Abstract
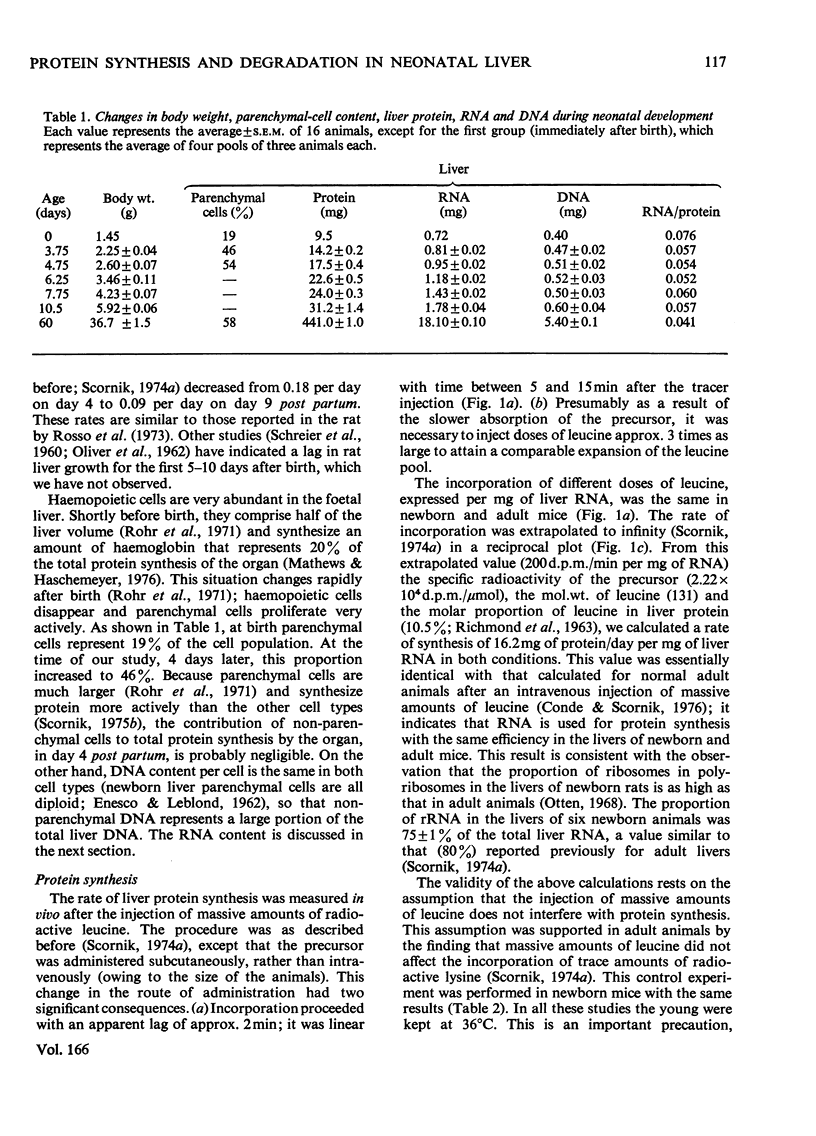
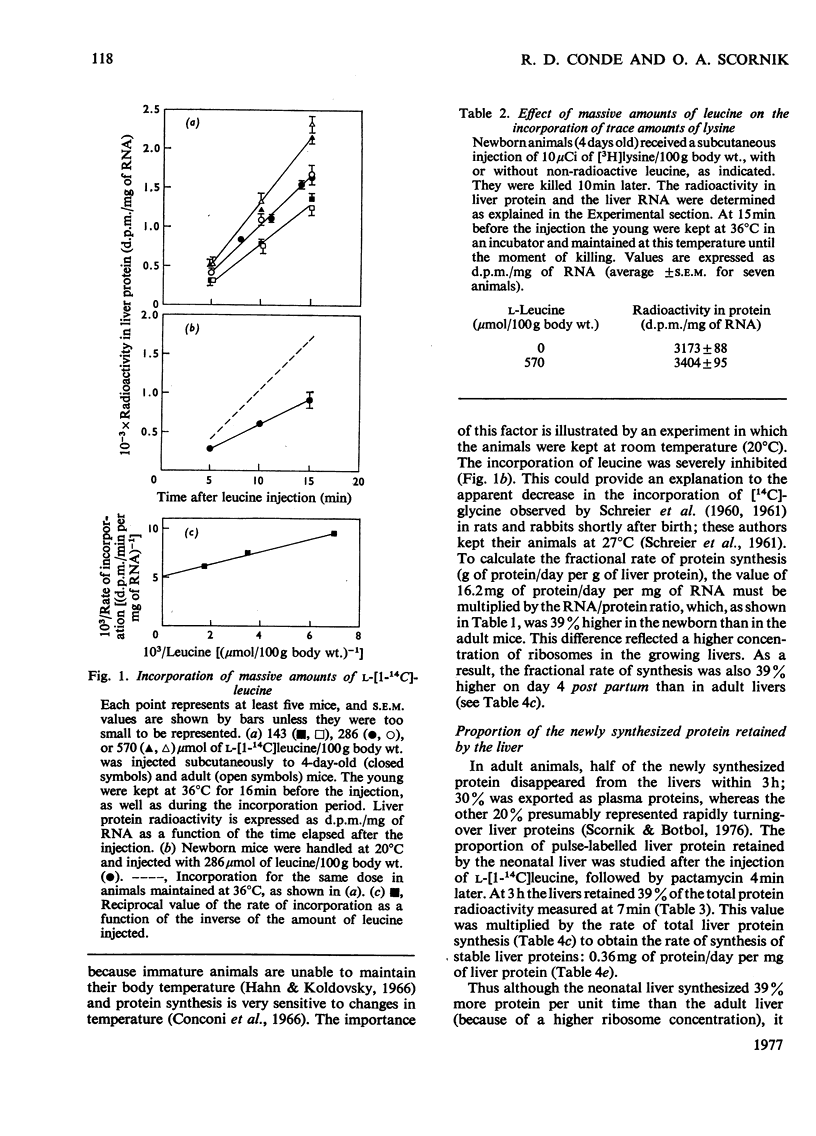
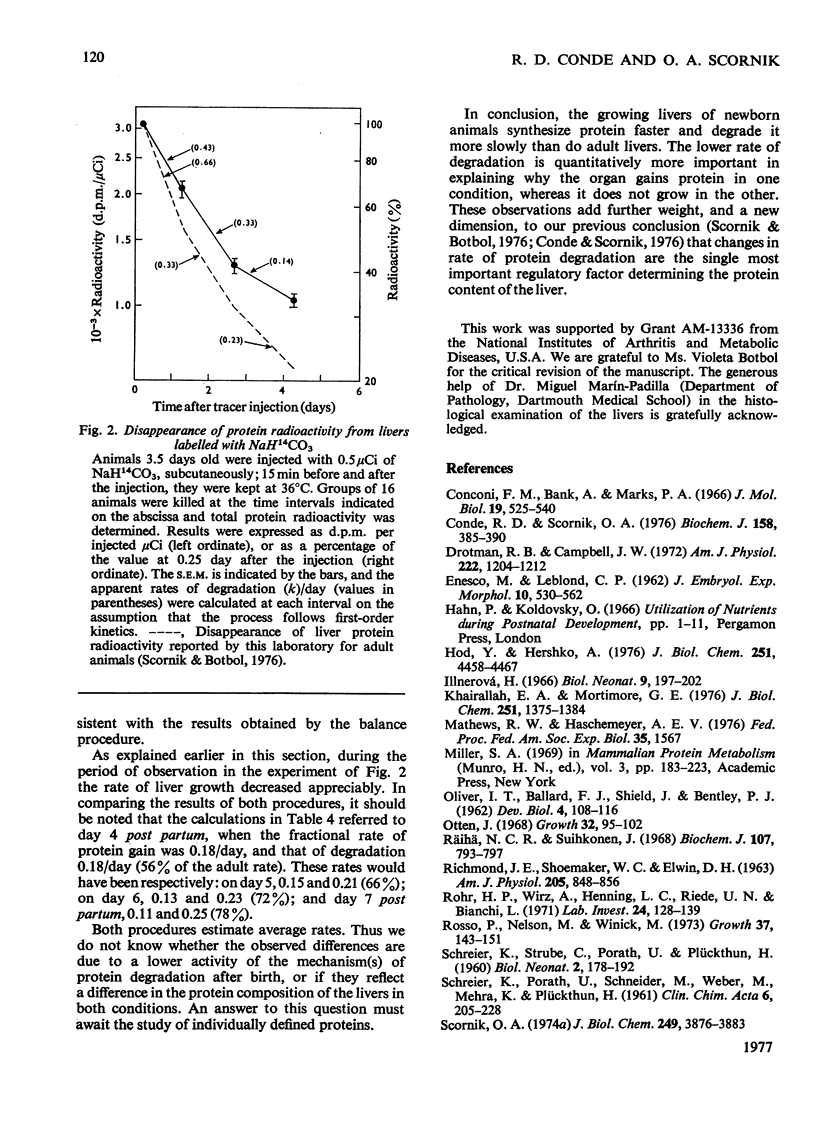
A study is presented of the liver protein gain during the early stages of postnatal development. Fractional rates of protein synthesis and degradation were determined in vivo in livers of 4-day-old mice. At this age, liver protein accumulated at a rate of 18% per day. Synthesis was measured after the injection of massive amounts of radioactive leucine. Degradation was extimated as the balance between synthesis and accumulation of stable liver proteins, or from the disappearance of radioactivity from liver protein previously labelled by the administration of NaH14CO3. We found that the neonatal livers: (1) synthesize 139% as much protein per unit time and unit mass as adult tissue, which is accounted for by a higher ribosome concentration (synthesis per mg of RNA was the same); (2) retain 39% of the newly synthesized protein as stable liver components (compared with 48% in adult mice); (3) degrade protein at 56% of the rate in the adult liver. This lower rate of degradation is quantitatively the most significant difference between the growing and non-growing liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conconi F. M., Bank A., Marks P. A. Polyribosomes and control of protein synthesis: effects of sodium fluoride and temperature of reticulocytes. J Mol Biol. 1966 Aug;19(2):525–540. doi: 10.1016/s0022-2836(66)80020-7. [DOI] [PubMed] [Google Scholar]
- Conde R. D., Scornik O. A. Role of protein degradation in the growth of livers after a nutritional shift. Biochem J. 1976 Aug 15;158(2):385–390. doi: 10.1042/bj1580385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drotman R. B., Campbell J. W. Protein arginine biosynthesis by mammalian liver tissue during postnatal development. Am J Physiol. 1972 May;222(5):1204–1212. doi: 10.1152/ajplegacy.1972.222.5.1204. [DOI] [PubMed] [Google Scholar]
- Hod Y., Hershko A. Relationship of the pool of intracellular valine to protein synthesis and degradation in cultured cells. J Biol Chem. 1976 Jul 25;251(14):4458–4457. [PubMed] [Google Scholar]
- Illnerová H. Urea formation in rats during postnatal development. Biol Neonat. 1965;9(1):197–202. doi: 10.1159/000239990. [DOI] [PubMed] [Google Scholar]
- Khairallah E. A., Mortimore G. E. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976 Mar 10;251(5):1375–1384. [PubMed] [Google Scholar]
- OLIVER I. T., BALLARD F. J., SHIELD J., BENTLEY P. J. Liver growth in early postpartum rat. Dev Biol. 1962 Feb;4:108–116. doi: 10.1016/0012-1606(62)90035-0. [DOI] [PubMed] [Google Scholar]
- Otten J. Rat liver polyribosomes during the first days of life. Growth. 1968 Jun;32(2):95–102. [PubMed] [Google Scholar]
- Richmond J. E., Shoemaker W. C., Elwyn D. H. Rates of biosynthesis of plasma and liver proteins. Am J Physiol. 1963 Nov;205(5):848–856. doi: 10.1152/ajplegacy.1963.205.5.848. [DOI] [PubMed] [Google Scholar]
- Rohr H. P., Wirz A., Henning L. C., Riede U. N., Bianchi L. Morphometric analysis of the rat liver cell in the perinatal period. Lab Invest. 1971 Feb;24(2):128–139. [PubMed] [Google Scholar]
- Rosso P., Nelson M., Winick M. Changes in cellular RNA content and alkaline ribonuclease activity in rat liver during development. Growth. 1973 Jun;37(2):143–151. [PubMed] [Google Scholar]
- Räihä N. C., Suihkonen J. Factors influencing the development of urea-synthesizing enzymes in rat liver. Biochem J. 1968 May;107(6):793–797. doi: 10.1042/bj1070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREIER K., PORATH U., SCHNEIDER M., WEBER M., MEHRA K., PLUECKTHUN H. [Studies on the developmental physiology of protein metabolism in different organs of the rabbit]. Clin Chim Acta. 1961 Mar;6:205–228. doi: 10.1016/0009-8981(61)90086-9. [DOI] [PubMed] [Google Scholar]
- SCHREIER K., STRUBE C., PORATH U., PLUECKTHUN H. [Studies of the physiological development of the protein metabolism of liver tissue]. Biol Neonat. 1960 Oct;2:178–192. [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rates of deaggregation of polyribosomes in normal and regenerating liver after the injection of pactamycin. Biochim Biophys Acta. 1974 Nov 20;374(1):76–81. doi: 10.1016/0005-2787(74)90200-7. [DOI] [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Vavrousek-Jakuba E. M., Miller S. A. Effect of starvation on protein synthesis in neonatal rat liver. J Nutr. 1975 Oct;105(10):1326–1333. doi: 10.1093/jn/105.10.1326. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON E. M., McCANCE R. A. Some effects of accelerating growth. I. General somatic development. Proc R Soc Lond B Biol Sci. 1960 May 17;152:188–206. doi: 10.1098/rspb.1960.0032. [DOI] [PubMed] [Google Scholar]
- Winick M., Noble A. Quantitative changes in DNA, RNA, and protein during prenatal and postnatal growth in the rat. Dev Biol. 1965 Dec;12(3):451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]


