Abstract
Background
Transcatheter aortic valve implantation (TAVI) has shown similar or improved clinical outcomes compared with surgical aortic valve replacement (SAVR) in patients with symptomatic severe aortic stenosis at low risk for surgical mortality. This cost-utility analysis compared TAVI with SAPIEN 3 versus SAVR in symptomatic severe aortic stenosis patients at low risk of surgical mortality from the perspective of the Swedish healthcare system.
Methods
A published, two-stage, Markov-based cost-utility model that captured clinical outcomes from the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated according to Recommended Therapies (SWEDEHEART) registry (2018–2020) was adapted from the perspective of the Swedish healthcare system using local general population mortality, utility and costs data. The model had a lifetime horizon. Model outputs included changes in direct healthcare costs and health-related quality of life from using TAVI as compared with SAVR.
Results
TAVI with SAPIEN 3 resulted in lifetime costs per patient of 940,541 Swedish krona (SEK) and lifetime quality-adjusted life years (QALYs) per patient of 7.16, whilst SAVR resulted in lifetime costs and QALYs per patient of 821,380 SEK and 6.81 QALYs, respectively. Compared with SAVR, TAVI offered an incremental improvement of +0.35 QALY per patient at an increased cost of +119,161 SEK per patient over a lifetime horizon, resulting in an incremental cost-effectiveness ratio of 343,918 SEK per QALY gained.
Conclusion
TAVI with SAPIEN 3 is a cost-effective option versus SAVR for patients with symptomatic severe aortic stenosis at low risk for surgical mortality treated in the Swedish healthcare setting. These findings may inform policy decisions in Sweden for the management of this patient group.
Keywords: Transcatheter aortic valve implantation, surgical aortic valve replacement, cost-effectiveness, aortic stenosis, low risk
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as the treatment of choice over surgical aortic valve replacement (SAVR) for patients with severe symptomatic aortic stenosis (sSAS) at intermediate- and high-surgical risk (1–6). As technology has evolved, improvements in patients’ outcomes, quality of life and reduced complication rates have been reported, leading to TAVI also being used in low-risk patients (7).
The Placement of Aortic Transcatheter Valves (PARTNER) 3 trial was a multicentre, randomised controlled study, which demonstrated that TAVI, using the SAPIEN 3 valve, provided meaningful benefits in patients with sSAS, who were considered at low risk of surgical mortality compared with SAVR (8, 9). In addition to a significant reduction in the composite outcome of death, stroke or rehospitalisation at 1 and 2 years, TAVI was also associated with significantly lower rates of stroke and new-onset atrial fibrillation (AF), shorter index hospitalisation, higher functional status and improved quality of life at 30-days compared with SAVR (9). Furthermore, there were no significant differences between the groups in major vascular complications, new permanent pacemaker implantations or moderate or severe paravalvular regurgitation (8, 9). Finally, in the recently published 5-year results, event rates for death, stroke or rehospitalisation remained low and very similar to the surgical arm (10). This expanding evidence base allows TAVI to be considered for patients at high (11), intermediate (12) and, increasingly, low risk of surgical mortality (13).
The updated 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines recommend TAVI in all patients aged ≥75 years with sSAS regardless of surgical risk status (recommendation class IA), providing there are no clinical or anatomical barriers and suitable femoral access (14).
Given the adoption of TAVI into various treatment guidelines, the demonstrated clinical benefits for patients, the high numbers of procedures performed to date and the potential for further adoption with the move towards use in lower risk patients, it is important to evaluate the implications on healthcare resources of potentially expanded TAVI use to inform clinicians and policymakers. To date, cost-effectiveness analyses have been published for France (15), Italy (16), Spain (17), Germany (18) and Belgium (19) that showed economic dominance or cost-effectiveness of TAVI with SAPIEN 3 versus SAVR in patients with sSAS at low surgical risk. These analyses used most of the clinical outcomes from the PARTNER 3 trial (9) and not from complete national clinical registries. Therefore, we conducted a cost-utility analysis using data from the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated according to Recommended Therapies (SWEDEHEART) registry (6) in combination with cost data from Sweden to adapt this Markov model and assess the value of TAVI with SAPIEN 3 versus SAVR in Swedish patients with sSAS at low risk of surgical mortality.
Methods
Model structure
A cost-utility analysis was conducted using methodology validated in previously published studies (15–19) to estimate changes in both direct healthcare costs and health-related quality of life with the use of TAVI with SAPIEN 3 versus isolated SAVR with any biological valve in sSAS patients at low risk of surgical mortality (as defined by a EuroSCORE II score <4%) from the perspective of the Swedish national healthcare system. For both TAVI and SAVR, patients between 70 and 80 years of age who received a registered procedure between 2018 and 2020 were included. Ethical approval of using Swedish registry data was granted (dnr 2017/455).
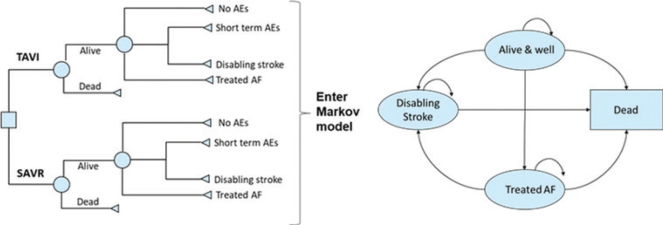
A two-stage model structure was used to form the basis of the cost-utility analysis, details of which have been published previously (15). In brief, early adverse events (AEs) linked to each procedure were captured primarily from the SWEDEHEART registry (6) and entered into a decision tree (Figure 1a) (15). Data on three events were not available from the registry and were obtained from the PARTNER 3 trial (9). The decision tree outcomes were subsequently fed into a Markov model, which included four distinct health states (‘Alive and well’, ‘Atrial fibrillation’, ‘Disabling stroke’ and ‘Dead’), to capture longer-term patient outcomes post-TAVI or SAVR intervention (Figure 1b) (15). Markov models are the most common model type used for economic evaluations of healthcare interventions (quantifying the costs and health benefits associated with an intervention) (20). These models are cohort-based and capture disease progression via transitions between mutually exclusive health states over discrete time periods (cycles). Their main strength is that they are simple and extremely adaptable, which is shown by their use across many clinical indications (21). For those unfamiliar with economic evaluation, a glossary of key terms can be found here: www.yhec.co.uk/glossary/ and a reader’s guide to facilitate reading and interpretation is detailed in Abbott et al. (22) The model structure was validated for the Swedish context by the authors, based on their clinical and health-economic expertise.
Figure 1.
The cost-effectiveness model had two stages: (A) early AEs from the PARTNER 3 trial were captured in a decision tree, which fed into (B) a Markov model that captured longer-term outcomes of patients, with four distinct health states.a
Reproduced from Gilard M, et al. (15); https://doi.org/10.1016/j.jval.2021.10.003 under the terms of the creative commons licence (Creative Commons Attribution License (CC BY)).
a‘Alive and well’: patients have undergone the procedure and survived with only short-term or no AEs; patients in this health state can transition to ‘disabling stroke’, ‘AF’ or ‘dead’ at any point during the model time horizon. ‘Treated AF’: patients have undergone the procedure and survived but developed AF requiring specific treatment; this can either occur within the first 30 days or during the rest of the time horizon of the model, and patients in this health state can transition to ‘disabling stroke’ or ‘dead’ at any point during the model time horizon. ‘Disabling stroke’: patients have undergone the procedure and survived but had a disabling stroke; this can either occur within the first 30 days or during the rest of the time horizon of the model, and patients in this health state can only transition into the ‘dead’ state at any point during the model time horizon. ‘Dead’: this is the absorbing state in the model: all patients in the model are at risk of dying due to general all-cause mortality; patients with treated AF and stroke are at an increased risk of dying.
AE, adverse event; AF, atrial fibrillation; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Considering that sSAS requires life-long valve replacement, a lifetime horizon was selected to reflect all possible consequences to individuals with sSAS over their lifetime, and a discounting factor per year of 3% was applied for both future costs and benefits (23).
Health-related quality of life was included in the analysis using quality-adjusted life years (QALYs) as an endpoint with EuroQol-5 Dimensions (EQ-5D) questionnaire utility decrements for the AF and stroke health states taken from the published literature and adjusted for age and population norms using Burström et al. (24).
Model inputs
Clinical events
In the base case, data on clinical events within 1 month after the procedure were extracted for the SAPIEN 3 (from a total of 603 procedures, 204 involved patients aged 70–80 years and logistic EuroSCORE <4% were included) and the SAVR (n = 1,375) groups from the SWEDEHEART sub-registers for TAVI and SAVR, the Swedish Transcatheter Cardiac Intervention Registry and the Swedish Cardiac Surgery Registry, respectively. With all Swedish centres (TAVI = 8 centres; SAVR = 8 centres) contributing data to the registries, they have complete national coverage. The registries hold information on clinical patient characteristics, echocardiographic findings, procedures and periprocedural outcomes. Details on the registries have been described in earlier publications (25, 26). For a few outcomes not covered by the registries, data were collected from the National Patient Registry using the International Classification of Disease – 10th revision (ICD-10) codes. Stroke was defined as an ICD-10 code starting with I63, and, thus, it was not possible to grade the severity of disability. For the remaining four outcomes (one for the TAVI arm and three for the SAVR arm), where data were not available in either of the registries, PARTNER 3 (9) outcomes were used (Supplementary Table 1). Data from the registry were also used to estimate the monthly probability of transitioning from ‘Alive and well’ to ‘Treated AF’, from ‘Alive and well’ to ‘Disabling stroke’ and from ‘Treated AF’ to ‘Disabling stroke’. Furthermore, data on the probability of aortic reintervention due to valve deterioration as well as rehospitalisation rates up to 3 years (post which rates were assumed to remain constant over the remaining time horizon of the model) were also obtained from the registry. For the TAVI arm, data were additionally extracted for the pooled SAPIEN 3 and SAPIEN 3 ultra sample (n = 373) for use within a scenario analysis.
The annual mortality risk for ‘alive and well’ by gender was obtained from National Life Tables Sweden (2022) (27) (Supplementary Table 2). The relative risk of death (hazard ratio [HR]) associated with being in the treated AF or disabling stroke health states was obtained from Odutayo et al. (28) and Dennis et al. (29), whilst the relative risk of undergoing an aortic reintervention was sourced from PARTNER 3 (9).
Cost inputs
The cost perspective was based on information from the Swedish Diagnosis Related Group (DRG) tariffs and from published literature (Table 1). Costs were indexed to 2022 unless otherwise stated. For the base case, costs associated with TAVI and SAVR procedures were estimated from the DRG tariffs and published literature (Table 2). Costs for rehabilitation following TAVI and SAVR were based on the Swedish DRG tariffs and from published literature, indexed to 2022. Costs associated with health states, 30-days AEs (myocardial infarction, non-disabling stroke, transient ischaemic attack, bleeding and acute kidney injury), intercurrent events (myocardial infarction, transient ischaemic attack and bleeding), rehospitalisation and pacemaker implantation were estimated from DRGs and/or published literature (Tables 1 and 2). Reintervention costs were assumed to be equal to the combined costs of the initial procedure and rehabilitation associated with the procedure.
Table 1.
Costs associated with TAVI and SAVR (procedure, complications and long-term).
| Unit cost components | TAVI with SAPIEN 3 | SAVR | Source |
|---|---|---|---|
| Procedure | 302,329 SEK | 244,750 SEK | Swedish Health Care Regions (2022) – Average of DRGs E04E and E03N across price lists for all regions |
| Acute post-operative complications | |||
| Reintervention | 302,329 SEK | Assumed equal to cost of initial procedure plus rehabilitation associated with procedure | |
| Associated to health states | |||
| Treated AF – Month 1 | 55,561 SEK | Hallberg et al. (30) | |
| Treated AF ≥ Month 2 | 2,111 SEK | ||
| Disabling stroke – Month 1 | 106,165 SEK | Lanitis et al. (31) | |
| Disabling stroke ≥ Month 2 | 4,284 SEK | ||
| Caregiver for disabling stroke – Month 1 | 69,491 SEK | ||
| Caregiver for disabling stroke ≥ Month 2 | 37,970 SEK | ||
| Alive and well – Year 1 | 8,215 SEK | Swedish Health Care Regions (2022) – Average of ‘besök kardiolog’ across price lists for all regions (assumed 2 visits in Year 1 and 1 visit Year 2+) | |
| Alive and well – Year 2+ | 4,108 SEK | ||
| Other costs considered | |||
| Pacemaker procedure | 77,451 SEK | Swedish Health Care Regions (2022) – Average of E26A, E26C and E26E across price lists for all regions | |
| Pacemaker complications (monthly) | 3,206 SEK | Swedish Health Care Regions (2022) – Average of X31O across price lists for all regions. | |
| Rehospitalisation | 61,340 SEK | Swedish Health Care Regions (2022) – Average of E47A, E47C and E47E across price lists for all regions | |
AF, atrial fibrillation; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table 2.
Breakdown of TAVI and SAVR procedure costs.
| TAVI with SAPIEN 3 | SAVR | Source | |
|---|---|---|---|
| Procedure | 301,079 SEK | 244,301 SEK | Swedish Health Care Regions (2022) – Average of E03N (TAVI) and E04E (SAVR) across price lists for all regions |
| Rehabilitation | 3,973 SEK | 3,973 SEK | Wittboldt et al. (32) |
| Rehabilitation rate | 2.8% | 11.3% | PARTNER 3 (9) |
| Rehabilitation | 111 SEK | 449 SEK | |
| Pacemaker insertion | 77,451 SEK | 77,451 SEK | Swedish Health Care Regions (2022) – Average of E26A, E26C and E26E across price lists for all regions |
| Permanent pacemaker insertion rate | 1.5% | 0% | SWEDEHEART registry (2018–2020) (6) |
| Pacemaker insertion | 1,139 SEK | 0 SEK | |
| Total procedure costs | 302,329 SEK | 244,750 SEK |
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Utilities
Age-adjusted population utility norms were used. An EQ-5D index value was used to document the population utility scores by age group (24) specific to the Swedish population. The small number of events in the PARTNER 3 trial meant that utility decrements were estimated using the published literature. Disabling stroke disutility was estimated based on a published study (31), whilst disutility for AF was estimated from Ref. (33). Disutility data were not included for intercurrent events in order to avoid a risk of double counting with the health state utilities applied to patients in the ‘treated AF’ and ‘disabling stroke’ states.
Model outputs
Details of the model outputs and assumptions have been published previously (15). All analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, WA). The model generated total per-patient costs and QALYs for each intervention over the patients’ lifetime, and an incremental cost-effectiveness ratio (ICER) for TAVI with SAPIEN 3 versus SAVR in Swedish low-risk patients with sSAS. In Sweden, there is no official willingness-to-pay (WTP) threshold. However, for the Tandvårds- och läkemedelsförmånsverket or The Swedish Dental and Pharmaceutical Benefits Agency that makes reimbursement decisions for prescription drugs as well as reviews cost-effectiveness analysis for the Council on New Therapies, the unofficial WTP threshold is 1,000,000 SEK for treatments of very high severity diseases (34). This is the WTP threshold assumed in this study, and it is based on published literature.
Sensitivity and scenario analyses
To evaluate uncertainty, univariate deterministic sensitivity analyses (DSAs) were performed by varying inputs using confidence intervals and ranges from the literature when available, and plausible ranges when data were unavailable (Supplementary Table 3). All parameters were changed, and the impact on the results explored. Overall parameter uncertainty was addressed using a probabilistic sensitivity analysis (PSA). Probability distributions for all input parameters were specified, and 1,000 Monte Carlo simulations were run using random draws of all parameters from within their assigned distributions (Supplementary Table 4). In addition, 13 scenario analyses were run to test a number of assumptions on the clinical inputs, time horizon, the discount factor and the survival data. Of these, three scenarios specially addressed the issues of development of postoperative AF and the use of non-vitamin K antagonist anticoagulants in SAVR patients. Scenario 14 considered a relative risk of mortality with treated AF of 1 to remove any survival benefit with TAVI. Scenario 15 assumed zero monthly costs associated with the health state of AF to avoid attributing a cost to SAVR not witnessed in clinical practice. Finally, Scenario 16 used late postoperative AF data and a HR of 5.1 for mortality associated with AF from PARTNER 3 (35).
Results
Base case
Compared with SAVR, TAVI is estimated to offer an incremental health benefit of +0.35 QALYs per patient at an incremental cost of +119,161 SEK per patient over a lifetime horizon. This represents an ICER of 343,918 SEK per QALY gained (Table 3). Overall, TAVI with SAPIEN 3 resulted in lifetime costs per patient of 940,541 SEK and lifetime QALY per patient of 7.16, and for SAVR, these values were 821,380 SEK and 6.81 QALYs, respectively (Table 3).
Table 3.
Base case results with acute and lifetime costs.
| Summary results | TAVI with SAPIEN 3 | SAVR | Incremental |
|---|---|---|---|
| Cost per patient | 940,541 SEK | 821,380 SEK | 119,161 SEK |
| Life year gained (undiscounted) | 11.79 | 11.36 | 0.43 |
| Median survival (years) | 13.75 | 12.00 | 1.75 |
| QALYs per patient | 7.16 | 6.81 | 0.35 |
| Incremental cost effectiveness ratio (ICER) | 343,918 SEK | ||
| Incremental net monetary benefit (NMB) | 277,320 SEK | ||
| Incremental net health benefit (NHB) | 0.23 | ||
| Acute phase cost (first hospitalisation and rehabilitation) | |||
| Index hospitalisation | 302,218 SEK | 244,301 SEK | 57,917 SEK |
| Rehabilitation * | 111 SEK | 449 SEK | −338 SEK |
| Acute phase costs | 302,329 SEK | 244,750 SEK | 57,579 SEK |
| Additional costs at 1 year | |||
| MI | 0 SEK | 525 SEK | −525 SEK |
| Costs of pacemaker complications | 500 SEK | 0 SEK | 500 SEK |
| Costs of hospitalizations | 30,130 SEK | 40,427 SEK | −10,297 SEK |
| Re-intervention costs | 192 SEK | 0 SEK | 192 SEK |
| Alive & well health state costs | 91,842 SEK | 73,438 SEK | 18,404 SEK |
| Treated AF health state costs | 2,195 SEK | 16,975 SEK | −14,780 SEK |
| Disabling stroke costs | 10,347 SEK | 9,393 SEK | 955 SEK |
| Death costs | 774 SEK | 932 SEK | −158 SEK |
| Total costs at 1 year | 438,310 SEK | 386,441 SEK | 51,869 SEK |
| Additional lifetime costs | |||
| Costs of pacemaker complications | 4,863 SEK | 0 SEK | 4,863 SEK |
| Costs of hospitalizations | 9,826 SEK | 4,019 SEK | 5,807 SEK |
| Re-intervention costs | 1,783 SEK | 1,722 SEK | 61 SEK |
| Alive and well health state costs | 406,043 SEK | 323,391 SEK | 82,652 SEK |
| Treated AF health state costs | 6,337 SEK | 41,784 SEK | −35,445 SEK |
| Disabling stroke costs | 56,770 SEK | 47,180 SEK | 9,592 SEK |
| Death costs | 16,609 SEK | 16,666 SEK | −57 SEK |
| Additional lifetime costs | 502,232 SEK | 434,749 SEK | 67,483 SEK |
| Total lifetime costs | 940,541 SEK | 821,380 SEK | 119,161 SEK |
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; AF, atrial fibrillation; MI, myocardial infarction; QALY, quality-adjusted life years.
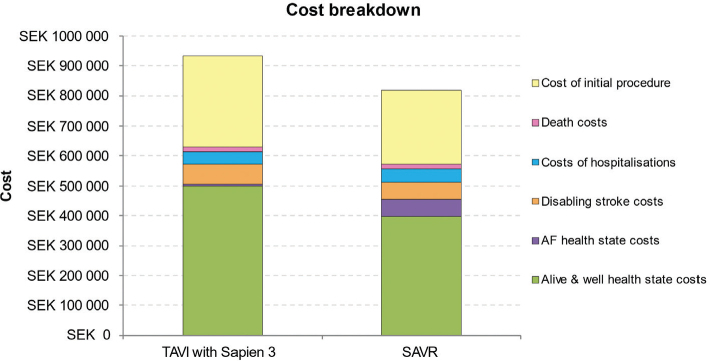
A detailed breakdown of costs revealed higher acute phase costs, alive and well health state costs, and disabling stroke costs for TAVI with SAPIEN 3 versus SAVR, but lower costs for treated AF and hospitalisation (Table 3; Figure 2).
Figure 2.
Cost breakdown for TAVI with SAPIEN 3 and for SAVR.
AF, atrial fibrillation; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Sensitivity analyses
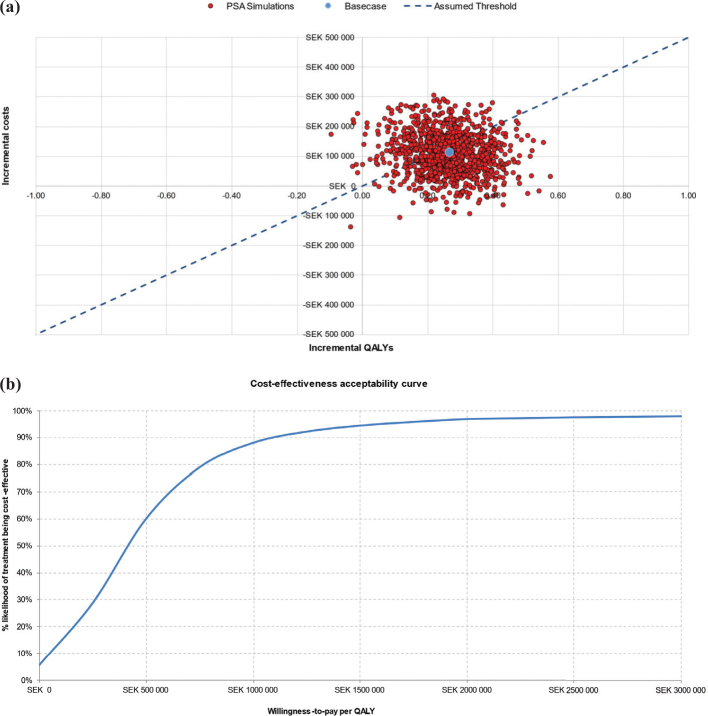
The findings of the PSA corroborate those of the base case analysis. At the assumed Swedish WTP threshold of 1,000,000 SEK/QALY, TAVI with SAPIEN 3 had an 88% probability of being cost-effective (Figure 3b). Assuming a more conservative cost-effectiveness threshold of 500,000 SEK/QALY, there is still a 50% likelihood of TAVI with SAPIEN 3 being cost-effective compared to SAVR (Figure 3a).
Figure 3.
Probabilistic sensitivity analysis: (a) cost-effectiveness scatter plot and (b) cost-effectiveness acceptability curve.
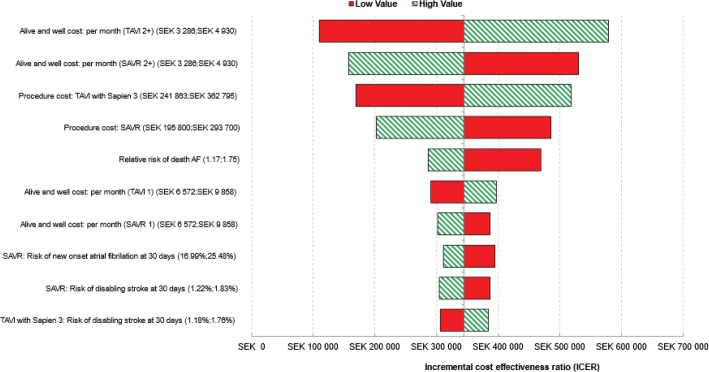
Univariate DSA demonstrated that TAVI with SAPIEN 3 remained cost-effective, compared with SAVR, regardless of plausible changes in individual model parameters (Figure 4). The model was most sensitive to ‘alive and well’ cost per month for both TAVI with SAPIEN 3 and with SAVR, procedure costs of TAVI with SAPIEN 3, the relative risk of death from AF, and procedure costs of SAVR (Figure 4).
Figure 4.
Tornado diagram showing the 10 parameters with greatest influence on the model (deterministic sensitivity analysis).
AF, atrial fibrillation; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
In the aforementioned Tornado plot, each horizontal bar summarises one univariate sensitivity analysis and represents the change in the ICER around the ‘base case’ value of a model parameter that is varied between two plausible extreme values (labelled ‘low’ and ‘high’). Corresponding to each horizontal bar, the varied model parameter can be seen in the y-axis with the ‘low’ and the ‘high’ values of this specific parameter reported within the brackets.
The results from the various scenario analyses demonstrated the comparative robustness of the model reported and are presented in Table 4.
Table 4.
Scenario analyses results.
| Scenarios | Incremental costs (TAVI vs SAVR) | Incremental QALYs (TAVI vs SAVR) | ICER (Incremental cost effectiveness ratio) | |
|---|---|---|---|---|
| Base case | 119,161 SEK | 0.35 | 343,918 SEK/QALY | |
| Scenario 1 | Survival data from PARTNER 3 | 180,021 SEK | 0.97 | 184,906 SEK/QALY |
| Scenario 2 | No survival benefit with TAVI | 116,597 SEK | 0.18 | 661,100 SEK/QALY |
| Scenario 3 | Including AE costs within 30 days | 108,149 SEK | 0.35 | 312,134 SEK/QALY |
| Scenario 4 | Time horizon = 5 years | 80,356 SEK | 0.09 | 829,043 SEK/QALY |
| Scenario 5 | Time horizon = 10 years | 100,791 SEK | 0.19 | 514,643 SEK/QALY |
| Scenario 6 | Time horizon = 15 years | 112,689 SEK | 0.28 | 399,557 SEK/QALY |
| Scenario 8 | Time horizon = 20 years | 117,751 SEK | 0.33 | 355,709 SEK/QALY |
| Scenario 9 | Time horizon = 30 years | 119,148 SEK | 0.35 | 344,026 SEK/QALY |
| Scenario 10 | Clinical inputs from SWEDEHEART registry data (2018–2020) for SAPIEN 3 and SAPIEN 3 ultra pooled sample | 125,253 SEK | 0.40 | 314,435 SEK/QALY |
| Scenario 11 | Costs and outcomes both discounted at 0 | 136,754 SEK | 0.46 | 295,216 SEK/QALY |
| Scenario 12 | Costs and outcomes both discounted at 5% | 110,379 SEK | 0.29 | 378,586 SEK/QALY |
| Scenario 13 | Costs discounted at 3% and outcomes at 0% | 110,379 SEK | 0.46 | 238,278 SEK/QALY |
| Scenario 14 | RR of death with treated AF= 1 | 112,625 SEK | 0.174 | 646,609 SEK/QALY |
| Scenario 15 | Monthly costs associated with treated AF health state= 0 | 169,387 SEK | 0.346 | 488,878 SEK/QALY |
| Scenario 16 | Using late POAF data from PARTNER 3 | 91,073 SEK | 0.293 | 310,670 SEK/QALY |
| Scenario 17 | Using 2022 values from KPP database for TAVI (277,327 SEK) and SAVR (243,462 SEK) | 96,243 SEK | 0.346 | 277,774 SEK/QALY |
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; AF, atrial fibrillation; POAF, postoperative atrial fibrillation; RR, relative risk.
Discussion
This analysis using real-world clinical outcomes from Sweden indicates that TAVI with SAPIEN 3 is expected to be a cost-effective valve replacement alternative versus SAVR for Swedish low surgical risk sSAS patients. The ICER of 343,918 SEK per QALY gained is within the assumed WTP threshold of 1,000,000 SEK per QALY. The incremental QALY gain of +0.35 is also within the range of those reported in previously published cost-effectiveness evaluations of TAVI and of pharmaceutical interventions in cardiology (36–38). Sensitivity analyses were used to assess uncertainty, and the results appeared robust.
A detailed breakdown of costs revealed higher acute phase costs, ‘alive and well’ health state costs, and disabling stroke costs for TAVI with SAPIEN 3 versus SAVR, but lower costs for treated AF and hospitalisation. Initial procedure costs for TAVI with SAPIEN 3 were higher than for SAVR in Sweden, which was also the case for Italy (16), Spain (17), Germany (18) and Belgium (19), whereas the initial cost for performing TAVI was lower than for SAVR in France (15). It is worth noting though that data from the registry show that the length of stay for TAVI is shorter as compared with SAVR (mean of 3 days for TAVI versus 8 days for SAVR). By implication, this should result in lower procedural costs for TAVI in comparison to that for SAVR. This corroborates our results from the base case that took a conservative approach of using DRG values for both the procedures.
These findings are consistent with others reported for TAVI with SAPIEN 3 versus SAVR in other countries in the European Union. For example, TAVI with SAPIEN 3 was shown to be dominant over SAVR in low-risk patients with sSAS in France (15, 39) and in Belgium (19) and was cost-effective over SAVR in Italy (16), Spain (17) and Germany (18). TAVI with SAPIEN 3 has also been reported to be dominant over SAVR in low-risk patients in Norway (40) and Ireland (41), and cost-effective compared with SAVR in Australia (42) and Canada (43).
Patients tend to prefer minimally invasive interventions as they are usually associated with less discomfort, lower risk of complications and/or rehospitalisation (44). The clinical benefits of TAVI with SAPIEN 3 in low-risk patients with sSAS have been established in PARTNER 3, which reported a lower risk of infection, fewer complications and shorter hospital stays, compared with SAVR, whilst also improving patients’ quality of life (8, 9).
From the healthcare provider perspective, TAVI provides efficiencies by reducing healthcare resource use, post-surgical complications and hospital stays. Reducing hospital stays allows higher patient intake capacity, which is an important consideration for health systems under high demand and with long waiting lists. TAVI presents benefits by reducing the recovery period to resuming normal activity that might not be accounted for in this analysis. Further indirect benefits may include a reduced need for caregiver support.
Limitations
This study comes with certain limitations. First, using data from registers comes with inherited weaknesses such as dependency on correct entries and on factors built into the registry. However, for both SWEDEHEART and the National Patient Register, high levels of data agreement have been reported (6, 45).
Second, the results cannot be generalised to all patients with aortic stenosis as the PARTNER 3 trial excluded patients with clinical frailty, bicuspid aortic valves or other unsuitable anatomical features that increased the risk of complications post-intervention. Caution must also be exercised when attempting to generalise the findings from this model to populations outside Sweden.
Third, in the base case, we made the conservative choice to use the average costs of DRG tariffs for the Swedish healthcare regions. This tariff is not specific to low-risk symptomatic severe aortic stenosis patients, and it is likely that it is overestimated for a low-risk TAVI patient treated under current practice. To account nevertheless for the uncertainty introduced with this choice, we used the alternative cost estimates for TAVI and SAVR procedures available from the Kostnad per patient (KPP) database (46) within a scenario analysis. It is worth noting that unlike the region’s price lists, which reflect current costs, the costs from the KPP database depend on the costing levels of the past years and reflect the costs of the entire intervention process. The results corroborate the findings of the base case.
Fourth, it is worth noting that there are inherent limitations in any cost-effectiveness analysis including: assumptions made in the presence of ‘best fit’ data or paucity of data; extrapolations modelled for time horizons beyond the scope of existing input data; and the potential for under- and over-estimations as a result of differences in healthcare systems and/or the intervention and treatment selection criteria within a specific system.
Finally, the cost-effectiveness findings from this study cannot be generalised to other TAVI devices beyond the SAPIEN 3 device.
Conclusion
In this analysis, leveraging real-world evidence from the SWEDEHEART registry, TAVI with SAPIEN 3 is likely to offer a cost-effective intervention versus SAVR in low-risk patients with sSAS in Sweden. These data may be helpful for informing clinicians, policymakers and healthcare budget holders in Sweden in their planning on how best to optimise both health outcomes and resource allocation in the management of patients with sSAS.
Supplementary Material
Acknowledgements
Editorial support was provided by Ian Wright of Zenith Healthcare Communications Ltd (Chester, United Kingdom), funded by Edwards Lifesciences.
Funding Statement
Funding This work was supported by Edwards Lifesciences Corporation. All authors had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Conflicts of interest
KN: No conflicts to report.
SJ: Proctoring fees from Medtronic.
OA: Speaker fees from Abbott, Medtronic and Meril. Proctoring fees from Abbott.
JB: No conflicts to report.
HB: Consultancy for Edwards Lifesciences and Boston Scientific.
PC: Employee of Edwards Lifesciences.
MG: Consulting honoraria for Boston Scientific and Medtronic.
HH: No conflicts to report.
NEN: No conflicts to report.
CM: Consulting compensation from Edwards Lifesciences for the Sweden specific costs, utilities and mortality inputs used in the model.
AS: Employee of Edwards Lifesciences.
MS: Proctoring/advisory board for Boston Scientific, Abbott Vascular, Edwards Lifesciences and Anteris.
TB: Compensation from Edwards Lifesciences for consulting on developing the model.
Data availability
The datasets used in this article are legally restricted because of Swedish privacy and secrecy laws and are therefore not publicly available.
Notes on contributors
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, provided critical revisions for important intellectual content at all stages in the development of this article and approved the final version submitted for publication. All authors take responsibility for the integrity of the work as a whole.
References
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 3.Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardio Thorac Surg. 2017;52:616–64. doi: 10.1093/ejcts/ezx324 [DOI] [PubMed] [Google Scholar]
- 4.Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382:799–809. doi: 10.1056/NEJMoa1910555 [DOI] [PubMed] [Google Scholar]
- 5.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–31. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 6.Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–21. doi: 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 7.Beyersdorf F, Vahanian A, Milojevic M, Praz F, Baldus S, Bauersachs J, et al. Erratum to: 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardio Thorac Surg. 2022;61:964. doi: 10.1093/ejcts/ezab557 [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–61. doi: 10.1016/j.jacc.2020.12.052 [DOI] [PubMed] [Google Scholar]
- 9.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 10.Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. 2023;389:1949–60. doi: 10.1056/NEJMoa2307447 [DOI] [PubMed] [Google Scholar]
- 11.Reynolds MR, Magnuson EA, Wang K, Lei Y, Vilain K, Walczak J, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102–9. doi: 10.1161/CIRCULATIONAHA.111.054072 [DOI] [PubMed] [Google Scholar]
- 12.Neyt M, Van Brabandt H, Devriese S, Van De Sande S. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open. 2012;2:e001032. doi: 10.1136/bmjopen-2012-001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thyregod HGH, Ihlemann N, Jorgensen TH, Nissen H, Kjeldsen BJ, Petursson P, et al. Five-year clinical and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation. 2019;139:2714–23. doi: 10.1161/CIRCULATIONAHA.118.036606 [DOI] [PubMed] [Google Scholar]
- 14.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 15.Gilard M, Eltchaninoff H, Iung B, Lefèvre T, Spaulding C, Dumonteil N, et al. Cost-effectiveness analysis of SAPIEN 3 transcatheter aortic valve implantation procedure compared with surgery in patients with severe aortic stenosis at low risk of surgical mortality in France. Value Health. 2022;25:605–13. doi: 10.1016/j.jval.2021.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Mennini FS, Meucci F, Pesarini G, Vandoni P, Lettino M, Sarmah A, et al. Cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in low surgical risk aortic stenosis patients. Int J Cardiol. 2022;357:26–32. doi: 10.1016/j.ijcard.2022.03.034 [DOI] [PubMed] [Google Scholar]
- 17.Pinar E, García de Lara J, Hurtado J, Robles M, Leithold G, Martí-Sánchez B, et al. Cost-effectiveness analysis of the SAPIEN 3 transcatheter aortic valve implant in patients with symptomatic severe aortic stenosis. Rev Esp Cardiol (English ed). 2022;75:325–33. doi: 10.1016/j.recesp.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Kuck KH, Leidl R, Frankenstein L, Wahlers T, Sarmah A, Candolfi P, et al. Cost-effectiveness of SAPIEN 3 transcatheter aortic valve implantation versus surgical aortic valve replacement in German severe aortic stenosis patients at low surgical mortality risk. Adv Ther. 2023;40:1031–46. doi: 10.1007/s12325-022-02392-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois C, Adriaenssens T, Annemans L, Bosmans J, Callebaut B, Candolfi P, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in severe aortic stenosis patients at low surgical mortality risk: a cost-effectiveness analysis in Belgium. Acta Cardiol. 2024;79:46–57. doi: 10.1080/00015385.2023.2282283 [DOI] [PubMed] [Google Scholar]
- 20.Dasari M, Cook J, Dhankhar P. Economic model and methodologies employed in recent nice single technology appraisal submissions. Value Health. 2019;22:S800–1. doi: 10.1016/j.jval.2019.09.2133 [DOI] [Google Scholar]
- 21.Komorowski M, Raffa J. Markov Models and Cost Effectiveness Analysis: Applications in Medical Research. In: Critical Data MIT , ed. Secondary analysis of electronic health records. Cham (CH: ): Springer International Publishing; 2016, pp. 351–67. [PubMed] [Google Scholar]
- 22.Abbott JH, Wilson R, Pryymachenko Y, Sharma S, Pathak A, Chua JYY. Economic evaluation: a reader’s guide to studies of cost‑effectiveness. Arch Physiother. 2022;12(1):28. doi: 10.1186/s40945-022-00154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.TVLR Guidelines . Tandvårds- och läkemedelsförmånsverkets allmänna råd. 2017. TLVAR 2017:1. Available from: https://www.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf [cited June 2024].
- 24.Burström K, Johannesson M, Diderichsen F. A comparison of individual and social time trade-off values for health states in the general population. Health Policy. 2006;76:359–70. doi: 10.1016/j.healthpol.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 25.Nilsson K, Buccheri S, Christersson C, Koul S, Nilsson J, Pétursson P, et al. Causes, pattern, predictors, and prognostic implications of new hospitalizations after transcatheter aortic valve implantation: a long-term nationwide observational study. Eur Heart J Qual Care Clin Outcomes. 2022;8:150–60. doi: 10.1093/ehjqcco/qcab026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternström L, et al. Validity of the Swedish Cardiac Surgery Registry. Interact Cardiovasc Thorac Surg. 2018;27:67–74. doi: 10.1093/icvts/ivy030 [DOI] [PubMed] [Google Scholar]
- 27.National life tables Sweden. 2022. Available from: https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101I/LivslangdEttariga/table/tableViewLayout1/ [cited September 2023].
- 28.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 29.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.STR.24.6.796 [DOI] [PubMed] [Google Scholar]
- 30.Hallberg S, Gandra SR, Fox KM, Mesterton J, Banefelt J, Johansson G, et al. Healthcare costs associated with cardiovascular events in patients with hyperlipidaemia or prior cardiovascular events: estimates from Swedish population-based register data. Eur J Health Econ. 2016;17:591–601. doi: 10.1007/s10198-015-0702-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanitis T, Kongnakorn T, Jacobson L, De Geer A. Cost-effectiveness of apixaban versus warfarin and aspirin in Sweden for stroke prevention in patients with atrial fibrillation. Thromb Res. 2014;134:278–87. doi: 10.1016/j.thromres.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 32.Wittboldt S, Leosdottir M, Ravn Fischer A, Ekman B, Bäck M. Exercise-based cardiac rehabilitation after acute myocardial infarction in Sweden – standards, costs, and adherence to European guidelines (The Perfect-CR study). Physiother Theory Pract. 2024;40(2):366–76. doi: 10.1080/09593985.2022.2114052 [DOI] [PubMed] [Google Scholar]
- 33.Sullivan PW, Slejko JF, Sculpher MJ. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31:800–4. doi: 10.1177/0272989X11401031 [DOI] [PubMed] [Google Scholar]
- 34.Siverskog J, Henriksson M. On the role of cost-effectiveness thresholds in healthcare priority setting. Int J Technol Assess Healthcare. 2021;37:e23. doi: 10.1017/S0266462321000015 [DOI] [PubMed] [Google Scholar]
- 35.Shahim B, Malaisrie SC, George I, Thourani VH, Biviano AB, Russo M, et al. Postoperative atrial fibrillation or flutter following transcatheter or surgical aortic valve replacement: PARTNER 3 trial. JACC Cardiovasc Interv. 2021;14:1565–74. doi: 10.1016/j.jcin.2021.05.026 [DOI] [PubMed] [Google Scholar]
- 36.Heathcote L, Srivastava T, Sarmah A, Kearns B, Sutton A, Candolfi P. A systematic review and statistical analysis of factors influencing the cost-effectiveness of transcatheter aortic valve implantation for symptomatic severe aortic stenosis. Clinicoecon Outcomes Res. 2023;15:459–75. doi: 10.2147/CEOR.S392566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polak TB, Cucchi DGJ, Darrow JJ, Versteegh MM. Incremental benefits of novel pharmaceuticals in the UK: a cross-sectional analysis of NICE technology appraisals from 2010 to 2020. BMJ Open. 2022;12(4):e058279. doi: 10.1136/bmjopen-2021-058279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisløff T, Hagen G, Hamidi V, Movik E, Klemp M, Olsen JA. Estimating QALY gains in applied studies: a review of cost-utility analyses published in 2010. Pharmacoeconomics. 2014;32:367–75. doi: 10.1007/s40273-014-0136-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.HAS-Sante . SAPIEN 3 treatment of severe symptomatic aortic stenosis in low-risk surgical patients in France. 2021. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2021-04/sapien3_9022021_avis_economique_vf2.pdf [cited September 2023].
- 40.Norwegian Institute of Public Health (NIPH) . Transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR) for patients with severe aortic stenosis and low surgical risk and across surgical risk groups: a health technology assessment. 2021. Available from: https://www.fhi.no/en/publ/2021/TAVI-vs-SAVR-for-patients-with-severe-aortic-stenosis-and-low-surgical-risk-and-across-surgical-risk-groups/ [cited September 2023].
- 41.Health Information and Quality Authority (HIQA) . Health technology assessment of transcatheter aortic valve implantation (TAVI) in patients with severe symptomatic aortic stenosis at low and intermediate risk of surgical complications. 2019. Available from: https://www.hiqa.ie/sites/default/files/2019-12/TAVI_HTA.pdf [cited September 2023].
- 42.Zhou JY, Liew D, Duffy SJ, Walton A, Htun N, Stub D. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in low-risk patients with severe aortic stenosis. Heart Lung Circ. 2021;30:547–54. doi: 10.1016/j.hlc.2020.09.934 [DOI] [PubMed] [Google Scholar]
- 43.Tam DY, Azizi PM, Fremes SE, Chikwe J, Gaudino M, Wijeysundera HC. The cost-effectiveness of transcatheter aortic valve replacement in low surgical risk patients with severe aortic stenosis. Eur Heart J Qual Care Clin Outcomes. 2021;7:556–63. doi: 10.1093/ehjqcco/qcaa058 [DOI] [PubMed] [Google Scholar]
- 44.Lytvyn L, Guyatt GH, Manja V, Siemieniuk RA, Zhang Y, Agoritsas T, et al. Patient values and preferences on transcatheter or surgical aortic valve replacement therapy for aortic stenosis: a systematic review. BMJ Open. 2016;6:e014327. doi: 10.1136/bmjopen-2016-014327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostnad per patient (KPP). Available from: https://skr.se/skr/halsasjukvard/ekonomiavgifter/kostnadperpatientkpp.1076.html [cited April 2024].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this article are legally restricted because of Swedish privacy and secrecy laws and are therefore not publicly available.