Abstract
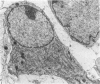
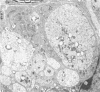
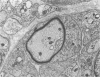
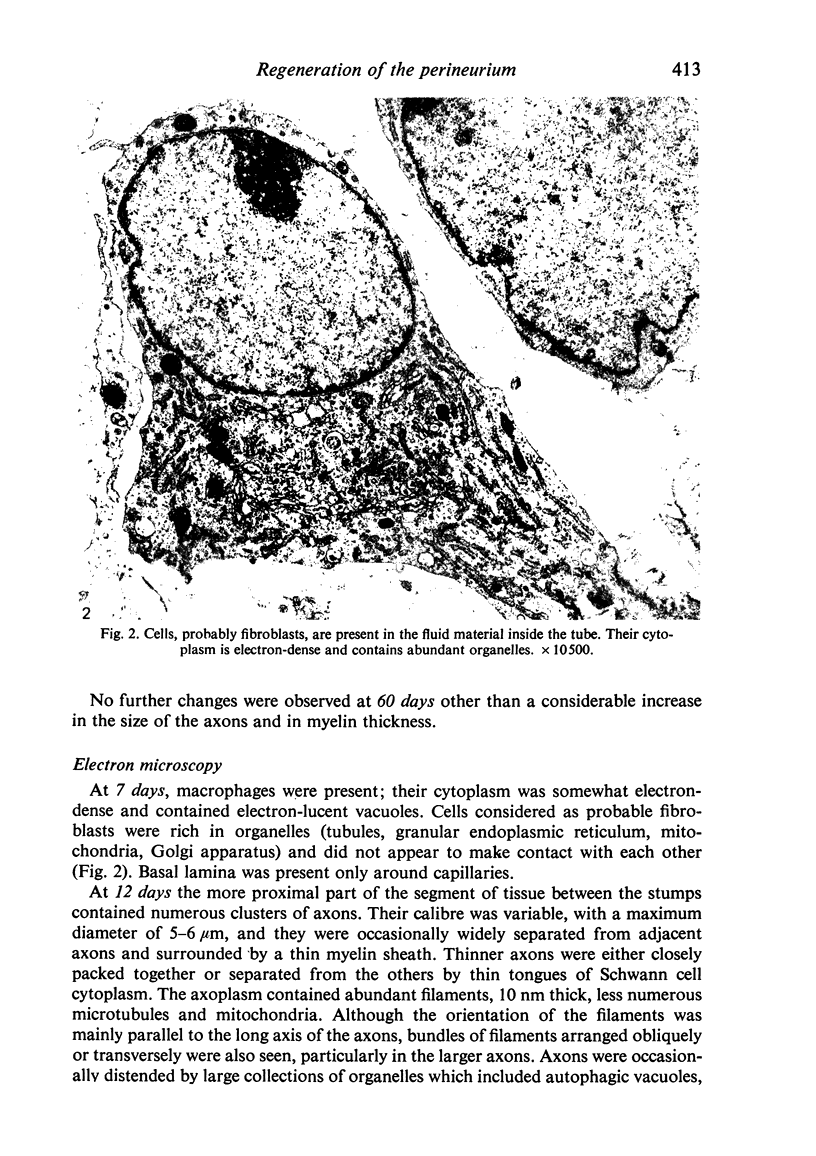
Sciatic nerves of mice were cut and the early regenerative stages were studied after the stumps had been encased within plastic tubes and kept separate by a gap of 5 mm. Only isolated cells were seen inside the tube after 7 days; after 12 days active regeneration and myelination were seen proximally; more distally, cells with long processes formed large spaces filled with collagen and less numerous Schwann cells. Zonulae occludentes and segments of basal lamina became more evident at a later stage. One month after the operation an almost complete regeneration of the nerve had taken place and perineurial cells were lined by a continuous basal lamina. The regeneration of the perineurium seemed to take place from fibroblasts; their cytoplasm as well as that of Schwann cells contained fibrillary material at this stage, sometimes in relation to segments of basal lamina. The results of this study indicate that both types of cells take part in the formation of endoneurial structures and that the early arrangement of fibroblasts contributes to the orderly longitudinal alignment of collagen fibrils.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunge M. B., Williams A. K., Wood P. M. Neuron-Schwann cell interaction in basal lamina formation. Dev Biol. 1982 Aug;92(2):449–460. doi: 10.1016/0012-1606(82)90190-7. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M., Uitto J., Jeffrey J. J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980 Jan;84(1):184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R. L., Tanzer M. L., Pfeiffer S. E. Collagen and procollagen production by a clonal line of Schwann cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1943–1946. doi: 10.1073/pnas.70.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. M., Hay E. D. Secretion of collagen by embryonic neuroepithelium at the time of spinal cord--somite interaction. Dev Biol. 1971 Dec;26(4):578–605. doi: 10.1016/0012-1606(71)90142-4. [DOI] [PubMed] [Google Scholar]
- Feasby T. E., Pullen A. H., Sears T. A. A quantitative ultrastructural study of dorsal root regeneration. J Neurol Sci. 1981 Mar;49(3):363–386. doi: 10.1016/0022-510x(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Bischhausen R. The fine structure of stumps of transected nerve fibers in subserial sections. J Neurol Sci. 1980 Jan;44(2-3):181–203. doi: 10.1016/0022-510x(80)90126-4. [DOI] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. COLLAGEN AND CELL PROTEIN SYNTHESIS BY AN ESTABLISHED MAMMALIAN FIBROBLAST LINE. Nature. 1964 Oct 24;204:347–349. doi: 10.1038/204347a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gamble H. J., Breathnach A. S. An electron-microscope study of human foetal peripheral nerves. J Anat. 1965 Jul;99(Pt 3):573–584. [PMC free article] [PubMed] [Google Scholar]
- Kelly D. E. Fine structure of desmosomes. , hemidesmosomes, and an adepidermal globular layer in developing newt epidermis. J Cell Biol. 1966 Jan;28(1):51–72. doi: 10.1083/jcb.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W. A comparative electron microscopic study of reactive, degenerating, regenerating, and dystrophic axons. J Neuropathol Exp Neurol. 1967 Jul;26(3):345–368. doi: 10.1097/00005072-196707000-00001. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Dahlin L. B., Danielsen N., Gelberman R. H., Longo F. M., Powell H. C., Varon S. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 1982 May;76(2):361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Gelberman R. H., Longo F. M., Powell H. C., Varon S. In vivo regeneration of cut nerves encased in silicone tubes: growth across a six-millimeter gap. J Neuropathol Exp Neurol. 1982 Jul;41(4):412–422. doi: 10.1097/00005072-198207000-00004. [DOI] [PubMed] [Google Scholar]
- Molander H., Olsson Y., Engkvist O., Bowald S., Eriksson I. Regeneration of peripheral nerve through a polyglactin tube. Muscle Nerve. 1982 Jan;5(1):54–57. doi: 10.1002/mus.880050110. [DOI] [PubMed] [Google Scholar]
- Morris J. H., Hudson A. R., Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. IV. Changes in fascicular microtopography, perineurium and endoneurial fibroblasts. Z Zellforsch Mikrosk Anat. 1972;124(2):165–203. doi: 10.1007/BF00335678. [DOI] [PubMed] [Google Scholar]
- Murray M. R., Stout A. P. Demonstration of the Formation of Reticulin by Schwannian Tumor Cells in Vitro. Am J Pathol. 1942 Jul;18(4):585–593. [PMC free article] [PubMed] [Google Scholar]
- NATHANIEL E. J., PEASE D. C. COLLAGEN AND BASEMENT MEMBRANE FORMATION BY SCHWANN CELLS DURING NERVE REGENERATION. J Ultrastruct Res. 1963 Dec;52:550–560. doi: 10.1016/s0022-5320(63)80084-2. [DOI] [PubMed] [Google Scholar]
- QUILLIAM T. A. Growth changes in sensory nerve fibre aggregates undergoing remyelination. J Anat. 1958 Jul;92(3):383–398. [PMC free article] [PubMed] [Google Scholar]
- Reale E., Luciano L., Spitznas M. Freeze-fracture faces of the perineurial sheath of the rabbit sciatic nerve. J Neurocytol. 1975 Jun;4(3):261–270. doi: 10.1007/BF01102112. [DOI] [PubMed] [Google Scholar]
- SHANTHAVEERAPPA T. R., BOURNE G. H. The 'perineural epithelium', a metabolically active, continuous, protoplasmic cell barrier surrounding peripheral nerve fasciculi. J Anat. 1962 Oct;96:527–537. [PMC free article] [PubMed] [Google Scholar]
- THOMAS P. K. THE DEPOSITION OF COLLAGEN IN RELATION TO SCHWANN CELL BASEMENT MEMBRANE DURING PERIPHERAL NERVE REGENERATION. J Cell Biol. 1964 Nov;23:375–382. doi: 10.1083/jcb.23.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]
- Thomas P. K., Bhagat S. The effect of extraction of the intrafascicular contents of peripheral nerve trunks on perineurial structure. Acta Neuropathol. 1978 Aug 7;43(1-2):135–141. doi: 10.1007/BF00685008. [DOI] [PubMed] [Google Scholar]
- Thomas P. K., Jones D. G. The cellular response to nerve injury. II. Regeneration of the perineurium after nerve section. J Anat. 1967 Jan;101(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Uzman B. G., Villegas G. M. Mouse sciatic nerve regeneration through semipermeable tubes: a quantitative model. J Neurosci Res. 1983;9(3):325–338. doi: 10.1002/jnr.490090309. [DOI] [PubMed] [Google Scholar]