Abstract
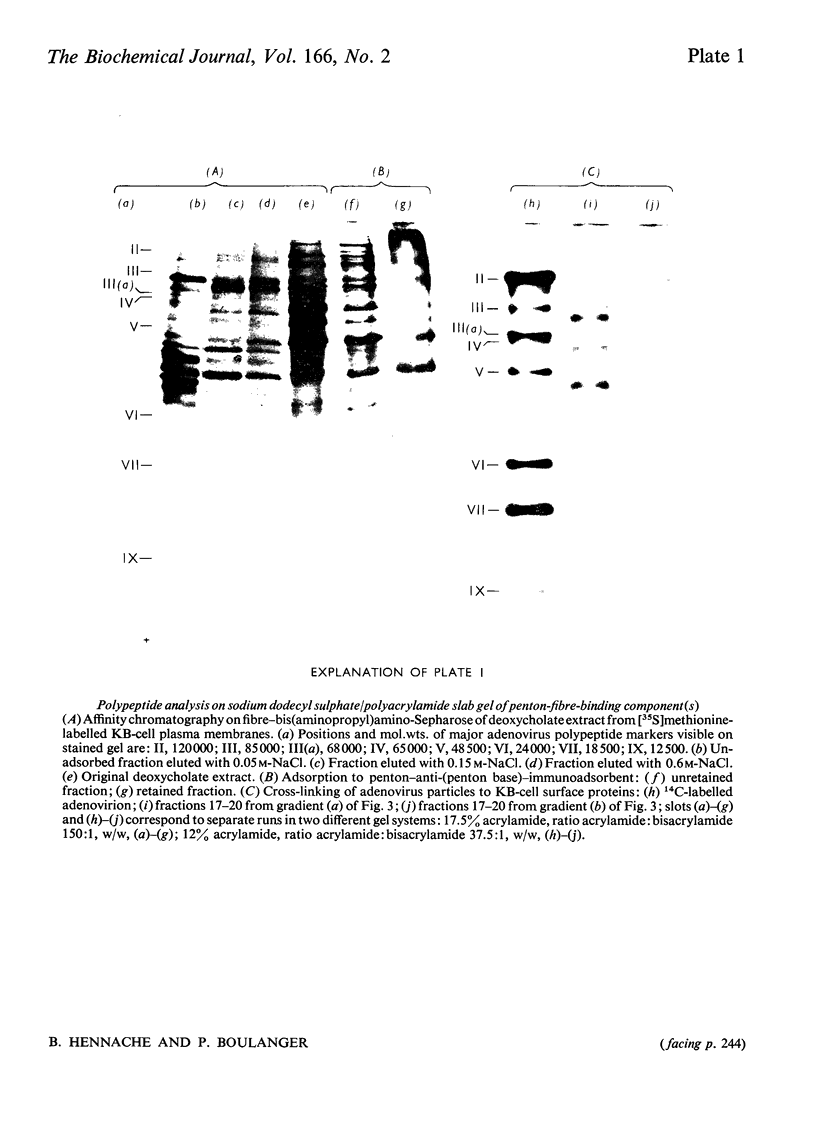
Three different approaches were used in an attempt to characterize the KB-cell receptor for adenovirus: affinity chromatography, immunoadsorption and cross-linking with a cleavable bifunctional reagent. The first system used an affinity gel consisting of adenovirus-fibre projection linked to Sepharose matrix by an intermediate bis(aminopropyl)amine arm, the amino groups of the fibre ligand being preserved by prior citraconylation. The second system consisted of adenovirus complete penton capsomere attached to anti-(penton base) antibody and cross-linked to polyacrylamide particles with glutaraldehyde. In this latter affinity model, the penton-fibre projection was appropriately oriented outwards, as in the virus particle. Both affinity systems permitted isolation from a KB-cell plasma-membrane extract of fibre-binding and penton-fibre-binding protein material, which inhibited adenovirus attachment. The penton–immunoadsorbent appeared more efficient and more specific than the affinity column of fibre–bis(aminopropyl)amino-Sepharose gel in specific activity of inhibition of adenovirus attachment. The third method consisted of reversibly cross-linking KB-cell receptor proteins with adenovirus particles by means of a cleavable di-imidoester and isolation of the complexes by sucrose-density-gradient centrifugation. Polypeptide analysis on sodium dodecyl sulphate/polyacrylamide gel of labelled KB-cell surface proteins, selected by the different procedures, showed that three major protein subunits of 78000, 42000 and 34000mol.wt. were common to the three selection systems. A possible model for the structure and function of the KB-cell receptor for adenovirus is discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Houdret N., Scharfman A., Lemay P. The role of surface sialic acid in adenovirus-cell adsorption. J Gen Virol. 1972 Sep;16(3):429–434. doi: 10.1099/0022-1317-16-3-429. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Puvion F. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur J Biochem. 1973 Nov 1;39(1):37–42. doi: 10.1111/j.1432-1033.1973.tb03100.x. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Hughes R. C. Solubilization and fractionation of glycoproteins and glycolipids of KB cell membranes. Biochem J. 1974 Jun;140(3):469–478. doi: 10.1042/bj1400469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters T. D., Hughes R. C. Surface labelling for human tumour KB cells. Iodination and fractionation of membrane glycoproteins. Biochem J. 1975 Jul;150(1):59–69. doi: 10.1042/bj1500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973 Dec;56(2):465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Boulanger P. A. Quantitation of adenovirus soluble antigens by crossed immunoelectrophoresis: application to serological characterization of mutants. Intervirology. 1975;5(3-4):162–172. doi: 10.1159/000149893. [DOI] [PubMed] [Google Scholar]
- Meager A., Butters T. D., Mautner V., Hughes R. C. Interactions of KB-cell glycoproteins with an adenovirus capsid protein. Eur J Biochem. 1976 Jan 15;61(2):345–353. doi: 10.1111/j.1432-1033.1976.tb10028.x. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Hartzell R. W., Rubin B. A. Partial characterization of the complementary sites involved in the reaction between adenovirus type 7 and erythrocyte receptors. Virology. 1970 Nov;42(3):789–793. doi: 10.1016/0042-6822(70)90326-0. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Philipson L., Höglund S. Structural proteins of adenoviruses. II. Purification and characterization of the adenovirus type 2 fiber antigen. Virology. 1968 Jun;35(2):204–215. doi: 10.1016/0042-6822(68)90261-4. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U. Structure and function of virion proteins of adenoviruses. Prog Exp Tumor Res. 1973;18:1–55. doi: 10.1159/000393160. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. W. Adenoviruses: the nature of the virion and of controlling factors in productive or abortive infection and tumorigenesis. Adv Virus Res. 1969;14:1–61. doi: 10.1016/s0065-3527(08)60556-4. [DOI] [PubMed] [Google Scholar]
- Singhal R. P., Atassi M. Z. Immunochemistry of sperm whale of myoglobin. IX. Specific interaction of peptides obtained by cleavage at arginine peptide bonds. Biochemistry. 1971 May 11;10(10):1756–1762. doi: 10.1021/bi00786a004. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Polyacrylamide-protein immunoadsorbents prepared with glutaraldehyde. FEBS Lett. 1972 Jun 1;23(1):24–28. doi: 10.1016/0014-5793(72)80274-6. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Bollen A., Sun T. T., Hershey J. W., Sundberg J., Pierce L. R. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973 Aug 14;12(17):3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]