Abstract
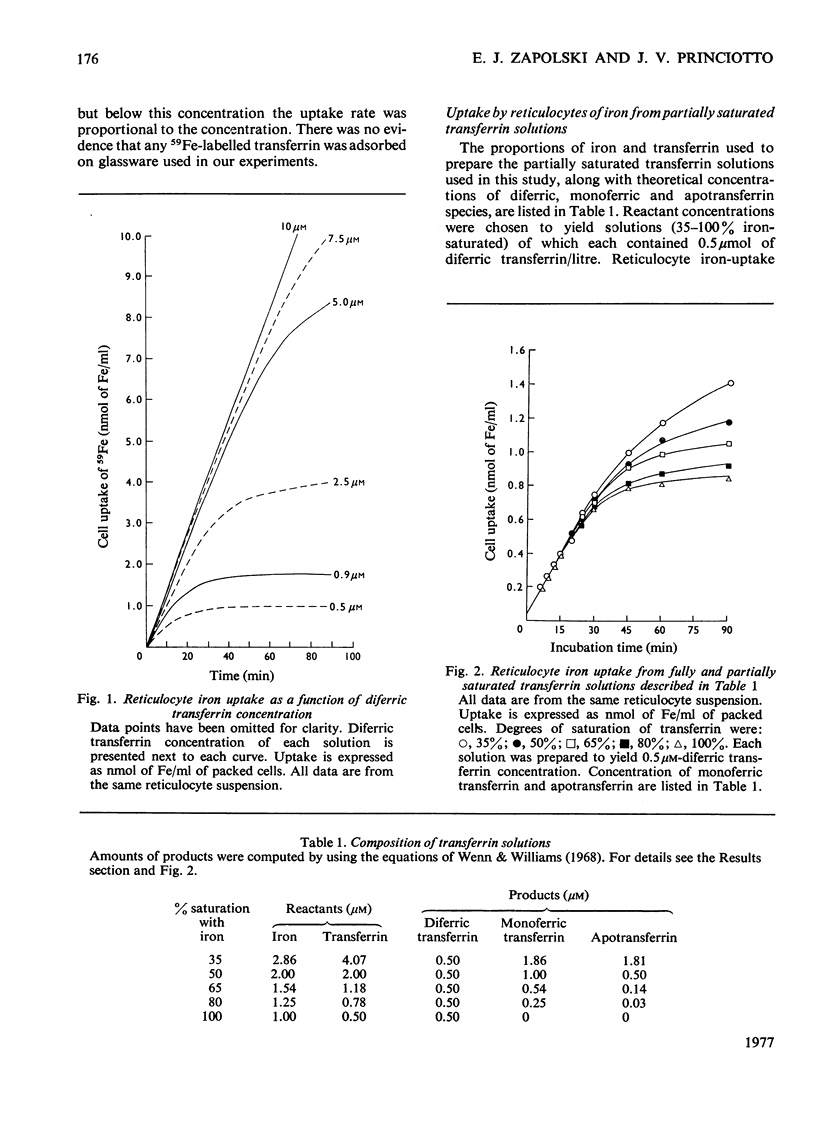
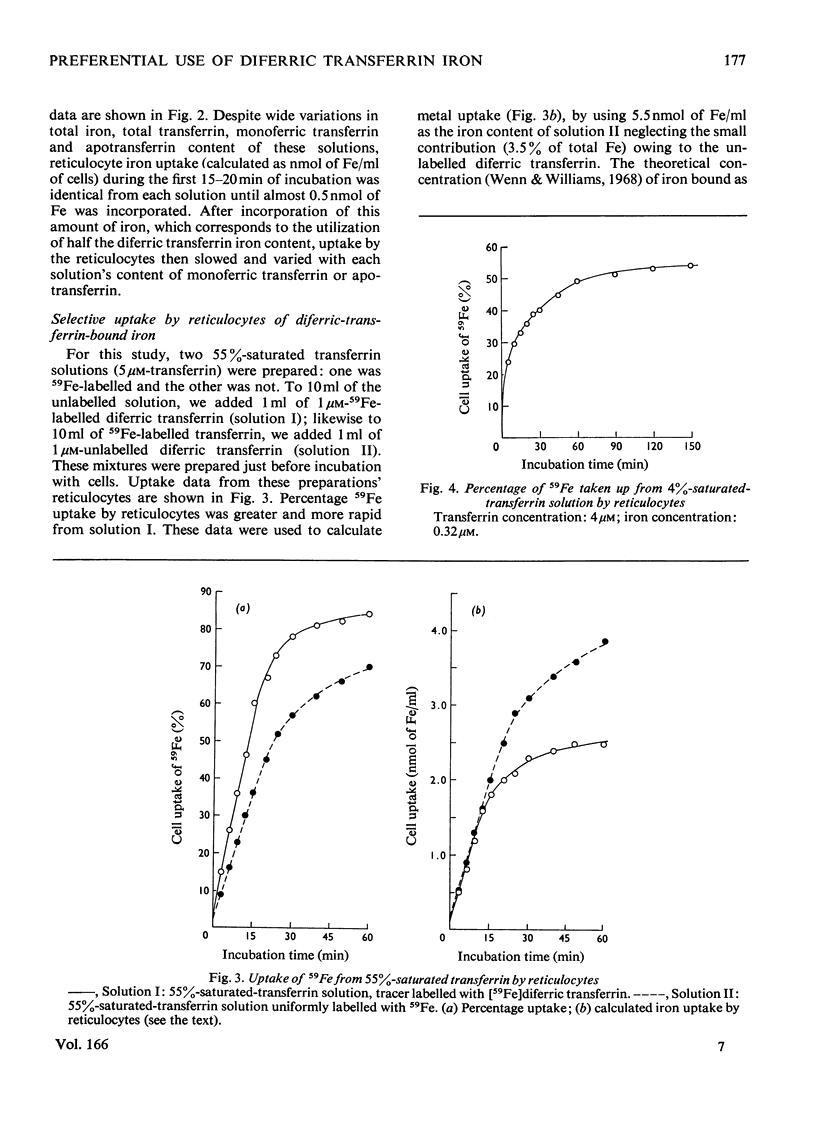
59Fe uptake by rabbit reticulocytes from human transferrin-bound iron was studied by using transferrin solutions (35, 50, 65, 80 and 100% saturated with iron) whose only common characteristic was their content of diferric transferrin. During the early incubation period, 59Fe uptake from each preparation by reticulocytes was identical despite wide variations in amounts of total transferrin, total iron, monoferric transferrin and apotransferrin in solution. During the later phase of incubation, rate of uptake declined and was proportional to each solution's monoferric transferrin content. Uptake was also studied in a comparative experiment which used two identical, partially saturated transferrin preparations, one uniformly 59Fe-labelled and the other tracer-labelled with [59Fe]diferric transferrin. In both experiments, iron uptake by reticulocytes corresponded to utilization of a ferric ion from diferric transferrin before utilization of iron from monoferric transferrin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Leibman A. The role of the anion-binding site of transferrin in its interaction with the reticulocyte. Biochim Biophys Acta. 1973 May 28;304(3):797–804. doi: 10.1016/0304-4165(73)90226-2. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A. The role of the anion-binding site of transferrin in its interaction with the reticulocyte. Biochim Biophys Acta. 1973 May 28;304(3):797–804. doi: 10.1016/0304-4165(73)90226-2. [DOI] [PubMed] [Google Scholar]
- Baker E., Morgan E. H. The kinetics of the interaction between rabbit transferrin and reticulocytes. Biochemistry. 1969 Mar;8(3):1133–1141. doi: 10.1021/bi00831a046. [DOI] [PubMed] [Google Scholar]
- Brown E. B., Okada S., Awai M., Chipman B. In vivo evidence for the functional heterogeneity of transferrin-bound iron. III. Studies of transferrin at high and low iron saturation. J Lab Clin Med. 1975 Oct;86(4):576–585. [PubMed] [Google Scholar]
- Egyed A. The significance of transferrin-bound bicarbonate in the uptake of iron by reticulocytes. Biochim Biophys Acta. 1973 May 28;304(3):805–813. doi: 10.1016/0304-4165(73)90227-4. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Function of transferrin. Nature. 1968 Jun 29;218(5148):1211–1214. doi: 10.1038/2181211a0. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Significance of the binding of iron by transferrin. Nature. 1967 Aug 5;215(5101):584–586. doi: 10.1038/215584a0. [DOI] [PubMed] [Google Scholar]
- Fletcher J. Variation in the availability of transferrin-bound iron for uptake by immature red cells. Clin Sci. 1969 Oct;37(2):273–297. [PubMed] [Google Scholar]
- Hahn D., Baviera B., Ganzoni A. M. Functional heterogeneity of the transport iron compartment I. In vivo radioiron clearance from high and low saturated transferrin. Acta Haematol. 1975;53(5):285–291. doi: 10.1159/000208194. [DOI] [PubMed] [Google Scholar]
- Hahn D. Functional behaviour of transferrin. Eur J Biochem. 1973 Apr;34(2):311–316. doi: 10.1111/j.1432-1033.1973.tb02760.x. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Functional equivalence of the two iron-binding sites of human transferrin. Nature. 1975 Oct 30;257(5529):821–823. doi: 10.1038/257821a0. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Iron-donating properties of transferrin. Biochemistry. 1975 Jan 28;14(2):262–268. doi: 10.1021/bi00673a011. [DOI] [PubMed] [Google Scholar]
- Hemmaplardh D., Kailis S. G., Morgan E. H. The effects of inhibitors of microtubule and microfilament function on transferrin and iron uptake by rabbit reticulocytes and bone marrow. Br J Haematol. 1974 Sep;28(1):53–65. doi: 10.1111/j.1365-2141.1974.tb06639.x. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., INMAN J. K., SIMMONS R. L., ALLEN D. W. Transfer of iron from serum iron-binding protein to human reticulocytes. J Clin Invest. 1959 Jan 1;38(1 Pt 1):161–185. doi: 10.1172/JCI103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., INMAN J. K., SIMMONS R. L., ALLEN D. W. Transfer of iron from serum iron-binding protein to human reticulocytes. J Clin Invest. 1959 Jan 1;38(1 Pt 1):161–185. doi: 10.1172/JCI103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. The effect of metal attachment to human apotransferrin on its binding to reticulocytes. Biochim Biophys Acta. 1969 Nov 11;194(1):25–33. doi: 10.1016/0005-2795(69)90175-5. [DOI] [PubMed] [Google Scholar]
- Lane R. S. Binding of transferrin and metal ions by suspensions of reticulocyte-rich rabbit blood. Biochim Biophys Acta. 1973 Aug 17;320(1):133–142. doi: 10.1016/0304-4165(73)90173-6. [DOI] [PubMed] [Google Scholar]
- Lane R. S. Differences between human Fe1-transferrin molecules. Br J Haematol. 1975 Mar;29(3):511–520. doi: 10.1111/j.1365-2141.1975.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Lane R. S. Iron uptake by rabbit reticulocytes. Br J Haematol. 1973 Mar;24(3):343–353. doi: 10.1111/j.1365-2141.1973.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Fluorescence and absorption studies of the binding of copper and iron to transferrin. J Biol Chem. 1969 Jul 10;244(13):3613–3617. [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- MORGAN E. H., LAURELL C. B. STUDIES ON THE EXCHANGE OF IRON BETWEEN TRANSFERRIN AND RETICULOCYTES. Br J Haematol. 1963 Oct;9:471–483. doi: 10.1111/j.1365-2141.1963.tb05471.x. [DOI] [PubMed] [Google Scholar]
- MORGAN E. H. THE INTERACTION BETWEEN RABBIT, HUMAN AND RAT TRANSFERRIN AND RETICULOCYTES. Br J Haematol. 1964 Oct;10:442–452. doi: 10.1111/j.1365-2141.1964.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Phillips J. L. "Specific" binding of radioiodinated transferrin to polypropylene culture tubes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):726–732. doi: 10.1016/0006-291x(76)90891-3. [DOI] [PubMed] [Google Scholar]
- Princiotto J. V., Zapolski E. J. Difference between the two iron-binding sites of transferrin. Nature. 1975 May 1;255(5503):87–88. doi: 10.1038/255087a0. [DOI] [PubMed] [Google Scholar]
- Princiotto J. V., Zapolski E. J. Functional heterogeneity and pH-dependent dissociation properties of human transferrin. Biochim Biophys Acta. 1976 May 28;428(3):766–771. doi: 10.1016/0304-4165(76)90207-5. [DOI] [PubMed] [Google Scholar]
- Wenn R. V., Williams J. The isoelectric fractionation of hen's-egg ovotransferrin. Biochem J. 1968 Jun;108(1):69–74. doi: 10.1042/bj1080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski E. J., Ganz R., Princiotto J. V. Biological specificity of the iron-binding sites of transferrin. Am J Physiol. 1974 Feb;226(2):334–339. doi: 10.1152/ajplegacy.1974.226.2.334. [DOI] [PubMed] [Google Scholar]
- Zapolski E. J., Princiotto J. V. Failure of rabbit reticulocytes to incorporate conalbumin or lactoferrin iron. Biochim Biophys Acta. 1976 Jan 14;421(1):80–86. doi: 10.1016/0304-4165(76)90171-9. [DOI] [PubMed] [Google Scholar]


