Abstract
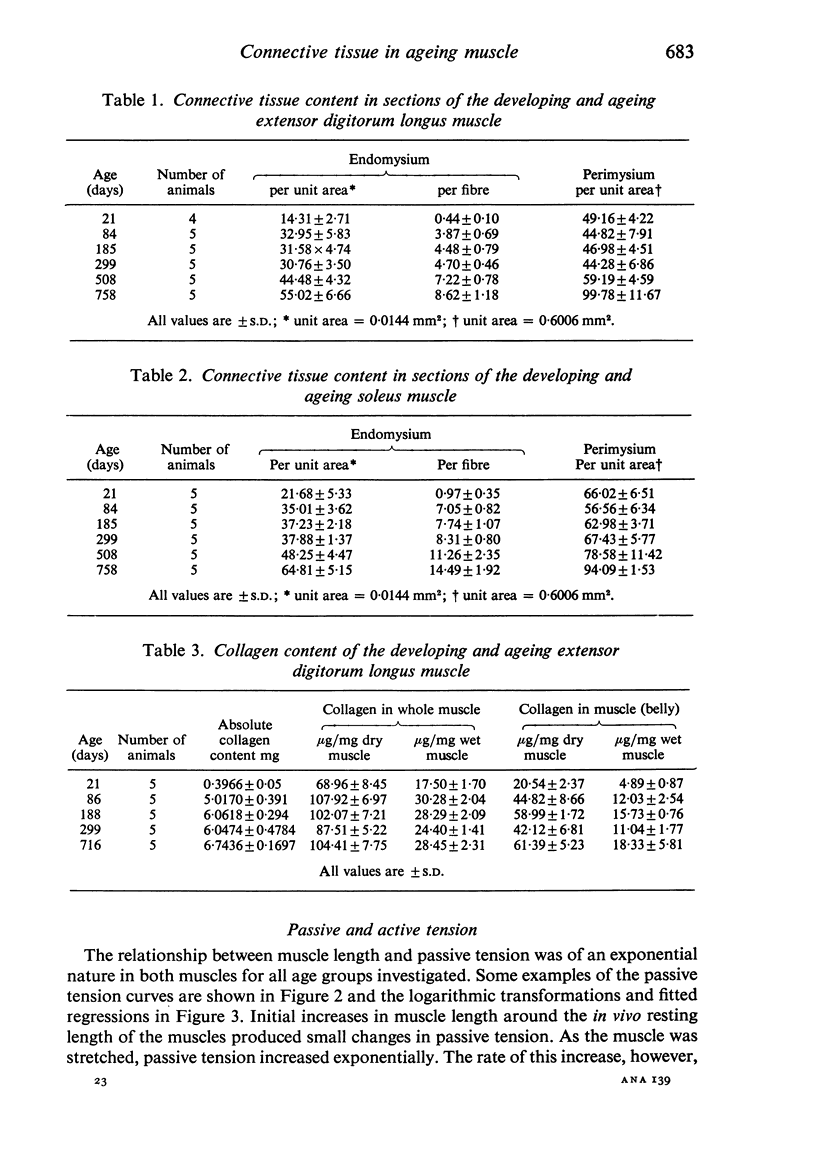
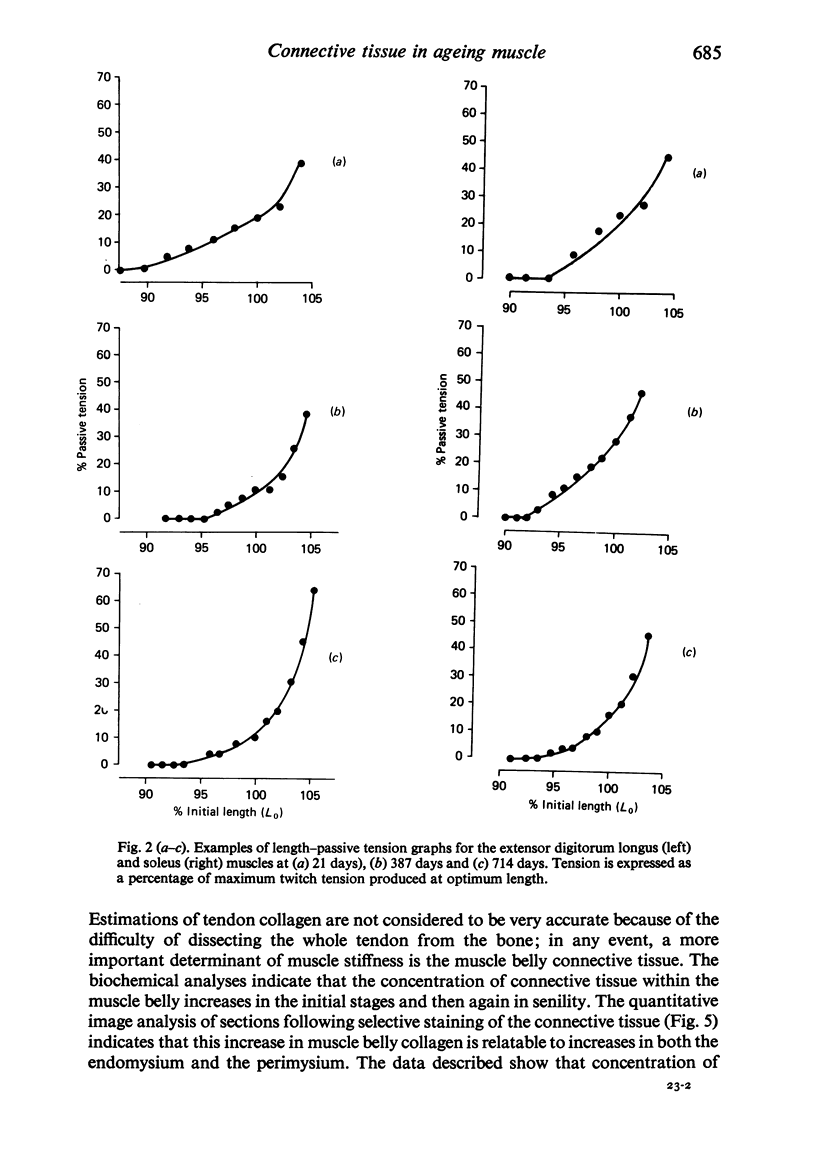
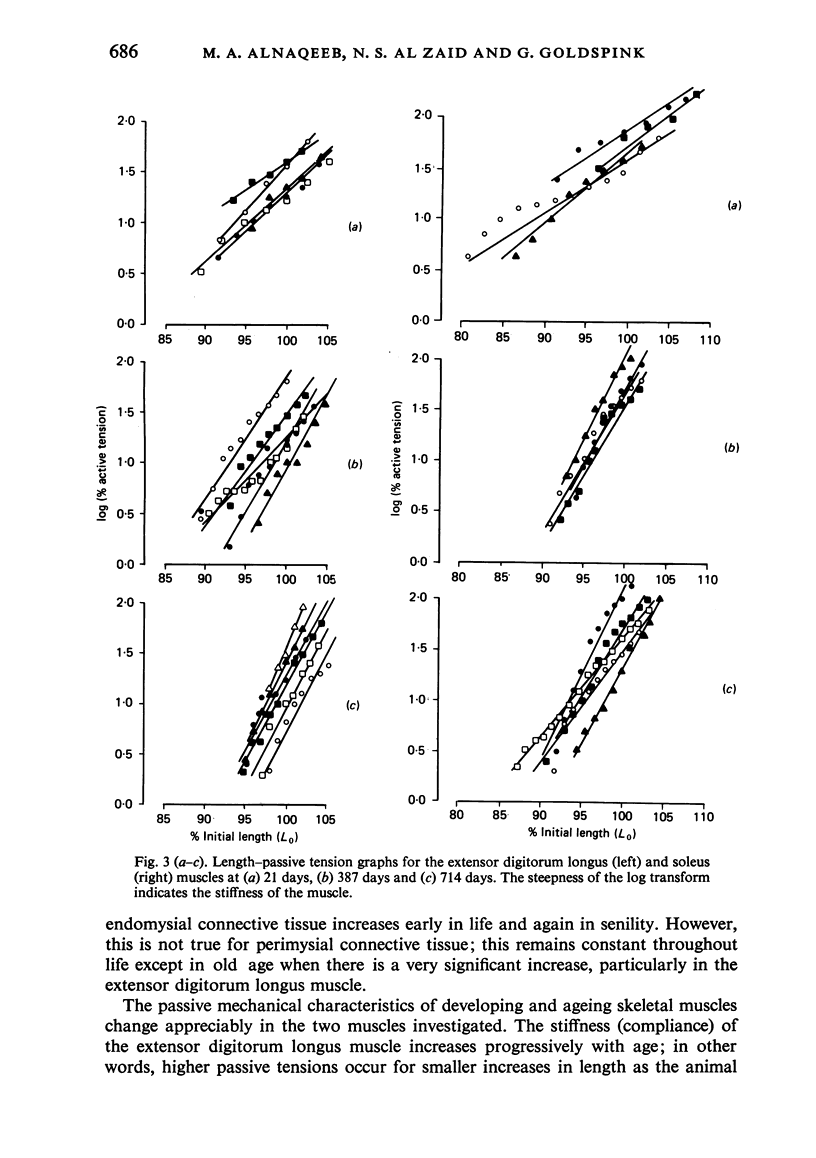
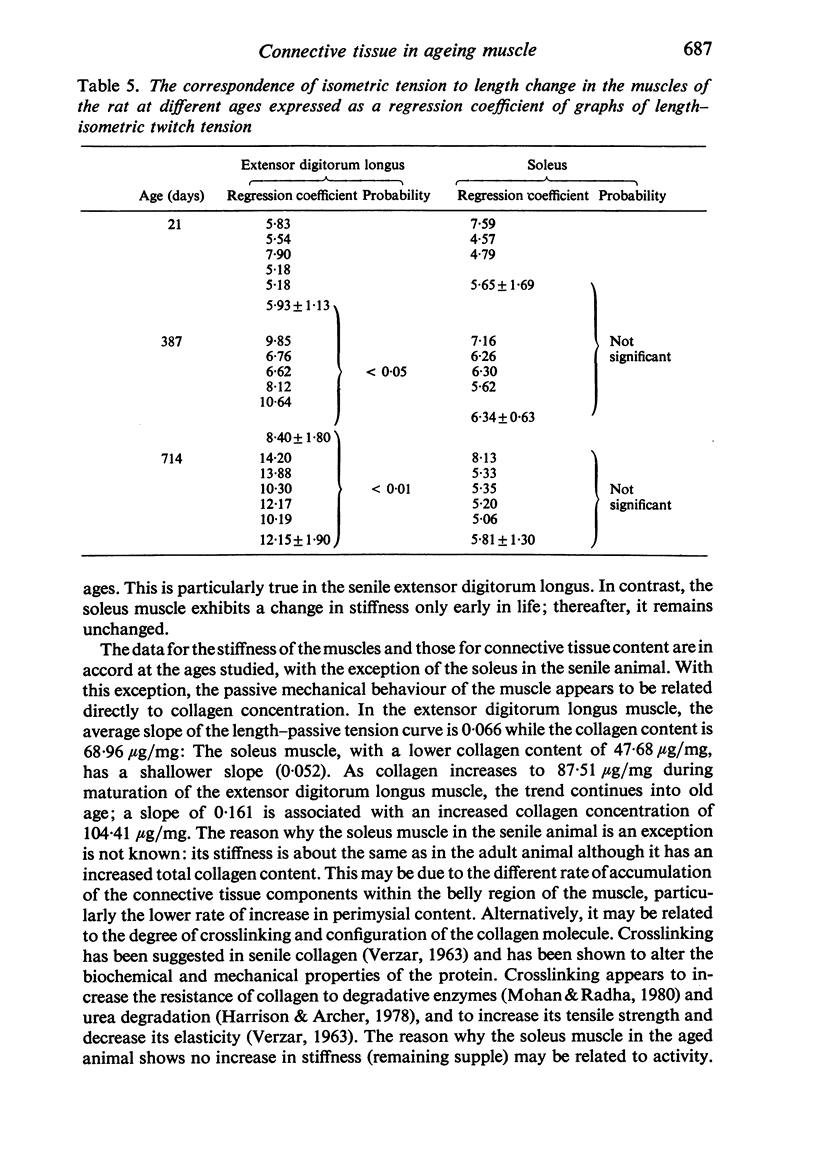
Selective staining of the connective tissue and image analysis showed that in the extensor digitorum longus and soleus muscles of the rat there was an increase in the thickness of the endomysium in both early growth and senility. The perimysium thickness was more or less constant throughout life except in senility when the concentration of this component also increased. The stiffness (length-passive tension) of these muscles was found to increase throughout life. Log transforms of the length-passive tension plots had particularly steep slopes in the senile extensor digitorum longus muscle. Except in the senile soleus muscle, the increase in stiffness was closely correlated with the increase in endomysium and perimysium and with total muscle collagen (as measured biochemically) with age. The relationship between the initial length and the active tension in the extensor digitorum longus muscle changed with age. The older muscles showed a greater decline in tension for each decrement of length resulting from the increased development of the connective tissue.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHIAKULAS J. J., PAULY J. E. A STUDY OF POSTNATAL GROWTH OF SKELETAL MUSCLE IN THE RAT. Anat Rec. 1965 May;152:55–61. doi: 10.1002/ar.1091520107. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., McComas A. J., Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973 Apr;36(2):174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYFUS J. C., SCHAPIRA G., BOURLIERE F. Modifications chimiques du muscle au cours de la sénescence chez le rat. C R Seances Soc Biol Fil. 1954 Jun;148(11-12):1065–1067. [PubMed] [Google Scholar]
- GRANT R. A. ESTIMATION OF HYDROXYPROLINE BY THE AUTOANALYSER. J Clin Pathol. 1964 Nov;17:685–686. doi: 10.1136/jcp.17.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Archer J. R. Measurement of changes in mouse tail collagen with age: temperature dependence and procedural details. Exp Gerontol. 1978;13(1-2):75–82. doi: 10.1016/0531-5565(78)90033-5. [DOI] [PubMed] [Google Scholar]
- Inokuchi S., Ishikawa H., Iwamoto S., Kimura T. Age-related changes in the histological composition of the rectus abdominis muscle of the adult human. Hum Biol. 1975 May;47(2):231–249. [PubMed] [Google Scholar]
- LANSING A. I. Some physiological aspects of ageing. Physiol Rev. 1951 Jul;31(3):274–284. doi: 10.1152/physrev.1951.31.3.274. [DOI] [PubMed] [Google Scholar]
- MacLennan W. J., Hall M. R., Timothy J. I., Robinson M. Is weakness in old age due to muscle wasting? Age Ageing. 1980 Aug;9(3):188–192. doi: 10.1093/ageing/9.3.188. [DOI] [PubMed] [Google Scholar]
- Mohan S., Radha E. Age-related changes in rat muscle collagen. Gerontology. 1980;26(2):61–67. doi: 10.1159/000212396. [DOI] [PubMed] [Google Scholar]
- SWEAT F., PUCHTLER H., ROSENTHAL S. I. SIRIUS RED F3BA AS A STAIN FOR CONNECTIVE TISSUE. Arch Pathol. 1964 Jul;78:69–72. [PubMed] [Google Scholar]
- Tomanek R. J., Lund D. D. Degeneration of different types of skeletal muscle fibres. I. Denervation. J Anat. 1973 Dec;116(Pt 3):395–407. [PMC free article] [PubMed] [Google Scholar]
- VERZAR F. The aging of collagen. Sci Am. 1963 Apr;208:104–114. [PubMed] [Google Scholar]



