Abstract
Electroplating is a widely used technology for anticorrosion materials and decorative coatings. In view of the transition to a circular economy, the current electroplating wastewater treatment disposing of heavy metal sludge and wastewater severely lacks sustainability. Authors recently reported the successful recycling of electroplating agents using hybrid semibatch/batch reverse osmosis technology (hybrid RO). Despite promising results, technology assessment to treat new, second-generation electrolytes, enhance boric acid recovery, close the water loops, and evaluate process robustness is still needed. This study investigates the viability of a high-pressure (120 bar) hybrid RO system, working with the DuPont XUS180808 membrane, to reclaim valuable second-generation plating components and water from electroplating rinses. The pilot-scale system showcased resilience in processing artificial electroplating wastewaters of variable concentration, achieving water recoveries of ≤87.7%, increasing chromium and sulfate to electrolyte levels (>6 g/L and >80 g/L), with low energy consumptions (≤2.7 kWh/m3), underlining its potential as a circular treatment in the chromium electroplating industry. A second-pass RO treatment strategy was explored, addressing residual boric acid in the permeate and leveraging solubility interactions to enhance its rejection, enabling water reuse. Based on these findings, an RO designed for industrial application was proposed for future scale-up and evaluation within a real-world production environment.
Keywords: Cr (III), electroplating, wastewater, reverse osmosis, zero heavy-metal discharge, zero liquid discharge
Short abstract
In this work, we identified the current obstacles to achieve full circularity in industrial electroplating operations. We provide a strategy to ensure the simultaneous recovery of clean water and resources.
Introduction
The main purpose of electroplating is to deposit layers of metal on the surface of parts, such as automotive trims or bathroom fittings, in order to make them corrosion-resistant and achieve an attractive finish. Plastic electroplating as an important example of this industry uses very sophisticated and complex aqueous electrolyte baths containing various inorganic and organic components. Injection-molded parts are consecutively submerged in different plating electrolytes, and thin metal layers are deposited via electrochemical reduction using direct current. After the deposition of each layer, plated parts are rinsed in separate rinse baths to avoid contamination of the subsequent plating electrolyte. The process is completed in the chromium electrolyte, followed by a last rinse, where its plating chemicals accumulate. Electroplating rinsewater is typically replaced after a few days and undergoes treatment before being discharged into the local sewage system.
This treatment is usually achieved by means of alkaline precipitation.1 During this operation, metal ions form insoluble metal hydroxides. After sedimentation, the solid particles are separated as metal sludge via filtration, and the cleaned water is discharged.2 Since the ban of carcinogenic, hexavalent chromium (Cr(VI))3 from industrial applications by the European REACH amendment in 2013,4 the industry has shifted toward establishing plating processes by integrating new plating electrolytes based on less-harmful trivalent chromium (Cr(III)).5 In these novel electrolytes, Cr(III) is in a stable complexed form for better charge transfer, using carboxylic acids as chelating ligands (e.g., oxalic acid,6 malic acid, glycolic acid, formic acid, or mixtures thereof),6,7 which severely affects the efficiency of the alkaline precipitation.8−10 Consequently, an energy-demanding pretreatment, involving harsh ultraviolet irradiation and H2O2 for the oxidation of the complexes, is now needed prior to precipitation to meet the discharge requirements. BIA Kunststoff- and Galvanotechnik GmbH & Co KG (BIA), a globally operating company producing chromium-plated trims and facings for the automotive industry, generates more than 30.000 m3 of wastewater per year. Their German facility, located in Solingen, reports that the pretreatment of Cr(III)-based rinsewater takes 4.3 h/m3 and causes an additional energy demand of 122 kWh/m3, resulting in 58,900 kWh annually. Furthermore, the process produces hazardous metal sludge,11 which is disposed of by a special waste disposal contractor, creating disposal costs and sending valuable plating components to waste. Consequently, the current treatment not only lacks sustainability but also becomes uneconomical. This issue affects not only BIA but the whole electroplating and tanning industry, which uses similar Cr(III) complexed solutions.8,10
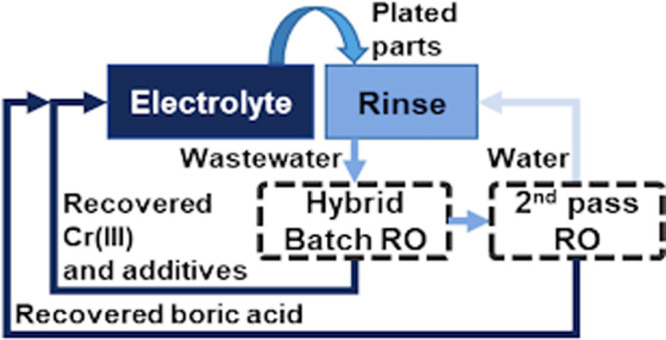
Although substantial research efforts focus on the absorption of chromium from wastewater,12 utilization of produced sludge,11 and the extension of plating bath lifetime,13 an ideal treatment approach must be of circular nature,14 enabling the reintegration of plating chemicals and water from the rinses into the plating process. A concept for a zero-liquid discharge (ZLD) approach with closed loops is displayed in Figure 1. Engstler et al. demonstrated the feasibility of a direct reverse osmosis (RO) treatment to recover Cr(III) and other electrolyte components from artificial electroplating rinsewater and reused them for electroplating purposes at a laboratory-scale (lab-scale).15
Figure 1.
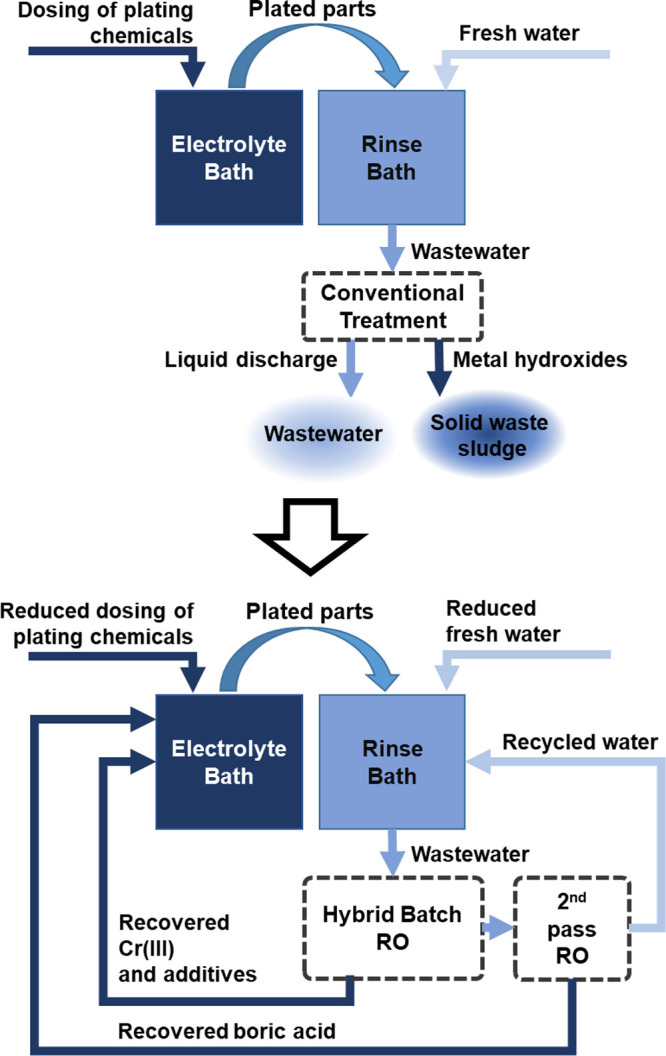
Proposed technology change from the current linear Cr(III) wastewater treatment (top) to a circular ZLD wastewater treatment process with resource recovery (bottom).
To enable the recirculation of plating chemicals from rinsewater into the electrolyte bath at an industrial scale, high rejection of plating components and high concentrations (high recovery) to avoid unnecessary dilution of the electrolyte bath are clear specifications of the RO process. High concentrations required for reuse result in high osmotic and therefore operational pressures. Capable RO systems need sophisticated engineering and tend to have a high energy consumption.16 The high-pressure hybrid semibatch/batch RO system (hybrid RO), developed by Davies and co-workers, aims for a specific energy consumption (SEC) close to the theoretical minimum.17 The system was improved through detailed modeling and optimization18 and was considered for high-recovery and low-energy desalination19 as well as for high-pressure (<120 bar)20 and ZLD applications.21 The working principle, described in detail in the Supporting Information, results in an innovative, high-pressure approach for treating hypersaline solutions, as presented in this work, which is a pressing field of research.22
Recently the system was assessed by treating 1.5 m3 of artificial electroplating wastewater. Karimi et al. used the pilot scale rig to treat artificial electroplating wastewaters based on a 1:10–1:20 diluted Cr(III) electrolyte.23 Chromium, sulfate, and total organic carbon (TOC) were recovered above the recycling requirement, using industrially relevant feed flows of 0.21–0.46 m3/h and transmembrane water fluxes of 6–14 L m–2 h–1 (LMH), while substantial energy savings were achieved with electrical SEC of <2.25 kWh/m3. The low SEC of the hybrid RO technology could be able to reduce the treatment cost for Cr(III)-based electroplating wastewater by up to 98% (cf. data from BIA quoted above). The rejection of the XUS180808 membrane for chromium, sulfate, and TOC was always >99.8% under these conditions.23 Only boric acid (B(OH)3) rejection showed lower values (70.6–78.7%),23 which is a well-known phenomenon caused by the small size of the neutral species.24,25 This leads to a lowered osmotic pressure of the RO retentate, enabling slightly higher concentrations of valuable chromium and sulfate under the constraints of a given maximum operation pressure. Consequently, 1–2 g/L boric acid remained in the permeate.23 Although a permeate of this quality could be partially used for the makeup of new electrolyte baths, residual boric acid prevents the circular reuse of it as rinsewater. Overall, successful scale-up was achieved in this work, but the technology still lacks maturity with regard to process robustness, the closing of the water loop, the reuse of boric acid, and the process analytical technology (PAT) strategy.
In the meantime, BIA completed the shift from Cr(VI) to Cr(III) with the implementation of an improved Cr(III) electrolyte, hereafter referred to as the second-generation electrolyte. Cr(III)-based electrolytes are under constant development since the reduction process26 and produced finishes differ from their Cr(VI)-based predecessors in optical appearance,27 topology,28 wear resistance,29 and adhesion properties,30 all of them tweaked with additives, leading to a growing complexity of newly developed electrolytes. Previous research, however, used the first-generation electrolyte.15,23Table 1 shows a comparison of the composition of the first and second generation (gen) of Cr(III) electrolytes.
Table 1. Inorganic Components, TOC, and Osmotic Pressure of 1st and 2nd Gen Plating Electrolyte.
| 1st gen electrolyte | 2nd gen electrolyte | |||
|---|---|---|---|---|
| range (g/L) | optimum (g/L) | range (g/L) | optimum (g/L) | |
| TOC | 6.9–10.3 | 8.6 | 4.3–6.5 | 5.4 |
| chromiuma | 8–12 | 10 | 6–9 | 7.5 |
| boric acida | 80–110 | 90 | 80–100 | 90 |
| sulfatea | 80–150 | 100 | 80–150b | 115 |
| osmotic pressure | 78 bar23 | ∼118 bar | ||
Supplier recommendations.
BIA uses higher sulfate content of 150–170 g/L.
The analysis of the new second gen electrolyte revealed that with approximately 118 bar, it yielded a significantly higher osmotic pressure compared to its predecessor. This increase in osmotic pressure is also anticipated in the rinse baths following the electrolyte, which is an important aspect for the recovery of chemicals in a pressure-driven treatment and calls for a robust technology capable of processing a wide variety of feeds. Despite modifications in concentration ranges, the primary constituents of the electrolyte remained unchanged. The lower bounds of the concentration range set the ideal values for the RO concentrate, potentially enabling its direct reuse in the plating bath.
Considerable efforts are still needed to close both loops (chemicals and water). Furthermore, the development of strategies to achieve the transition from an early stage separation technology to a mature fully circular hybrid RO technology raises the following questions:
-
1.
Which concentrations and physical properties (such as osmotic pressure and electrical conductivity) are to be expected in real electroplating rinsewater?
-
2.
Is the XUS180808 membrane still a viable option for the treatment of rinses originating from the second gen electrolyte?
-
3.
How sensitive is the efficiency of the process against naturally occurring variations in feed concentration and operational conditions (feed temperature, permeate flux, and pump speed) at the industrial site?
-
4.
Can we design a second pass treatment with improved boric acid rejection for its recovery and simultaneously close the water loop?
-
5.
Can we establish correlations between in situ measurable parameters (e.g., electrical conductivity) and key performance indicators such as osmotic pressure and concentration factors for process monitoring purposes?
The present work aims to answer these five leading questions, concluded by giving the final RO design, which should be regarded as a deployable technology helping to achieve full circularity in electroplating operations. Experimental details are provided in the Supporting Information.
Results and Discussion
Characterization of Feed Water at BIA
At the start of the research project, BIA was already testing Cr(III)-based electrolytes of the first gen in their facility in Slovakia. Their goal was the full upgrade of their chromium electroplating processes to Cr(III)-based processes. The first analysis of rinsewater samples from this site is displayed in the Supporting Information of this work. The results indicated a high derivation of mass concentrations across samples. Detected sulfate concentrations spanned from 2.02 to 9.85 g/L, while boric acid was present in amounts ranging from 1.10 to 8.28 g/L. Chromium concentrations varied from 0.14 to 0.84 g/L, and TOC levels were measured between 0.11 and 0.67 g/L. Despite these variations, the proportional ratio of components within each sample mirrored the 10:9:1 ratio of sulfate, boric acid, and chromium from the electrolyte (see Table 1, 1st gen electrolyte). Based on the observed concentration ranges, a 1:10–1:20 diluted electrolyte was used to mimic rinsewater in previous investigations.15,23 This approach allowed for the preparation of fresh electrolyte mixtures with controlled concentrations using original chemicals in the laboratory setting, avoiding the logistical challenges of transporting several cubic meters of contaminated wastewater.
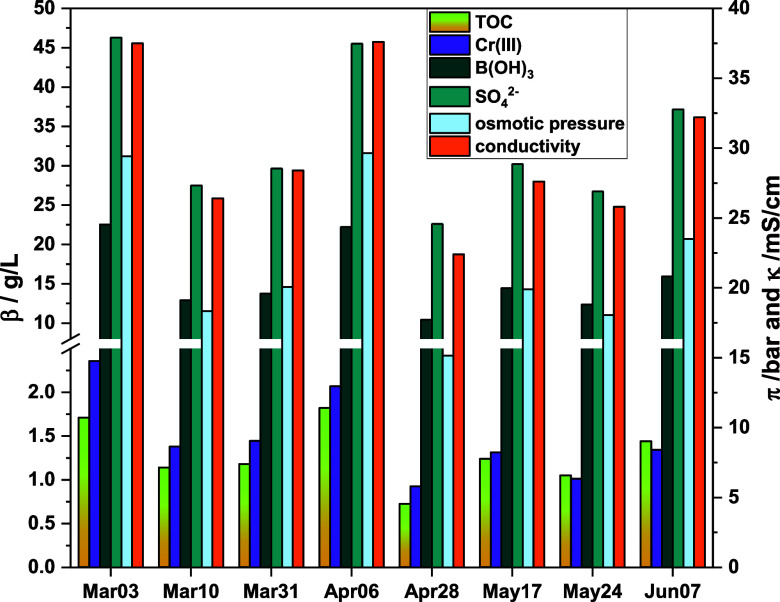
In 2022, the second gen electrolyte was first established in the BIA German production facility in Solingen. For 8 weeks of plating production, Cr(III) rinsewater samples were taken prior to discharge. Water from the rinse bath subsequent to the Cr(III) plating electrolyte was analyzed with the ICP-OES and TOC difference method. Additionally, electrical conductivity and osmotic pressure were determined (refer to Experimental in Supporting Information). The results are depicted in Figure 2. The water from this rinse bath is designated as the feed for the IntelWATT project’s hybrid RO rig, to be designed and built for implementation at BIA’s facility in Germany.
Figure 2.
Mass concentration of boric acid, sulfate, chromium, TOC and osmotic pressure of rinsewater samples of an industrial plating line using 2nd generation Cr(III) electrolyte. Samples were taken at maximum degree of contamination, right before rinsewater tanks were drained. The samples were kindly provided by BIA Kunststoff- and Galvanotechnik GmbH & Co. KG, Solingen (Germany).
The analyzed rinsewater samples exhibited mass concentrations within the following ranges: 0.72–1.71 g/L for TOC, 0.93–2.36 g/L for chromium, 10.4–22.5 g/L for boric acid, and 22.6–46.3 g/L for sulfate. These variations in concentration can be attributed to the fluctuating workload on the plating line. Despite this variability, the relative proportions of each component remained relatively stable, reflecting the composition of the preceding chromium(III) electrolyte bath, which contained concentrations of 90–100 g/L boric acid, 6–9 g/L chromium, and 150–170 g/L sulfate at that time. Rinse water displayed 10.9–23.7% of the boric acid, 12.4–31.5% of the chromium, and 14.1–28.9% of the sulfate found in the electrolyte bath (2nd gen, compare Table S1). Variations in the ratios, as found for chromium in sample Mar03 compared to Jun07, stem from the dosing of single components when their accepted minimum is reached. This is particularly relevant for chromium, which is depleted in the plating process. The concentrations found in these rinsewater samples were significantly higher compared to rinsewater samples of the first generation (compare graph in Supporting Information) and closer to a 1:5 dilution of the electrolyte. This is due to a less frequent change of the rinsewater in the German facility.
Osmotic pressures were observed to vary between 15.1 and 29.6 bar. In the context of a hybrid RO operating at 120 bar, concentration factors (CFs) of 4.0–7.9 could be anticipated. However, due to the nonlinear relationship between concentration and osmotic pressure at elevated concentrations,31 along with less than 100% rejection of boric acid,15 the reaching of higher CFs is conceivable.
Conductivity levels were measured in the range of 22.4–37.6 mS/cm, with trends that seem to correlate closely with sulfate concentrations. The possibility of predicting sulfate and boric acid concentrations based on conductivity measurements will be analyzed in the section “Monitoring Strategies”.
Lab-Scale Evaluation of XUS180808 Membrane with Second Gen Electrolyte
The XUS180808 membrane′s retention capabilities for boric acid and chromium were assessed using simulated rinsewater of a second gen electrolyte in lab-scale experiments. The RO lab plant used a flat-sheet configuration, with rejection values (R) presented in Figure 3.
Figure 3.
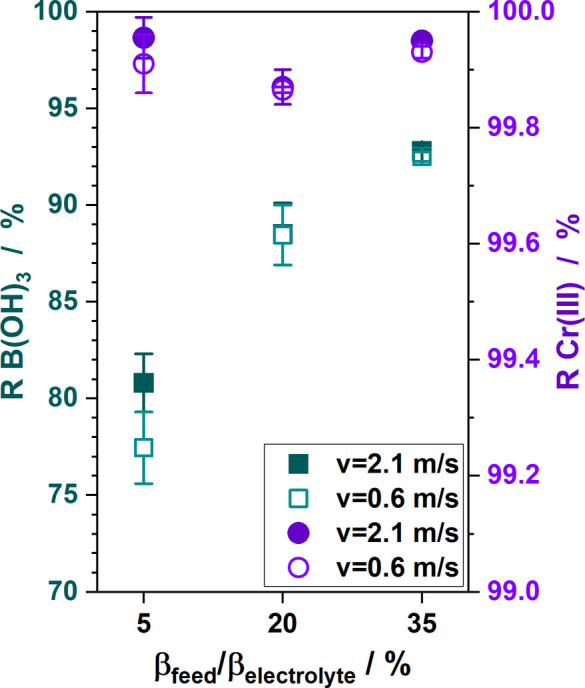
Rejection of boric acid (squares) and chromium (dots) with increasing feed concentration and different feed cross-flow velocities in lab-scale RO experiments using a XUS180808 membrane flat-sheet.
The feed flow velocities did not notably influence the rejection of chromium and boric acid in the observed range. From the geometry of the membrane cell, Reynold numbers of 981 and 3269 were calculated, indicating laminar flow for 0.6 m/s and transition flow regime for 2.1 m/s. Chromium rejection was consistently above 99.87% across all feed concentrations, a value that aligns with those reported for a first gen electrolyte.23
An increase in boric acid rejection from 80.8 to 92.1% was noted with rising feed concentration. This dependence of boric acid rejection on feed concentration was also observed in previous work15 and is a known phenomenon in the presence of 1:1 salts such as sodium chloride.32 Recent studies have shown that boric acid rejection increases in solutions containing sodium chloride and boric acid within a pH range of 4–8.22.33 The investigated electrolytes, with pH values of 3.5–3.9, yield diluted rinses within this specific pH spectrum.34,35 They do not contain sodium chloride but other salts, like sodium sulfate, in high concentrations. The presence of ions like sodium or lithium is known to enhance boric acid solubility,36 a phenomenon also attributed to sulfate ions.37,38 Our findings suggest that sodium sulfate may contribute to the elevated rejection of boric acid.
However, given the complex matrix of inorganic (e.g., chromium hydroxide sulfate) and organic components (e.g., glycolic acid, formic acid, and resorcinol) within the feed, this effect warrants further investigation. The influence of sodium sulfate on the boric acid rejection was investigated and a strategy for a second-pass RO permeate treatment was developed from it, which is documented in a later section of this work.
The membrane permeance was determined with 1.16–1.26 L*m–2 h–1 bar–1 consistent through the course of the experiments and closely matching with the literature value of 1.39 L*m–2 h–1 bar–1.21 Sodium chloride rejection exceeded 98.8% in all salt rejection experiments, indicating no immediate signs of membrane fouling or chemical degradation.
The results verify the suitability of the membrane for treatment of this electrolyte combination, laying the groundwork for further investigations in the hybrid RO rig at the pilot scale.
Investigating the Robustness of the Hybrid RO Treatment at Pilot Scale
Previous investigations highlighted notable enhancements in energy efficiency while yielding a large enrichment of chromium, sulfate, and TOC in the retentate.23 Advancing this technology toward practical application necessitates an understanding of its performance under nonideal working conditions and the natural variances encountered in an operational plating facility like BIA. A factorial design of experiments (DOE) was employed, consisting of 11 experiments, including three center points, across four factors, to assess these dynamics:
-
1.
Feed water concentration variability: To simulate fluctuations and higher concentrations of rinsewater from the second-generation electrolyte, artificial rinsewater was prepared by diluting the electrolyte with water in ratios of 1:5, 1:7.5, and 1:10.
-
2.
Temperature fluctuations: Although the feedwater tank is temperature-controlled (T = 25 °C), temperature variations may arise with seasonal ambient changes in BIA nonclimate-controlled workshop. The feedwater temperature range of 20–30 °C was selected to account for these variations.
-
3.
Permeate flux adjustments: To achieve the high concentration factor needed for the reuse of components in the electrolyte bath, the hybrid RO system operates at slightly higher fluxes in the semibatch phase and low fluxes in the batch phase of operation. Standard operations have utilized fluxes of 10 LMH in semibatch and 5.7 LMH in batch mode. Adjusting these values by ±15% to 11.5 LMH/6.6 LMH simulates operation near the industrial rig’s planned maximum capacity (12.75 LMH), whereas 8.5 LMH/4.8 LMH allows exploration of an energy-saving operation mode.
-
4.
Pump flow rate variations: The recirculation pump ensures the uniform mixing and flow of the feed during the treatment. Increasing pump flow rates to 55 L/min can mitigate fouling as well as overpressure from concentration polarization at the cost of higher energy consumption.20 Conversely, reduced flow rates (35 L/min) were used to mimic the effects of diminished pump performance over the years.
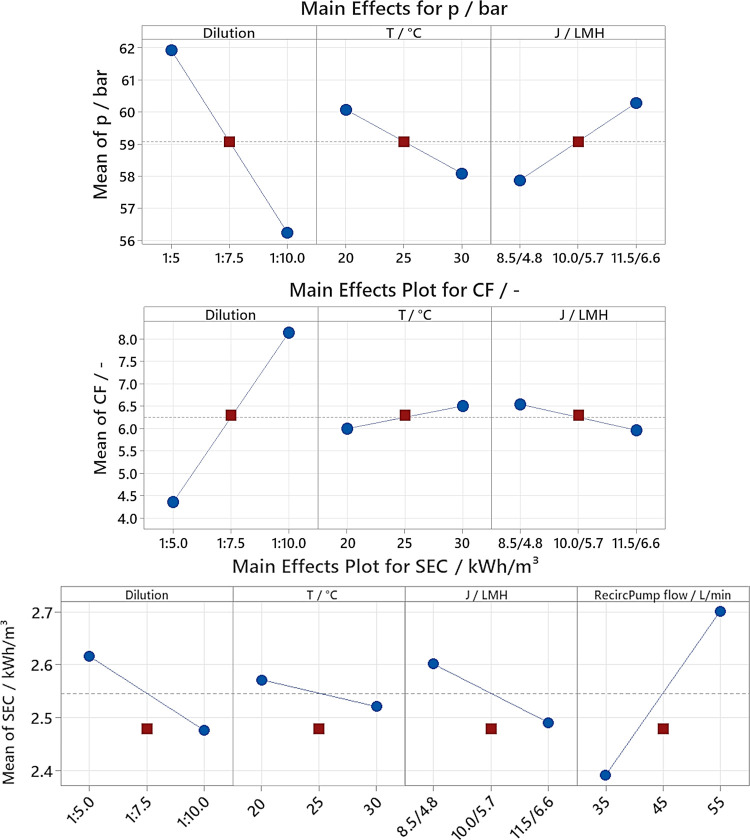
Energy consumption, average operational pressure, and achieved concentration factor (via volume displacement) were systematically monitored, with findings detailed in Figure 4.
Figure 4.
Main effect plots of the average pressure p, the concentration factor CF, and the electrical SEC with feed dilution, feed temperature T, permeate flux J, and recirculation pump flow.
The average pressure was affected by the feed dilution, feed temperature, and permeate flux (upper row of Figure 4, R2 = 95.18%, standard deviation S = 0.84 bar). Specifically, reducing the feed concentration from a 1:5 to a 1:10 dilution resulted in a decrease in average operational pressure from 61.9 to 56.2 bar due to diminished osmotic pressure. Increasing the temperature from 20 to 30 °C reduced the average pressure by 2.0 bar, attributable to enhanced membrane permeance. The findings highlight that strict temperature control is not needed and rinsewater with fluctuating temperatures can be processed. Increasing the flux from 8.5 LMH/4.8 LMH to 11.5 LMH/6.6 LMH induced a pressure increase of 2.3 bar, a manageable increase within the system’s operational ceiling of 120 bar. The variation of recirculation pump flow did not significantly affect operational pressures, indicating minimal influence of concentration polarization within the experimented flux range. The recirculation pump flow creates cross-flow velocities in the RO module from 0.046 to 0.072 m/s (Re = 22 and 35) during the batch phase, as the feed flow is low, and slightly higher during the semibatch phase.
The feed dilution and corresponding concentration factor are depicted in the middle row on the left in Figure 4, depicting enhanced recovery values for more diluted rinsewater. Specifically, according to the volume displacement, the diluted feeds were reconcentrated by factors of 8.14 ± 0.53, 6.30 ± 0.11, and 4.36 ± 0.27, translating to r = 87.7%, r = 84.4%, and r = 77.1%, respectively. The resulting concentrations of boric acid, sulfate, and chromium in RO concentrates are listed in Table 2. The influence of feed temperature and flux is marginal and in the range of the experiment variance (S = 0.20), suggesting negligible concentration polarization at the employed low fluxes (4.8/5.7/6.6 LMH) in the batch phase.
Table 2. Mean Concentrations of RO Feeds and RO Concentrates (c) from the Robustness Tests.
| β Cr(III) (g/L) | β B(OH)3 (g/L) | β SO42– (g/L) | |
|---|---|---|---|
| feed (1:10) | 0.86 ± 0.02 | 8.05 ± 0.10 | 13.41 ± 0.04 |
| feed (1:7.5) | 1.13 ± 0.00 | 14.72 ± 0.20 | 16.01 ± 0.20 |
| feed (1:5) | 1.64 ± 0.04 | 13.83 ± 0.19 | 23.25 ± 0.52 |
| conc. 1 | 6.26 ± 0.51 | 31.30 ± 0.51 | 87.19 ± 7.79 |
| conc. 2 | 6.77 ± 0.26 | 39.22 ± 1.72 | 92.97 ± 3.36 |
| conc. 3 | 6.26 ± 0.35 | 39.67 ± 2.34 | 86.02 ± 4.73 |
A comprehensive analysis presented in the bottom row of Figure 4 (R2 = 99.84%, S = 0.011 kWh/m3) identifies all experimental factors as significantly (p ≤ 0.05) influencing the SEC. The variance (S = 0.011 kWh/m3) was derived from the center point experiments, performed in triplicate, as is usual for factorial DOE experiments. However, this analysis encountered challenges with the experimental data not conforming to a clear linear trend. Therefore, the real variance of the experiments could be higher. The influence of temperature and flux was minor, although the higher osmotic pressure of less diluted rinsewater increased the SEC by 0.14 kWh/m3, which is an increase of 6.2% compared to the previously reported 2.25 kWh/m3.23 This increase has to be considered for the change from first gen rinsewater to second gen rinsewater. Increasing temperatures, however, reduced the energy consumption by only 0.05 kWh/m3, due to lowered membrane resistance.
The alterations in recirculation pump flow had the most pronounced impact on the energy consumption, with an increase of 0.30 kWh/m3. Given the reported energy consumption of 2.25 kWh/m3 for the process,23 this increase of 13% is the most noteworthy, and optimizing the recirculation pump flow should be considered as long as other performance parameters are not inflicted. Nevertheless, the SEC is three times lower compared to a high-concentration (87 g/L LiCl) RO process treating wastewater with 7.71 kWh/m3.39
With concentrations >6 g/L for Cr and >80 g/L for sulfate, the hybrid RO was able to increase concentrations from artificial rinsewater of different dilutions (1:10/1:7.5/1:5) to concentrations levels of the plating electrolyte, even under suboptimal conditions. Such RO concentrates could be directly reused in the plating process. Lower boric acid rejection led to less concentration increase. In the acidic rinse waters (approximately pH 3.7–4.9) boric acid is present in its neutral form (pKa = 9.22) allowing the small, nonhydrated molecules to partially pass through the membrane.24 These molecules can form hydrogen bonds with functional groups in the barrier layer of the membrane, leading to a diffusive trans-membrane transport analogous to that of water.25 Although this behavior leads to a contaminated permeate, which has to be further purified (see next section), it lowers the osmotic pressure of the retentate during the treatment, thus enabling a concentration increase of the remaining components, which would otherwise not be possible using an applied operation pressure of 110 bar.
The presented data indicate that the dilution of the feed has the most significant impact on the concentration factor and pressure of the operation. The impacts of temperature and flux are lesser. The energy consumption, however, is mostly affected by feed dilution and cross-flow that is driven by the recirculation pump. The latter is a process parameter that can be chosen by the operator and should be kept low as long as sufficient mixing can be provided and other phenomena, such as concentration polarization, do not inflict the treatment.
The hybrid RO was capable of producing a concentrate of sufficient quality for direct reuse in electroplating from rinsewater of varying concentration and temperature, within the ranges of maximum fluctuations that would be expected in real application, with only minor increases in energy consumption. In summary, the DoE confirmed the robustness of the process given the uncontrollable variation of operating conditions likely to occur.
Treatment of First Stage RO Permeate
Remaining boric acid in the permeate of the RO process poses a challenge for the closed circularity approach. Although this boric acid-containing water can be used for the makeup of new electrolytes, the more ambitious direct reuse of this water in the rinse tanks is favored. Ideally, water for reuse should contain less than 1 g/L of boric acid.23 A strategy for the treatment of the RO permeate in a second RO pass was investigated. Permeate from the rinsewater of first gen electrolyte contained 1–2 g/L boric acid,23 while the permeate from the second gen electrolyte showed an increase to 2.7 g/L.
In RO operations, the increase of pH is known to enhance boric acid rejection significantly, by shifting the boric acid-borate equilibrium to the charged borate with a higher hydrodynamic radius.40 The pH adjustment, e.g., with sodium hydroxide, prior to treatment is not possible due to the risk of chromium hydroxide precipitation as well as the continuous addition of sodium ions in the loop. Consequently, pH increase of the permeate of the first stage, followed by a second stage RO with subsequent neutralization, is not a conceivable option for this application.
Here, we investigate a novel one-step approach for a second-stage RO without the introduction of foreign substances: Sodium sulfate is present in the plating electrolyte with up to 222 g/L (150 g/L sulfate) and found in the rinsewater with up to 68.5 g/L (46.3 g/L sulfate). The literature documents an increase in the solubility of boric acid with increasing sodium sulfate concentration. The reason for this phenomenon is not fully understood and was in literature attributed to the presence of either sodium ions41 or sulfate ions.38 The stabilization of boric acid with sodium is dependent on the pH value,42 and is highest between pH 4–8,33 which applies for diluted electrolytes (initially pH 3.5–3.9). However, the behavior under pH values lower than this range was not reported.
It was assumed that ionic interactions lead to an increase in both solubility and rejection. This opens up the possibility of influencing the rejection of boric acid without significant pH changes. Boric acid rejection for feeds of different concentrations is displayed in Figure 5.
Figure 5.
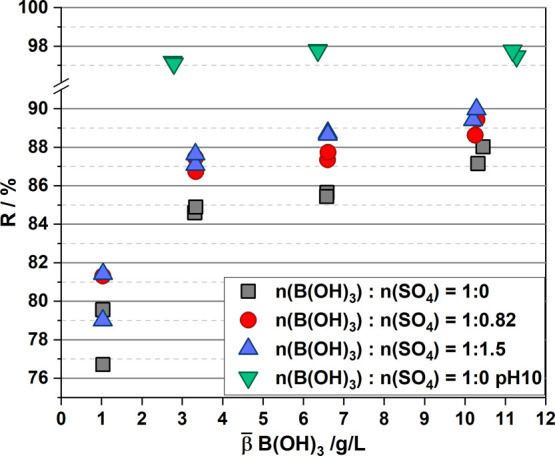
Rejection of boric acid with increased sulfate content: without addition of sulfate (gray squares), with addition of sulfate in the stoichiometric ratio found in rinses and 2nd gen electrolyte (1:0.82, red dots) and with a stoichiometric ratio of 1:1.5 (blue triangles). A feed at pH 10 (green triangles) is given for comparison.
The rejection for boric acid solutions (gray squares) increased from 76.7 to 88.0% with increasing B(OH)3 concentration from 1.0 (0.016) to 10.5 g/L (0.17 mol/L), mirroring a RO treatment with 90.5% recovery. Increased rejection with feed concentration was observed in previous investigations using multi-ion solutions15 and is reported for a single-component solution with the presented data. The behavior can be explained with the formation of polyborates, reported for concentrations ≥0.025 mol/L,43 explaining the rapid increase in rejection using the feed with 3.2 g/L (0.052 mol/L) compared to the feed with 1 g/L boric acid (0.016 mol/L). The formation of polyborates occurs with proton release (Bro̷nsted acid pK = 9.23),43 instead of electron pair acceptor interaction (Lewis acid pK = 6.84),44 which usually occurs for monomeric boric acid in an aqueous solution.
Under the addition of Na2SO4 in the stoichiometry that is found in the plating electrolyte (1:0.82, red dots), the values were increased to 81.4% for the feed with 1 g/L up to 89.5% (10.5 g/L, r = 90.5%). Rejection was further increased by almost doubling the sulfate content (1:1.5, blue triangles) with 87% for the lowest boric acid concentration and 89.9% for the highest boric acid concentration. For comparison, rejections of ≥97% were found for solutions at pH 10. In experiments with the highest Na2SO4 addition (blue triangles), sulfate increased the rejection of boric acid on average by 2.5%. This improvement appeared to be stoichiometry-dependent rather than concentration-dependent, suggesting that the full potential for rejection enhancement might not yet be fully realized. We assume that this effect can be attributed to an increased hydration radius due to ionic interactions. This behavior was reported for sulfate and boric acid in electrodialysis by Ezechi et al.45 and in solvent extraction of boric acid, where sodium sulfate is coextracted with boric acid using 2-ethyl-1-hexanol.38 To the knowledge of the authors, it is a not yet reported phenomenon in RO.
Considering the application, feeds with 1–3 g/L B(OH)3 are expected. Based on the data presented in Figure 5, a feed containing 1 g/L boric acid and sulfate (ratio 1:1.5) would lead to a mean rejection of 86.5% during its concentration increase to 10.5 g/L, while a feed with initially 3.2 g/L would have a mean rejection of 88.6% during analogous treatment. Although having different mean concentrations during the treatment (5.75 and 6.85 g/L, respectively), second-pass permeates would both contain 0.78 g/L boric acid. Feeds containing the same amount of boric acid, but no sulfate, would face mean rejections of 84.0 and 86.0% resulting in permeates with 0.92 and 0.96 g/L boric acid, which are just slightly below the reuse limit of 1 g/L.
Incorporating sulfate into the feed notably increases its osmotic pressure, which is displayed in detail in the Supporting Information. The addition of sulfate increased the osmotic pressure from 0.4 to 1.9 bar for a feed with 1 g/L boric acid. For the feeds with 10 g/L boric acid, the osmotic pressures increased from 5.7 bar up to 16.1 bar. For feeds adjusted to pH 10, the osmotic pressures were lower, ranging from 2.1 to 5.3 bar with increasing boric acid content. The osmotic pressures of the feeds are throughout lower than those found in the rinsewater treatment (cf. Figure 2). Determining the energy consumption of the presented procedure on a larger scale is of interest for industrial applications and may be conducted in future experiments.
Despite lower rejection values compared to treatments after the pH shift, the new procedure at unchanged pH opens up an alternative process. The retentate, rich in boric acid and sulfate, can be recycled into the plating electrolyte and/or the makeup of the new electrolyte solution, whereas the permeate can be recycled into the rinse baths. This allows the closing of the water loop.
Monitoring Strategies
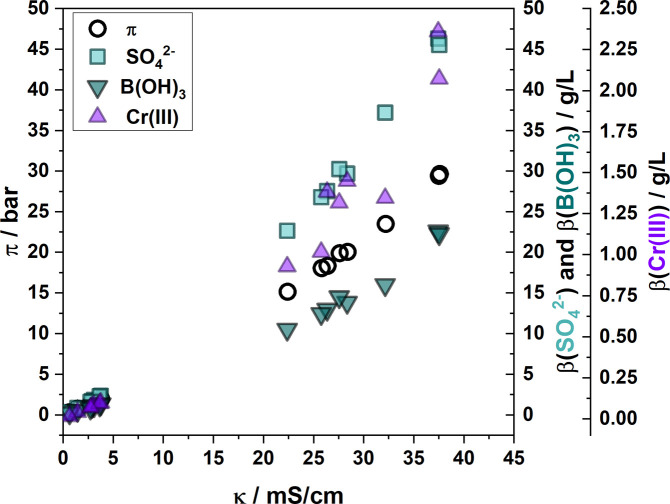
In the optimization of the final RO system at BIA, a direct determination of concentrations in the RO feed is preferred. At pilot scale experiments, the concentration was determined with a combination of ICP and titration, neither of which provided immediate results. Investigating the possibility to predict the concentration of sulfate and other components from the feed’s conductivity, the data presented in Figure 2 was expanded by incorporating additional samples of a sparingly used plating line. These samples exhibited lower degrees of contamination; all data are listed in Figure 6.
Figure 6.
Osmotic pressure π and mass concentrations β of sulfate, boric acid, and chromium as a function of rinsewater conductivity. Data in the range of 20–40 mS/cm was collected from the samples displayed in Figure 2. Data in the range of 0–5 mS/cm was obtained from rinsewater samples of lower contamination.
Trends in the mass concentration of chromium, boric acid, and sulfate as well as the osmotic pressure of the solutions had similar trends and were closely fitted by quadratic functions over the observed conductivity range, turning approximately linear between 22.5 and 37.5 mS/cm. The latter comprises the data from rinsewater samples before discharge (cf. Figure 2).
The function of sulfate concentration as a function of conductivity was found to be quadratic with y = 0.016x2 + 0.622x (R2 = 0.9997), simplifying to a linear correlation within the higher concentration range (22.6–46.2 g/L) that is expected as feed for the treatment of rinsewater y = 1.588x – 14.03 (R2 = 0.9924). The accurate fit can be attributed to sodium sulfate being the main constituent of the electrolyte and its dissociation into three ions. The observation is self-evident since sodium sulfate is used in electroplating to increase the conductivity of the electrolyte for even charge transfer.6 The high value for the intercept with the y-axis underlines that the linear correlation is only valid for this narrow concentration range and predictions outside this range being unreliable.
Efforts to correlate the chromium concentration with the conductivity of rinsewater resulted in quadratic and linear fits of poor quality (R2 = 0.9766 and R2 = 0.8621). Due to the variable consumption and replenishment of chromium in the plating process, these metrics should not be connected with a linear or other simplistical function.
Surprisingly, boric acid, present in its neutral state, thus not directly contributing to the electrical conductivity, shows a quadratic correlation similar to that of sulfate with y = 0.009x2 + 0.0239x – 0.100 (Figure 6, R2 = 0.9966) over the shown concentration range and an approximate linear correlation for higher concentrations (y = 0.796x – 8.104; R2 = 0.9666).
This pattern once more displays the uniform drag-in of plating components during the rinsing process. The correlation between the concentrations of sulfate and boric acid is plotted in the Supporting Information. Sulfate and boric acid are not consumed in the plating process. Due to the consistent composition of the plating electrolyte, the concentrations can be connected linearly with y = 2.116x + 0.038 (R2 = 0.9959, refer to Figure S6 in the Supporting Information). In respect to the industrial RO rig, this finding enables the estimation of sulfate and boric acid concentrations from conductivity measurements in the RO feed tank.
The electrical conductivity of the rinsewater is affected by all ions in the solution. Additionally, ionic interactions and dissociation equilibria affect this property. Therefore, predicting the concentration of single components from this characteristic will always be speculative. However, the presented data suggest that the procedure is feasible in a limited concentration range and could be used as a first and fast rinsewater characterization, in addition to more time-consuming techniques.
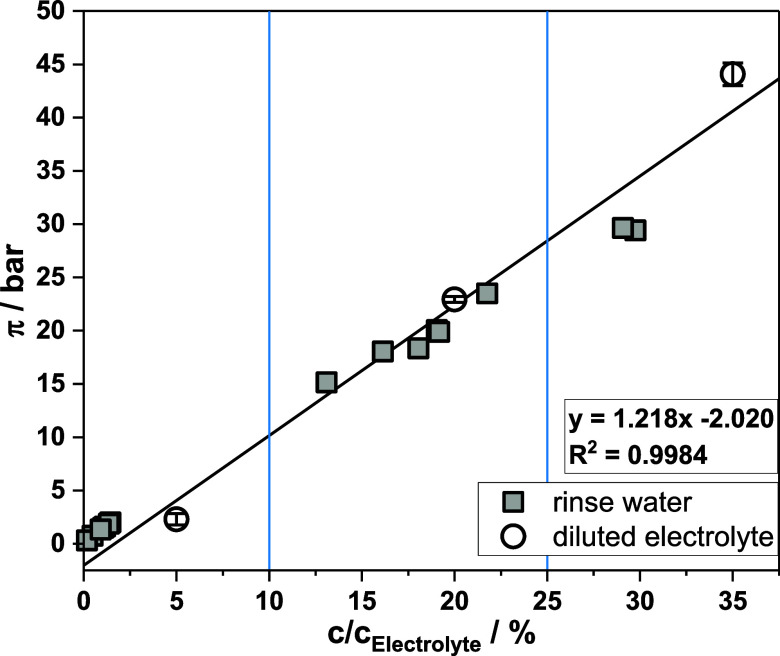
The osmotic pressures of rinsewater samples and diluted electrolytes are displayed in Figure 7. The linear regression was calculated from diluted electrolyte samples (white dots, y = 1.218x – 2.020, R2 = 0.9984). Rinse water samples with a relative concentration of 12.5–22.5% were in good agreement with the linear function. The derivation was 1.8 bar (Δπ/π = 8.2%), on average. Samples in this concentration range were taken right before discharge and displayed the expected feed for RO treatment. In contrast, rinsewater samples with considerably lower concentrations (≤1.8%) showed a maximum deviation of 2.4 bar. Two samples with high concentrations of 29.1 and 29.8% deviated up to 4.8 bar (Δπ/π = 16.3%); for one of the samples, it had been confirmed that it was used for 4 days instead of 2–2.5 days.
Figure 7.
Osmotic pressure of diluted plating electrolyte (white dots) and rinsewater samples (gray squares) with relative concentration. Linear regression based on electrolyte samples. Errors for rinsewater were ≤0.15 bar. Expected concentration range of regular rinsewater samples before discharge depicted with blue lines.
The results show that rinsing water before discharge is indistinguishable in its osmotic pressure from electrolyte samples with a 1:4.5 to 1:8 dilution.
The direct measurement of the electrolytes’ osmotic pressure was possible neither with a freeze point osmometer nor with a water activity meter due to boric acid crystallization from the oversaturated solution. Extrapolation from the diluted electrolyte, using the equation of the linear regression line, the electrolyte (c/cElectrolyte = 100%) was estimated to have an osmotic pressure of 118 bar. This is significantly higher than the 78 bar of the first-generation electrolyte. Although the second gen electrolyte uses an increased sulfate content, the value may be assumed too high.
In highly concentrated solutions, ionic interactions affect dissociation equilibria, thereby affecting osmotic pressure. The interplay between sulfate and boric acid exemplifies such interactions, which was discussed in an earlier section of this work. The same applies to the dissociation behavior of boric acid within these electrolytes: With roughly 1.46 mol/L boric acid in the electrolyte and an average of 0.32 mol/L in rinsewater, boric acid is able to form polyborates.43 Possible effects were also stated in a previous section of this work. The behavior of glycolic and formic acids, serving as organic complexing agents for chromium(III), further complicates the solution dynamics. Their protonation, dissociation, or complexation states are influenced by the concentrations of chromium and the complexing agents, as well as the solution’s temperature and pH. Given the broader variance in chromium concentrations compared to other constituents, and considering that diluted rinsewater is treated at ambient temperatures (20–30 °C), as opposed to the operational temperatures of the electrolyte (50–60 °C),35 accurately determining these equilibria proves challenging.
Despite the complex nature of the electrolyte, our findings affirm that RO concentrates, attained using operating pressures of 110 bar, are adequately concentrated for direct reuse in the plating bath.
Final RO Design
The pilot scale hybrid RO system used in this study, located at the University of Birmingham (U.K.), serves as a starting point for the development of a wastewater treatment rig at BIA plating site in Solingen, Germany. Based on the same hybrid semibatch/batch working principle, adjustments have to be made with regard to the facility and production process.
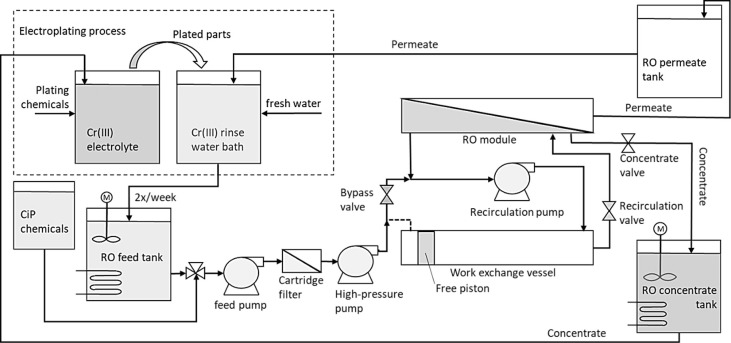
At BIA plastic parts for the automotive industry are plated in three 8 h shifts a day for 5 days per week, on three electroplating lines. One of the last steps in the process is the deposition of a decorative chromium layer by submerging the parts in the plating electrolyte and subsequently rinsing in a rinse bath. The schematic workflow of the RO wastewater treatment plant and its suggested connection to the electroplating infrastructure is displayed in Figure 8 and Major Unit Operations and sensors are summarized in the Supporting Information.
Figure 8.
Scheme of the industrial RO wastewater treatment plant connected to the electroplating line (dashed box).
The rinsewater of a bath (1.5 m3) was discharged to the RO feed tank 1–2 times a week. Heating (25 °C) and stirring in the feed and concentrate tank prevents possible boric acid precipitation. The wastewater treatment is located in the basement of the facility of the company, so the feed tank will be fed by gravity. The membrane module will be equipped with two 8 in. spiral-wound XUS180808 (DuPont XUS180808, DuPont de Nemours Inc.) elements in series, doubling the membrane area of the pilot (30.6m2) to a total of 61.2 m2. Membrane specifications are documented in Table S2 in the Supporting Information. The maximum permeate flux of the system is 12.75 LMH, limited by the capacity of the high-pressure RO pump. Considering an operation like that in the pilot-scale system with different fluxes per phase, the average flux will be lower. Assuming an average flux of 10 LMH, the treatment time of one rinse bath was 2.5 h. This not only enables the treatment of a rinsewater batch within an 8h shift of an operator, including preparations and rinsing of the system but also gives the needed capacity to treat the wastewater of more than one plating line in the future. Both criteria were asked from BIA.
For simplicity of design, the housing of the membrane module and the work exchanging vessel are designed identically: 2.5 m in length and capable of withstand 120 bar of operating pressure. This results in a sweep piston length of 2.2 m2 and a pressure vessel volume of 65 L. The possibility of a cleaning-in-place (CiP) setup was integrated into to design.
Conductivity sensors in the feed, permeate, and concentrate outlet are considered to determine the salt content of the streams going to respective tanks. The possibility to derive the concentration of plating chemicals from the rinsewater conductivity was investigated in this paper. Both the level and temperature are monitored in the tanks. The permeate flow is derived from the level difference over time. According to the high-quality standards of the automotive industry, RO permeate and concentrate are stored batchwise until chemical analysis proves their suitability for reuse. Challenges related to up-scaling projections have been analyzed and discussed.46
Conclusions
In this work, we have identified and answered open questions related to the successful transition from an early stage to a mature RO technology that enables full circularity in Cr(III) electroplating.
Industrial electroplating rinsewater showed high fluctuations in concentrations of organic and inorganic plating components due to different utilization of the rinses (number of plated pieces, shape of the pieces, frequency of rinse discharge). The ratio of components, however, mirrored the involved electrolytes, and conductivity measurements seem to be a useful tool for the prediction of the osmotic pressure as well as sulfate and boric acid concentrations.
We confirmed that the XUS180808 high-pressure RO membrane works very well for the recovery of valuable plating components from the first and second generations. Although of higher concentration and osmotic pressure than the previously investigated first gen rinsewater, the pilot-scale RO treatment was able to increase chromium and sulfate of an artificial second gen rinsewater to the electrolyte level, enabling its direct reuse in the plating process.
A post-treatment procedure for the RO permeate was investigated at the lab-scale involving the addition of sodium sulfate to the feed, which is known to influence boric acid solubility. An increased boric acid rejection of 2.5% on average was reported. Permeates of the second stage achieved the quality for reuse, theoretically closing the loop of the water stream. A second pass RO will to some degree increase the ≤2.7 kWh/m3 SEC (1st pass), but enables a ZLD process design drastically reducing the SEC of the current process (122 kWh/m3).
Future investigations should evaluate the second stage RO treatment on a pilot scale. Although observed osmotic pressures are lower than those found in the rinsewater treatment, the determination of the energy consumption is of interest for the industrial implementation.
Hybrid semibatch/batch RO, as presented in our article, is one of several options for implementing high-recovery RO in the general category of batch and semibatch systems. Overall, we believe it is an important topic of further research to compare the pros and cons of different approaches such as SCRO (Semi-Closed RO).47
With the industrial implementation, a long-term evaluation of the plant performance, especially the membrane rejection capabilities, permeance, and proposed cleaning-in-place procedure, should be evaluated. Alongside a life cycle sustainability assessment (LCSA), the impact of the new procedure has to be evaluated, giving a full picture of connected costs and savings as well as the environmental implications.
Acknowledgments
The authors are indebted to the Institute of Advanced Studies at the University of Birmingham for awarding Stéphan Barbe a Distinguished Visiting Fellowship and facilitating the collaboration with Philip Davies. Thanks to CUT Membrane Technology GmbH (Erkrath, Germany) for providing the lab-scale RO rig.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsestwater.4c00556.
Working principle of hybrid RO; experimental details, materials and methods, including photographs of experimental setup, characterization of first gen rinsewater, osmotic pressure of RO permeates and second pass feed, mass concentration of sulfate and boric acid in rinsewater, and major unit operations of final RO design (PDF)
Data Fig3_beta_R (XLSX)
Data Fig2_Fig6_Fig7_rinse water analysis (XLSX)
Data Fig4_DOE (XLSX)
Data Fig5_concentrations_and_rejections (XLSX)
Data Fig8_osmP_rinses_diluted electrolyte (XLSX)
Author Contributions
R.E.: conceptualization, formal analysis, investigation, methodology, writing—original draft, and writing—review and editing. E.H.: formal analysis, investigation, and writing—review and editing. S.Y.: formal analysis and investigation. F.H.: funding acquisition and investigation. M.W.: investigation. M.U.: supervision and writing—review and editing. P.D. and S.B.: conceptualization, writing—review and editing, supervision, project administration, and funding acquisition. The manuscript was written through contributions of all authors./All authors have given approval to the final version of the manuscript.
The presented research was performed as part of the IntelWATT research project, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 958454.
The authors declare no competing financial interest.
Supplementary Material
References
- Azmi A. A.; Jai J.; Zamanhuri N. A.; Yahya A. Precious Metals Recovery from Electroplating Wastewater: A Review. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 358 (1), 12024. 10.1088/1757-899X/358/1/012024. [DOI] [Google Scholar]
- Rajoria S.; Vashishtha M.; Sangal V. K. Treatment of electroplating industry wastewater: a review on the various techniques. Environmental science and pollution research international 2022, 29 (48), 72196–72246. 10.1007/s11356-022-18643-y. [DOI] [PubMed] [Google Scholar]
- Cohen M. D.; Kargacin B.; Klein C. B.; Costa M. Mechanisms of chromium carcinogenicity and toxicity. Critical reviews in toxicology 1993, 23 (3), 255–281. 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Barroso J. M.COMMISSION REGULATION (EU) No 348/2013 of 17 April 2013:Amending Annex XIV to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off. J. Eur. Union 2013. (1272). [Google Scholar]
- Pechova A.; Pavlata L. Chromium as an essential nutrient: a review. Veterinarni Medicina 2007, 52 (1), 1–18. 10.17221/2010-VETMED. [DOI] [Google Scholar]
- Xu L.; Pi L.; Dou Y.; Cui Y.; Mao X.; Lin an; Fernandez C.; Peng C. Electroplating of Thick Hard Chromium Coating from a Trivalent Chromium Bath Containing a Ternary Complexing Agent: A Methodological and Mechanistic Study. ACS Sustainable Chem. Eng. 2020, 8 (41), 15540–15549. 10.1021/acssuschemeng.0c04529. [DOI] [Google Scholar]
- Büker L.; Dickbreder R.; Böttcher R.; Sadowski S.; Bund A. Investigation of The Reaction Kinetics of Chromium(III) Ions with Carboxylic Acids In Aqueous Solutions and The Associated Effects on Chromium Deposition. J. Electrochem. Soc. 2020, 167 (16), 162509. 10.1149/1945-7111/abd1f4. [DOI] [Google Scholar]
- Ye Y.; Shan C.; Zhang X.; Liu H.; Wang D.; Lv L.; Pan B. Water Decontamination from Cr(III)-Organic Complexes Based on Pyrite/H2O2: Performance, Mechanism, and Validation. Environ. Sci. Technol. 2018, 52 (18), 10657–10664. 10.1021/acs.est.8b01693. [DOI] [PubMed] [Google Scholar]
- Kamar M. T.; Elattar H.; Mahmoud A. S.; Peters R. W.; Mostafa M. K. A critical review of state-of-the-art technologies for electroplating wastewater treatment. Int. J. Environ. Anal. Chem. 2024, 104, 4143. 10.1080/03067319.2022.2098486. [DOI] [Google Scholar]
- Yu X.; Hou Y.; Ren X.; Sun C.; Wang M. Research progress on the removal, recovery and direct high-value materialization of valuable metal elements in electroplating/electroless plating waste solution. Journal of Water Process Engineering 2022, 46, 102577 10.1016/j.jwpe.2022.102577. [DOI] [Google Scholar]
- Kabtamu D. M.; Wu Y.-N.; Chen Q.; Zheng L.; Otake K.-I.; Matović L.; Li F. Facile Upcycling of Hazardous Cr-Containing Electroplating Sludge into Value-Added Metal–Organic Frameworks for Efficient Adsorptive Desulfurization. ACS Sustainable Chem. Eng. 2020, 8 (33), 12443–12452. 10.1021/acssuschemeng.0c03110. [DOI] [Google Scholar]
- Liu S.; Mishra S. B.; Zhang Y.; Qi L. Uptake of Hexavalent Chromium in Electroplating Wastewater by Hydrothermally Treated and Functionalized Sand and Its Sustainable Reutilization for Glass Production. ACS Sustainable Chem. Eng. 2017, 5 (2), 1509–1516. 10.1021/acssuschemeng.6b02185. [DOI] [Google Scholar]
- García V.; Margallo M.; Aldaco R.; Urtiaga A.; Irabien A. Environmental Sustainability Assessment of an Innovative Cr (III) Passivation Process. ACS Sustainable Chem. Eng. 2013, 1 (5), 481–487. 10.1021/sc3001355. [DOI] [Google Scholar]
- Lin J.; Ye W.; Huang J.; Ricard B.; Baltaru M.-C.; Greydanus B.; Balta S.; Shen J.; Vlad M.; Sotto A.; Luis P.; van der Bruggen B. Toward Resource Recovery from Textile Wastewater: Dye Extraction, Water and Base/Acid Regeneration Using a Hybrid NF-BMED Process. ACS Sustainable Chem. Eng. 2015, 3 (9), 1993–2001. 10.1021/acssuschemeng.5b00234. [DOI] [Google Scholar]
- Engstler R.; Reipert J.; Karimi S.; Vukušić J. L.; Heinzler F.; Davies P.; Ulbricht M.; Barbe S. A Reverse Osmosis Process to Recover and Recycle Trivalent Chromium from Electroplating Wastewater. Membranes 2022, 12 (9), 853. 10.3390/membranes12090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Park K.; Yang D. R.; Hong S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Applied Energy 2019, 254, 113652 10.1016/j.apenergy.2019.113652. [DOI] [Google Scholar]
- Davies P. A.; Wayman J.; Alatta C.; Nguyen K.; Orfi J. A desalination system with efficiency approaching the theoretical limits. Desalination and Water Treatment 2016, 57 (48–49), 23206–23216. 10.1080/19443994.2016.1180482. [DOI] [Google Scholar]
- Park K.; Burlace L.; Dhakal N.; Mudgal A.; Stewart N. A.; Davies P. A. Design, modelling and optimization of a batch reverse osmosis (RO) desalination system using a free piston for brackish water treatment. Desalination 2020, 494, 114625 10.1016/j.desal.2020.114625. [DOI] [Google Scholar]
- Park K.; Davies P. A. A compact hybrid batch/semi-batch reverse osmosis (HBSRO) system for high-recovery, low-energy desalination. Desalination 2021, 504, 114976 10.1016/j.desal.2021.114976. [DOI] [Google Scholar]
- Hosseinipour E.; Harris E.; El Nazer H. A.; Mohamed Y. M.A.; Davies P. A. Desalination by batch reverse osmosis (RO) of brackish groundwater containing sparingly soluble salts. Desalination 2023, 566, 116875 10.1016/j.desal.2023.116875. [DOI] [Google Scholar]
- Hosseinipour E.; Karimi S.; Barbe S.; Park K.; Davies P. A. Hybrid semi-batch/batch reverse osmosis (HSBRO) for use in zero liquid discharge (ZLD) applications. Desalination 2022, 544, 116126 10.1016/j.desal.2022.116126. [DOI] [Google Scholar]
- Davenport D. M.; Deshmukh A.; Werber J. R.; Elimelech M. High-Pressure Reverse Osmosis for Energy-Efficient Hypersaline Brine Desalination: Current Status, Design Considerations, and Research Needs. Environ. Sci. Technol. Lett. 2018, 5 (8), 467–475. 10.1021/acs.estlett.8b00274. [DOI] [Google Scholar]
- Karimi S.; Engstler R.; Hosseinipour E.; Wagner M.; Heinzler F.; Piepenbrink M.; Barbe S.; Davies P. A. High-pressure batch reverse osmosis (RO) for zero liquid discharge (ZLD) in a Cr(III) electroplating process. Desalination 2024, 580, 117479 10.1016/j.desal.2024.117479. [DOI] [Google Scholar]
- Jin X.; Tang C. Y.; Gu Y.; She Q.; Qi S. Boric acid permeation in forward osmosis membrane processes: modeling, experiments, and implications. Environ. Sci. Technol. 2011, 45 (6), 2323–2330. 10.1021/es103771a. [DOI] [PubMed] [Google Scholar]
- Cengeloglu Y.; Arslan G.; Tor A.; Kocak I.; Dursun N. Removal of boron from water by using reverse osmosis. Sep. Purif. Technol. 2008, 64 (2), 141–146. 10.1016/j.seppur.2008.09.006. [DOI] [Google Scholar]
- Danilov F. I.; Protsenko V. S.; Butyrina T. E.; Vasil’eva E. A.; Baskevich A. S. Electroplating of chromium coatings from Cr(III)-based electrolytes containing water soluble polymer. Prot Met 2006, 42 (6), 560–569. 10.1134/S0033173206060075. [DOI] [Google Scholar]
- Leimbach M.; Tschaar C.; Zapf D.; Kurniawan M.; Schmidt U.; Bund A. Relation between Color and Surface Morphology of Electrodeposited Chromium for Decorative Applications. J. Electrochem. Soc. 2019, 166 (6), D205–D211. 10.1149/2.0871906jes. [DOI] [Google Scholar]
- Leimbach M.; Tschaar C.; Schmidt U.; Bund A. Low-frequency pulse plating for tailoring the optical appearance of chromium layers for decorative applications. J. Appl. Electrochem. 2020, 50 (4), 489–499. 10.1007/s10800-020-01406-3. [DOI] [Google Scholar]
- Liang A.; Li Y.; Liang H.; Ni L.; Zhang J. A favorable chromium coating electrodeposited from Cr(III) electrolyte reveals anti-wear performance similar to conventional hard chromium. Mater. Lett. 2017, 189, 221–224. 10.1016/j.matlet.2016.12.022. [DOI] [Google Scholar]
- Giovanardi R.; Orlando G. Chromium electrodeposition from Cr(III) aqueous solutions. Surf. Coat. Technol. 2011, 205 (15), 3947–3955. 10.1016/j.surfcoat.2011.02.027. [DOI] [Google Scholar]
- Duroudier J.-P., Ed. Thermodynamics: Chapter 3—Solution Activity Coefficients; Elsevier Ltd, 2017. 10.1016/B978-1-78548-176-5.50003-0. [DOI] [Google Scholar]
- Biesheuvel P. M.; Porada S.; Elimelech M.; Dykstra J. E. Tutorial review of reverse osmosis and electrodialysis. J. Membr. Sci. 2022, 647, 120221 10.1016/j.memsci.2021.120221. [DOI] [Google Scholar]
- Moutafis I.; Scoullos I. M.; Kouvelos E.; Sapalidis A.. The Study of Boron Rejection Relation to Salinity and pH by RO membrane:scientific poster. IntelWATT workshop on European leadership in action: Enabling technologies to boost freshwater preservation.
- MacDermid Enthone GmbH . Technical Data Sheet Trilyte Flash SF Electrolyte (accessed 2023-11-17).
- MacDermid Enthone . TECHNICAL DATA SHEET TriMac BLUE Electrolyte: Decorative, trivalent chromium process for Plating on Plastic; Process Code: 081013; IMDS ID: 997301019/2 (accessed 2023-05-31).
- Alavia W.; Lovera J.; Graber T. A. Thermodynamic modeling of the solubility of boric acid in the systems boric acid+lithium sulfate+water, boric acid+sodium sulfate+water and boric acid+potassium sulfate+water at 293.15–313.15K. Fluid Phase Equilib. 2015, 398, 63–71. 10.1016/j.fluid.2015.04.012. [DOI] [Google Scholar]
- Di Giacomo G.; Brandani P.; Brandani V.; Del Re G. Solubility of boric acid in aqueous solutions of sulfate salts. Desalination 1992, 89 (2), 185–202. 10.1016/0011-9164(92)80100-N. [DOI] [Google Scholar]
- Balinski A.; Recksiek V.; Kelly N. Solvent Extraction of Boric Acid: Comparison of Five Different Monohydric Alcohols and Equilibrium Modeling with Numerical Methods. Processes 2021, 9 (2), 398. 10.3390/pr9020398. [DOI] [Google Scholar]
- Qiu Y.; Ruan H.; Tang C.; Yao L.; Shen J.; Sotto A. Study on Recovering High-Concentration Lithium Salt from Lithium-Containing Wastewater Using a Hybrid Reverse Osmosis (RO)–Electrodialysis (ED) Process. ACS Sustainable Chem. Eng. 2019, 7 (15), 13481–13490. 10.1021/acssuschemeng.9b03108. [DOI] [Google Scholar]
- Magara Y.; Tabata A.; Kohki M.; Kawasaki M.; Hirose M. Development of boron reduction system for sea water desalination. Desalination 1998, 118 (1–3), 25–33. 10.1016/S0011-9164(98)00076-9. [DOI] [Google Scholar]
- Medina W. A.Effect of Lithium and Sodium Sulfates on the Crystallization of Boric Acid from Aqueous Solutions: Kinetic and Thermodynamic Effects. Dissertation; Universidad de Antofagasta, 2015. (accessed 2022-07-03). [Google Scholar]
- Mac L.; Mak D.; Krej F.; Solubility H. Solubility of Trihydrogenboric Acid in Aqueous Solutions Containing Various Cations at Different pH Values. Chem. Papers 1991, 47, 23–27. [Google Scholar]
- Kezia K.; Lee J.; Hill A. J.; Kentish S. E. Convective transport of boron through a brackish water reverse osmosis membrane. J. Membr. Sci. 2013, 445, 160–169. 10.1016/j.memsci.2013.05.041. [DOI] [Google Scholar]
- Crapse K. P.; Kyser E. A. III. Literature Review of Boric Acid solubility Data: Prepared for the U.S. Department of Energy under contract number DE-AC09-08SR22470 (accessed 2022-02-14).
- Ezechi E. H.; Isa M. H.; Kutty S. R. B. M. Boron in Produced Water: Challenges and Improvements: A Comprehensive Review. J. of Applied Sciences 2012, 12 (5), 402–415. 10.3923/jas.2012.402.415. [DOI] [Google Scholar]
- Hosseinipour E.; Davies P. A. Free-piston batch reverse osmosis (RO): Modelling and scale-up. Desalination 2024, 591, 117980 10.1016/j.desal.2024.117980. [DOI] [Google Scholar]
- Mo Z.; Li D.; She Q. Semi-closed reverse osmosis (SCRO): A concise, flexible, and energy-efficient desalination process. Desalination 2022, 544, 116147 10.1016/j.desal.2022.116147. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.