, 1014–1017 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Sendall, K. M. et al. Acclimation of photosynthetic temperature optima of temperate and boreal tree species in response to experimental forest warming. Glob. Change Biol.21, 1342–1357 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Battaglia, M., Beadle, C. & Loughhead, S. Photosynthetic temperature responses of Eucalyptus globulus and Eucalyptus nitens. Tree Physiol.16, 81–89 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Slot, M., Rifai, S. W. & Winter, K. Photosynthetic plasticity of a tropical tree species, Tabebuia rosea, in response to elevated temperature and [CO2]. Plant Cell Environ.44, 2347–2364 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Stocker, B. D. et al. P-model v1.0: an optimality-based light use efficiency model for simulating ecosystem gross primary production. Geosci. Model. Dev.13, 1545–1581 (2020). [Google Scholar]
- 44.Luo, Y., Gessler, A., D’Odorico, P., Hufkens, K. & Stocker, B. D. Quantifying effects of cold acclimation and delayed springtime photosynthesis resumption in northern ecosystems. New Phytol.240, 984–1002 (2023). [DOI] [PubMed]
- 45.Dusenge, M. E. et al. Limited thermal acclimation of photosynthesis in tropical montane tree species. Glob. Change Biol.27, 4860–4878 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Doughty, C. E. et al. Tropical forests are approaching critical temperature thresholds. Nature621, 105–111 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Pastorello, G. et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data7, 225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, N. G., McNellis, R. & Dukes, J. S. No acclimation: instantaneous responses to temperature maintain homeostatic photosynthetic rates under experimental warming across a precipitation gradient in Ulmus americana. AoB PLANTS12, plaa027 (2020).
- 49.Cunningham, S. C. & Read, J. Do temperate rainforest trees have a greater ability to acclimate to changing temperatures than tropical rainforest trees? New Phytol.157, 55–64 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Prentice, I. C., Dong, N., Gleason, S. M., Maire, V. & Wright, I. J. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecol. Lett.17, 82–91 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Zeng, Y. et al. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ.3, 477–493 (2022). [Google Scholar]
- 52.Mäkelä, A. et al. Developing an empirical model of stand GPP with the LUE approach: analysis of eddy covariance data at five contrasting conifer sites in Europe. Glob. Change Biol.14, 92–108 (2008). [Google Scholar]
- 53.Mengoli, G. et al. Ecosystem photosynthesis in land‐surface models: a first‐principles approach incorporating acclimation. J. Adv. Model Earth Syst.14, e2021MS002767 (2022). [Google Scholar]
- 54.Loveys, B. R. et al. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol.9, 895–910 (2003). [Google Scholar]
- 55.Li, B. et al. BESSv2.0: a satellite-based and coupled-process model for quantifying long-term global land–atmosphere fluxes. Remote Sens. Environ.295, 113696 (2023). [Google Scholar]
- 56.Harrison, S. P. et al. Eco‐evolutionary optimality as a means to improve vegetation and land‐surface models. New Phytol.231, 2125–2141 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Ryu, Y. et al. Integration of MODIS land and atmosphere products with a coupled-process model to estimate gross primary productivity and evapotranspiration from 1 km to global scales. Glob. Biogeochem. Cycles10.1029/2011GB004053 (2011).
- 58.Knauer, J., El-Madany, T. S., Zaehle, S. & Migliavacca, M. Bigleaf—an R package for the calculation of physical and physiological ecosystem properties from eddy covariance data. PLoS ONE13, e0201114 (2018). [DOI] [PMC free article] [PubMed]
- 59.Stocker, B. D. et al. Quantifying soil moisture impacts on light use efficiency across biomes. New Phytol.218, 1430–1449 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lian, X. et al. Multifaceted characteristics of dryland aridity changes in a warming world. Nat. Rev. Earth Environ.2, 232–250 (2021). [Google Scholar]
- 61.Baldocchi, D., Chu, H. & Reichstein, M. Inter-annual variability of net and gross ecosystem carbon fluxes: a review. Agric. For. Meteorol.249, 520–533 (2018). [Google Scholar]
- 62.Lasslop, G. et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Glob. Change Biol.16, 187–208 (2010). [Google Scholar]
- 63.Keenan, T. F. et al. Widespread inhibition of daytime ecosystem respiration. Nat. Ecol. Evol.3, 407–415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papale, D. et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: algorithms and uncertainty estimation. Biogeosciences3, 571–583 (2006). [Google Scholar]
- 65.Lloyd, J. & Taylor, J. A. On the temperature dependence of soil respiration. Funct. Ecol.8, 315 (1994). [Google Scholar]
- 66.Wutzler, T. et al. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences15, 5015–5030 (2018). [Google Scholar]
- 67.Meek, D. W., Hatfield, J. L., Howell, T. A., Idso, S. B. & Reginato, R. J. A generalized relationship between photosynthetically active radiation and solar radiation. Agron. J.76, 939–945 (1984). [Google Scholar]
- 68.Gunderson, C. A., O’hara, K. H., Campion, C. M., Walker, A. V. & Edwards, N. T. Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Glob. Change Biol.16, 2272–2286 (2010). [Google Scholar]
- 69.Fisher, J. B., Whittaker, R. J. & Malhi, Y. ET come home: potential evapotranspiration in geographical ecology. Glob. Ecol. Biogeogr.20, 1–18 (2011). [Google Scholar]
- 70.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
- 71.Kattge, J. et al. TRY—a global database of plant traits. Glob. Change Biol.17, 2905–2935 (2011). [Google Scholar]
- 72.Chen, J. L., Reynolds, J. F., Harley, P. C. & Tenhunen, J. D. Coordination theory of leaf nitrogen distribution in a canopy. Oecologia93, 63–69 (1993). [DOI] [PubMed] [Google Scholar]
- 73.Wright, I. J., Reich, P. B. & Westoby, M. Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat.161, 98–111 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Wang, H. et al. Towards a universal model for carbon dioxide uptake by plants. Nat. Plants3, 734–741 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Liu, J. Evidence for widespread thermal acclimation of canopy photosynthesis. Zenodo10.5281/zenodo.13854273 (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Texts 1 and 2, Tables 1–3, Figs. 1–9, and References.
Data Availability Statement
The dataset of FLUXNET2015 flux sites under the CC-BY-4.0 policy is publicly available for download at http://fluxnet.fluxdata.org. Remote-sensing canopy structure data from the MODIS MCD43A and MOD15A2H products are freely accessible at https://lpdaac.usgs.gov/products/mcd43a3v006/ and https://lpdaac.usgs.gov/products/mod15a2hv006/. BESS flux products are publicly available at https://www.environment.snu.ac.kr/data/.
The corresponding R code scripts used in this study are available via Zenodo at 10.5281/zenodo.13854273 (ref. 75). The code for the deviation of Amax from the FLUXNET2015 database is available via GitHub at https://github.com/trevorkeenan/inhibitionPaperCode. The code for modelling optimality-based Vcmax is available via GitHub at https://github.com/chongya/SVOM.