Abstract
Post-Acute Sequelae of SARS-CoV-2 infection (PASC), commonly known as Long COVID, represents a significant and complex health challenge with a wide range of symptoms affecting multiple organ systems. This review examines the emerging evidence suggesting a critical role of the gut and gut-brain axis in the pathophysiology of Long COVID. It explores how changes in the gut microbiome, disruption of gut barrier integrity, and the persistence of SARS-CoV-2 antigens within the gastrointestinal tract may contribute to the prolonged and varied symptoms seen in Long COVID, including chronic inflammation and neuropsychiatric disturbances. The review also summarizes key insights gained about Long COVID, highlighting its multifactorial nature, which involves immune dysregulation, microvascular damage, and autonomic nervous system dysfunction, with the gut playing a central role in these processes. While progress has been made in understanding these mechanisms, current evidence remains inconclusive. The challenges of establishing causality, standardizing research methodologies, and addressing individual variations in the microbiome are discussed, emphasizing the need for further longitudinal studies and more comprehensive approaches to enhance our understanding of these complex interactions. This review underscores the importance of personalized approaches in developing effective diagnostic and therapeutic strategies for Long COVID, while also acknowledging the significant gaps in our current understanding. Future research should aim to further unravel the complex interplay between the gut and Long COVID, ultimately improving outcomes for those affected by this condition.
Keywords: Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), Long COVID, Post COVID, Gut microbiome, Gut-brain axis, Immune dysregulation, SARS-CoV-2 antigen persistence, Gastrointestinal symptoms, Neuropsychiatric symptoms, Chronic inflammation, Microvascular damage, Autonomic nervous system dysfunction, Dysbiosis, COVID-19 pathophysiology, Personalized medicine, Diagnostic biomarkers
Introduction
Post-Acute Sequelae of SARS-CoV-2 infection (PASC), commonly referred to as Long COVID, has emerged as one of the most complex and challenging health issues following the COVID-19 pandemic [1]. Unlike the acute phase of COVID-19, which primarily affects the respiratory system, Long COVID is characterized by a wide array of symptoms that can persist for weeks, months, or even longer, affecting various organ systems [2]. Symptoms of Long COVID include chronic fatigue, cognitive impairments often described as “brain fog,” shortness of breath, chest pain, gastrointestinal issues, and a host of other manifestations that vary greatly among individuals [3]. Notably, these symptoms can occur regardless of the initial severity of the SARS-CoV-2 infection, meaning that even those with mild or asymptomatic COVID-19 can experience significant long-term effects [4].
Understanding the pathophysiology of Long COVID has proven to be an intricate endeavor. The condition is believed to arise from a combination of factors, including persistent viral reservoirs, immune dysregulation, microvascular damage, and autonomic nervous system dysfunction. The heterogeneity of symptoms and their variability across different individuals suggest that Long COVID is likely a multifactorial syndrome with complex interactions among various biological systems [5]. This complexity poses significant challenges in developing effective diagnostic and therapeutic strategies. Current research is increasingly focusing on the potential role of the gut and its microbiome in the pathophysiology of Long COVID, offering new insights that could lead to novel approaches for treatment [6-8].
The gut, often referred to as the “second brain,” plays a crucial role in maintaining overall health. It is deeply involved in digestion, immune regulation, and communication with the central nervous system (CNS) via the gut-brain axis [9]. The gut microbiome—a diverse community of microorganisms residing in the gastrointestinal tract—is essential for these functions and has been implicated in a wide range of diseases, particularly those with an inflammatory or autoimmune component [10]. Given this context, researchers are now investigating how alterations in the gut microbiome and gut function might contribute to the pathophysiology of Long COVID [11,12].
The gut-brain axis, a bidirectional communication network, is central to this investigation. This system involves neural, hormonal, and immunological pathways that facilitate constant interaction between the gut and the brain [13]. Disruptions in the gut-brain axis have been implicated in various conditions, including irritable bowel syndrome (IBS), depression, anxiety, and neurodegenerative diseases [14,15]. The role of the gut microbiome in modulating brain function and behavior has become a critical area of study, particularly in the context of Long COVID, where neuropsychiatric symptoms are prevalent [12,16].
This review aims to provide a comprehensive exploration of the current understanding of the gut’s involvement in the pathophysiology of Long COVID. It will examine the impact of acute COVID-19 on the gut, the potential persistence of SARS-CoV-2 antigens within the gastrointestinal tract, and the subsequent effects on immune function and overall health. Furthermore, the review will delve into the role of serotonin in the gut-brain axis and its implications for the neurological and gastrointestinal symptoms seen in Long COVID. By synthesizing the available evidence, this review seeks to offer insights that could inform future research and therapeutic strategies, with a focus on the potential for gut-targeted interventions in the management of Long COVID.
Overview of Gut Physiology and the Gut-Brain Axis
The human gut hosts a vast and diverse community of microorganisms, collectively known as the gut microbiome [17]. This complex ecosystem includes bacteria, viruses, fungi, and archaea, with bacteria being the most abundant [18]. The gut microbiome plays a critical role in maintaining overall health by aiding in digestion, synthesizing essential vitamins, regulating immune function, and protecting against pathogens [19]. The composition of the gut microbiome is unique to each individual and is influenced by various factors, including diet, genetics, environment, and lifestyle [20]. The microbiome consists besides others of four major bacterial phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. Firmicutes and Bacteroidetes are the most dominant in Western societies, and a balance appears crucial for maintaining gut health [21]. An imbalance in this microbial community, known as dysbiosis, has been linked to numerous health conditions, such as inflammatory bowel disease, obesity, diabetes, and neurological disorders [22,23]. The gut microbiome is not static; it can change in response to various external factors, making it a dynamic and responsive component of human physiology [24].
Emerging research has highlighted the role of the gut microbiome in regulating the immune system. The gut-associated lymphoid tissue (GALT), a part of the immune system, is located in the gut and interacts closely with the microbiome [25]. The gut microbiome influences the development and function of immune cells, including T cells, B cells, and macrophages, and plays a key role in maintaining immune tolerance [26,27]. Disruptions in the gut microbiome can lead to immune dysregulation, contributing to the development of inflammatory and autoimmune diseases [26]. This connection between the gut microbiome and immune function appears to be particularly relevant in the context of Long COVID, where chronic inflammation and immune dysregulation are central features [11,12].
The gut-brain axis is a bidirectional communication network between the gastrointestinal tract and the CNS [28]. This complex system involves multiple pathways, including neural, hormonal, and immunological signaling, that facilitate constant interaction between the gut and the brain [29]. The vagus nerve, a key player in the complex neural communication within the gut-brain axis, serves as a primary conduit for transmitting signals between the gut and the brain [30].
A critical aspect of the gut-brain axis is the role of the gut microbiome in modulating brain function and behavior [31]. The gut microbiome produces a variety of metabolites, including short-chain fatty acids (SCFAs) and neurotransmitters such as serotonin, which can influence brain function [32,33]. Notably, approximately 90% of the body’s serotonin, a neurotransmitter that plays a pivotal role in mood regulation, is produced in the gut [32]. Serotonin not only affects gut motility and secretion but also has a significant impact on the CNS, influencing mood, cognition, and overall mental health [6,9]. Recent studies, including ours, have identified serotonin reduction as a feature of Long COVID [6,34]. The gut-brain axis is also deeply involved in immune regulation. The GALT plays a crucial role in maintaining immune homeostasis and protecting against pathogens. The gut microbiome interacts with GALT to modulate immune responses, which can have far-reaching effects on systemic inflammation and immune function [29,35]. Disruptions in the gut-brain axis have been implicated in various conditions, including IBS, depression, anxiety, and neurodegenerative diseases [36]. Data supports that disturbances in this system also contribute to the persistent symptoms observed in Long COVID [6,7,12].
In summary, the gut and the gut-brain axis is implicated in the control of symptoms in the acute phase of COVID-19 infection and Long COVID [37]. The alterations induced by the virus in these systems may set the stage for the development and persistence of Long COVID, highlighting the importance of understanding these connections in the broader context of COVID-19 pathophysiology.
Impact of Acute COVID-19 on the Gut and Potential Implications for Long COVID
SARS-CoV-2, the virus responsible for COVID-19, primarily infects the respiratory system, but it can also affect the gastrointestinal tract [38]. The virus gains entry into human cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor, which is abundantly expressed not only in the lungs but also along the epithelial cells of the gastrointestinal tract [38], particularly in the small intestine. This broad expression of ACE2 in the gut makes it a significant target for SARS-CoV-2, potentially leading to direct viral infection and replication within the gastrointestinal tract ultimately causing inflammation [39,40]. Additionally, SARS-CoV-2 RNA has been detected in stool samples of infected individuals, further confirming the virus’s presence in the gut [41,42].
Gastrointestinal symptoms are common in COVID-19 and can occur even in the absence of respiratory symptoms. Studies suggest that up to 20-30% of COVID-19 patients experience gastrointestinal symptoms, with diarrhea being the most frequently reported [43-45]. These symptoms are often associated with worse clinical outcomes, as they may indicate a higher viral load or more extensive systemic involvement [41,43,44].
The exact mechanisms by which SARS-CoV-2 induces gastrointestinal symptoms are not fully understood, but they are likely multifactorial [46]. Direct viral invasion of the gut epithelium can disrupt the intestinal barrier, leading to inflammation and altered gut motility [38,47]. Inflammation within the gut, combined with systemic immune responses, may exacerbate these symptoms and contribute to the overall severity of the disease [48]. Moreover, the presence of the virus in the gut might promote viral shedding in stool, raising concerns about fecal-oral transmission, although the extent of this transmission route remains unclear [49].
The impact of acute COVID-19 on the gut has become an area of intense study. SARS-CoV-2, the virus responsible for COVID-19, enters host cells via the ACE2 receptor, which is abundantly expressed in the gut lining [50]. This can lead to direct viral infection of the gastrointestinal tract, contributing to a range of gastrointestinal symptoms observed during acute COVID-19, such as diarrhea, nausea, vomiting, and abdominal pain [43].
Acute COVID-19 and Long COVID have been shown to significantly disrupt the gut microbiome, leading to dysbiosis and inflammation [39,51-53]. Studies report a decrease in beneficial bacterial species such as Faecalibacterium prausnitzii and an increase in pathogenic bacteria, including Enterococcus and Rothia [11,12,47,54]. This imbalance can impair gut barrier function, resulting in increased gut permeability, often referred to as “leaky gut,” which allows microbial products and inflammatory mediators to enter the bloodstream [55]. Such gut barrier dysfunction can trigger systemic inflammation, potentially exacerbating the severity of COVID-19 and contributing to its systemic manifestations [55,56]. Additionally, there have been reports of new-onset pancreatic insufficiency and diabetes following COVID-19 [57-59]. These conditions may contribute to gastrointestinal symptoms, such as malabsorption, and metabolic issues in Long COVID, though the mechanisms remain speculative [60,61].
The disruption of the gut-brain axis during acute COVID-19 may have widespread long-term consequences, affecting both neurological and gastrointestinal health [6,62]. Changes in the gut microbiome can alter serotonin signaling and other key signaling molecules, impacting mood and cognitive functions [6,63,64]. This can contribute to a range of neuropsychiatric symptoms observed in Long COVID, such as “brain fog,” anxiety, and depression [6,63,64]. Furthermore, this disruption may also lay the groundwork for the development of chronic gastrointestinal disorders, enhancing the complexity and persistence of Long COVID symptoms [62]. Additionally, immune dysregulation induced by acute COVID-19, exacerbated by gut-derived inflammation, may persist and continuously play a role in the ongoing symptoms of Long COVID [8,39,65,66].
The gut barrier is a critical component of the body’s defense system [67], preventing the translocation of harmful pathogens and toxins from the gut into the bloodstream. In acute COVID-19, and potentially in the aftermath, the integrity of this barrier can be compromised. SARS-CoV-2 infection, coupled with the inflammatory responses it triggers, can lead to increased gut permeability, a condition often referred to as “leaky gut” [68].
When the gut barrier is breached, microbial products such as lipopolysaccharides (LPS) and other endotoxins can enter the circulation, leading to systemic inflammation [69]. This systemic inflammatory response is a hallmark of severe COVID-19 and is associated with complications such as acute respiratory distress syndrome (ARDS), multiorgan failure, and increased mortality [70,71]. In the context of the gut, this inflammatory cascade may further damage the gut lining, perpetuating a cycle of inflammation and barrier dysfunction [72].
The gut is a major site of immune activity, with a substantial portion of the body’s immune cells residing in the GALT [73]. During acute COVID-19, the immune response in the gut is likely activated not only by the presence of the virus but also by the associated dysbiosis and barrier dysfunction [74]. This activation can lead to the production of pro-inflammatory cytokines, further contributing to both local and systemic inflammation [75]. In Long COVID, the persistence of gut-derived inflammation may contribute to the ongoing systemic inflammation that is a hallmark of the condition [65,76,77]. Additionally, the gut’s immune activity could influence the development of autoimmunity, which is increasingly being recognized as a possible contributor to the long-term sequelae of COVID-19, including Long COVID.
Conditions such as IBS, Small Intestinal Bacterial Overgrowth (SIBO), and Mast Cell Activation Syndrome (MCAS) have been proposed as contributors to the gastrointestinal symptoms observed in Long COVID [78,79]. These conditions, often linked to altered gut motility and immune responses, may overlap with the gastrointestinal manifestations commonly seen in Long COVID patients. IBS and SIBO are associated with changes in gut motility and bacterial overgrowth, while MCAS can contribute through chronic low-grade inflammation and immune dysregulation [79]. The interplay of these factors, alongside potential autonomic dysfunction affecting gut motility, could contribute to the persistence of gut-related symptoms in Long COVID, highlighting the multifactorial nature of this condition [78].
In summary, the impact of acute COVID-19 on the gut is multifaceted, involving direct viral invasion, dysbiosis, barrier dysfunction, and heightened immune responses. These changes not only contribute to the gastrointestinal symptoms experienced during the acute phase but may also have lasting effects that influence the development and persistence of symptoms in Long COVID, which is poorly understood. Understanding intestinal disease mechanisms in Long COVID appears crucial for developing targeted therapies.
SARS-CoV-2 Antigen Persistence
The persistence of SARS-CoV-2 antigens was first identified in the gut, marking a significant breakthrough in understanding the potential long-term effects of the virus [8,66]. This discovery emerged from studies that detected viral RNA and protein fragments in the gastrointestinal tract of patients well after their recovery from the acute phase of COVID-19 [8,66]. Unlike many viral infections that are fully cleared by the immune system, SARS-CoV-2 has demonstrated a tendency to persist in the body, with the gastrointestinal tract being one of the easiest organs to study [8,80-82]. Our endoscopy study published in 2022 was pivotal, being the first to establish a direct link between persisting SARS-CoV-2 antigen fragments in the gut and Long COVID [8]. Building on this foundation, our follow-up study further demonstrated that clearance of gut mucosal SARS-CoV-2 antigen fragments is linked to the resolution of Long COVID symptoms [34], suggesting that targeted therapeutic strategies focusing on viral clearance from the gut could be supportive in managing Long COVID [34].
Since these findings, multiple studies have corroborated the evidence of SARS-CoV-2 persistence in the gut and other tissues associated with Long COVID [8,66,81,83]. Research has demonstrated the presence of viral RNA and proteins in the stool of COVID-19 patients weeks to months after their initial infection, even in the absence of gastrointestinal and respiratory symptoms [42,84]. Postmortem analyses have also detected viral antigens in the intestinal tissues, the respiratory tract, lymph nodes, and other organs of patients who had recovered from COVID-19, further supporting the idea that the gut may serve as a reservoir for the virus [81]. Additionally, SARS-CoV-2 antigen persistence was also reported in plasma [80,85]. The identification of viral persistence in the gut provided a critical insight into the possible reservoirs where the virus might evade the immune system, contributing to ongoing symptoms [8].
The persistence of SARS-CoV-2 antigens in the gastrointestinal tract may be driven by several underlying mechanisms [86]. The virus’s ability to evade immune detection by residing within specific, less accessible cell types is one possibility [87,88]. The unique immune environment of the gut, which tolerates a wide array of foreign antigens, may further enable the virus’s survival without eliciting a strong immune response. Additionally, the virus may enter a latent state within certain cells, remaining dormant but capable of reactivation [89,90]. While much evidence suggests that viral remnants—non-infectious fragments [8]—persist after the acute infection, there are reports indicating the presence of low levels of active, replicating virus [88]. This raises important questions about whether these remnants are merely inert or if low-grade viral replication is occurring. Immune dysfunction, whether due to underlying conditions or immunosuppressive medications, may play a significant role in this persistence, potentially allowing the virus to replicate at low levels and contribute to prolonged symptoms [88,90,91]. In this context, Gut Secretory IgA (SIgA) deficiency, a component of mucosal immunity, has also been speculated to contribute to the persistence of viral antigens in the gut [92,93]. Understanding these dynamics is crucial for addressing long-term viral persistence and its clinical implications.
While SARS-CoV-2 antigen persistence has been observed in multiple organs [81], the gut presents a unique environment that may facilitate this persistence. The gastrointestinal tract is constantly exposed to a variety of external antigens, which the immune system must tolerate to maintain homeostasis. This immune tolerance might allow SARS-CoV-2 antigens to persist in the gut longer than in other tissues where the immune response is more robust [94]. Additionally, the gut’s complex microbiome and its interactions with the immune system might influence the persistence of the virus, either by protecting it from immune detection or by creating an environment conducive to viral latency [47].
The persistence of viral antigens in the gut can lead to chronic immune activation [8,95]. This heightened state of alertness can drive ongoing inflammation, which is a key feature of Long COVID [96]. The continuous presence of viral antigens may stimulate the production of pro-inflammatory cytokines and other immune mediators, contributing to the systemic inflammation observed in some Long COVID patients [97,98]. This ongoing immune activation can perpetuate symptoms such as fatigue, muscle pain, and cognitive dysfunction, which are commonly reported in Long COVID [95,99-101].
The concept of targeting antigen persistence in the gut as a therapeutic approach for treating Long COVID is intriguing but remains speculative due to weak supporting data. Therapies aimed at modulating the gut environment, such as probiotics, prebiotics, or dietary interventions, with the goal of reducing antigen retention and dampening chronic inflammation, cannot be recommended based on current evidence [102,103]. Similarly, fecal microbiota transplantation (FMT) to ameliorate Long COVID symptoms is highly experimental [104]. A potentially more promising strategy is the use of antiviral drugs to eliminate residual viral RNA or proteins from the gut, but the evidence for their efficacy is limited. Although Nirmatrelvir-Ritonavir has shown post-acute benefits when administered during acute COVID-19 [105], particularly against cardiovascular and respiratory complications, a recent study found that while generally safe, it did not significantly improve specific Long COVID symptoms [106]. The ongoing RECOVER-VITAL study (ClinicalTrials.gov-NCT05595369) is further investigating the ability of Nirmatrelvir-Ritonavir to alleviate persistent Long COVID symptoms, with results expected by October 2025.
Until then, the effectiveness of such interventions remains uncertain. A deeper understanding of antigen persistence in the gut could eventually lead to biomarkers for identifying patients at risk of Long COVID and guide more personalized treatments, but this area of research is still evolving [11,107].
Pathophysiology of Long COVID
Besides antigen persistence, the pathophysiology of Long COVID is a complex and multifactorial process, involving a combination of immune dysregulation, vascular and organ damage, chronic inflammation, and neurotransmitter imbalances [5,108]. This intricate interplay of factors not only challenges our understanding of Long COVID but also highlights the broad spectrum of clinical manifestations associated with the condition [109-111]. The multifactorial nature of Long COVID underlines the difficulty in pinpointing a single causative mechanism, suggesting that multiple pathways contribute to the persistent symptoms observed in patients [5,108].
These symptoms, which span across different organ systems, highlight the extensive impact of Long COVID [112]. Commonly reported symptoms include chronic fatigue, respiratory issues, neurological disturbances such as brain fog and headaches, as well as cardiovascular and musculoskeletal complaints [112].
The severity and duration of these symptoms vary greatly, with some individuals experiencing mild and transient issues, while others suffer from debilitating and persistent conditions [107,113]. The heterogeneity of symptoms also complicates clinical research and the development of therapeutic strategies. It suggests that Long COVID may not be a single entity but rather a syndrome resulting from multiple overlapping pathophysiological processes [114]. This variability underscores the importance of personalized medicine approaches in managing Long COVID, where treatment is tailored to the specific symptoms and underlying mechanisms affecting each individual patient [115]. Moreover, the onset of symptoms can be delayed, with some individuals only developing symptoms weeks or even months after their initial recovery from acute COVID-19 [116,117].
Similar to Long COVID, several other gastrointestinal and systemic disorders—such as IBS, SIBO, MCAS, leaky gut syndrome, fibromyalgia, postural orthostatic tachycardia syndrome (POTS), and chronic fatigue syndrome (CFS)—often lack clear diagnostic markers, making accurate diagnosis particularly challenging [118,119]. Distinguishing Long COVID from these conditions, sometimes imprecisely labeled as functional disorders due to the absence of robust diagnostic tools, presents a critical challenge in post-COVID management. This complexity arises from overlapping symptoms and the multifactorial nature of these conditions [120-122]. The differential diagnosis primarily relies on a comprehensive clinical history, including a confirmed SARS-CoV-2 infection, which crucially links symptoms to a preceding viral event, unlike many other functional disorders that may not have such identifiable triggers [123]. However, even with a clear history of COVID-19, distinguishing Long COVID can be fraught with difficulties due to symptom commonalities with disorders like CFS or fibromyalgia, such as fatigue, cognitive disturbances, and musculoskeletal pain [124]. Although certain symptoms like prolonged loss of taste or smell and specific imaging findings may suggest Long COVID, these are not universally present in all cases. Furthermore, the inherent variability in how individuals experience and report symptoms adds another layer of complexity, making it challenging to definitively pinpoint Long COVID in every situation [109]. A multidisciplinary approach involving various specialties is essential, yet even this comprehensive strategy may not always yield clear distinctions, underscoring the need for ongoing research and development of more precise diagnostic criteria [125].
Understanding the pathophysiologic mechanisms underlying Long COVID is essential for developing effective treatments. The following sections delve into the most relevant and impactful factors that contribute to the development and persistence of this condition.
Immune Dysregulation: One of the central hypotheses in understanding the pathophysiology of Long COVID is immune dysregulation. In many Long COVID patients, there is evidence of chronic inflammation and an ongoing immune response long after the resolution of the acute infection [95,126,127]. This chronic inflammation may be driven by several factors, including the persistence of viral antigens, autoimmunity, and a dysregulated immune response [8,127,128]. Autoimmunity has been proposed as a contributing factor to the development of symptoms such as joint pain, rashes, and neurological issues [129-131]. The persistence of immune activation, characterized by elevated levels of inflammatory cytokines, could lead to tissue damage and chronic symptoms [126,132,133].
Viral Persistence: Another key hypothesis is that Long COVID symptoms may be driven by the persistent presence of SARS-CoV-2 viral antigens in the body [8,80,82,85]. These viral reservoirs could exist in various tissues, including the gut, lungs, and lymph nodes, where they continue to stimulate the immune system and drive inflammation [65,81,96,134]. The gut, in particular, has been identified as a potential reservoir for these viral antigens, as discussed in previous sections. The persistence of viral components may not only contribute to ongoing immune activation but also interfere with the normal function of the affected organs, leading to a wide range of symptoms [135,136].
Microvascular Damage: Microvascular damage is another proposed mechanism contributing to Long COVID. SARS-CoV-2 has been shown to cause endothelial damage and inflammation, leading to the formation of microthrombi (small blood clots) and impaired blood flow in small vessels [137,138]. This microvascular injury can result in tissue hypoxia and damage, particularly in organs with high metabolic demands, such as the brain, heart, and muscles [139,140]. Symptoms such as fatigue, cognitive dysfunction, and muscle pain could be linked to this impaired blood flow and tissue damage [141].
Autonomic Nervous System Dysfunction: Dysfunction of the autonomic nervous system (ANS) is increasingly recognized as a significant factor in Long COVID [142]. The ANS controls involuntary bodily functions, including heart rate, blood pressure, and digestion. Dysautonomia, or autonomic dysfunction, can manifest as symptoms like tachycardia, dizziness, and gastrointestinal disturbances [143,144]. Many Long COVID patients report symptoms consistent with dysautonomia, including those resembling POTS [142,145]. The precise mechanisms underlying ANS dysfunction in Long COVID are still under investigation, but they may be related to direct viral effects on autonomic pathways, immune-mediated nerve damage, or persistent inflammation [146].
Role of Serotonin: Approximately 90% of the body’s serotonin, a crucial neurotransmitter, is produced in the gut, primarily by enterochromaffin cells [147]. Serotonin plays a vital role in regulating various gastrointestinal functions, including gut motility, secretion, and the coordination of reflexes that control digestion [148]. Beyond its role in the gut, serotonin is a critical mediator in the gut-brain axis, influencing not only gastrointestinal function but also brain activity and behavior [32,149]. Serotonin produced in the gut can affect mood, cognition, and overall mental health by interacting with the CNS via neural and humoral pathways [32]. Disruptions in serotonin signaling are associated with conditions such as depression, anxiety, and cognitive impairments [150]. These mental health issues are commonly reported in Long COVID patients, suggesting that alterations in gut serotonin production and signaling may contribute to the neuropsychiatric symptoms observed in this condition [6,151,152].
There is emerging evidence that acute COVID-19 can alter serotonin levels in the body [6,34], potentially through mechanisms such as gut inflammation, dysbiosis, and changes in the function of enterochromaffin cells [6]. The inflammatory response triggered by SARS-CoV-2 may disrupt the normal production and regulation of serotonin in the gut, leading to downstream effects on both gastrointestinal and neurological health [6,63]. Therefore, alterations in serotonin signaling could play a significant role in the persistence of symptoms in Long COVID. In the context of serotonin depletion and the quest for effective treatments, the role of Selective Serotonin Reuptake Inhibitors (SSRIs) in managing post-acute COVID-19 syndrome has become increasingly prominent [153]. SSRIs, by blocking serotonin reuptake and thereby increasing brain serotonin levels [154], directly address this deficiency, potentially alleviating a broad array of Long COVID symptoms. Their anti-inflammatory properties further enhance their therapeutic appeal given the role of inflammation in the condition [155].
Clinical Implications and Therapeutic Potential
Postviral illnesses, including but not limited to those caused by SARS-CoV-2, encompass a significant portion of the global health burden, manifesting in prolonged and debilitating conditions that can drastically affect individuals’ quality of life [130,156]. These conditions, which arise after recovery from various viral infections such as influenza, Epstein-Barr virus, and others, include a range of persistent symptoms like chronic fatigue, neurocognitive issues, and multisystem complaints [130]. Their impact is extensive, not only on health systems but also on economic productivity due to prolonged illness and disability [157]. The recent pandemic has brought renewed attention to postviral syndromes, emphasizing the need for healthcare systems to recognize and address these conditions as part of a broader approach to post-infection health complications [130]. This awareness is crucial for developing strategies to manage and mitigate the long-term effects of viral diseases globally.
Building on the insights into the widespread impact of postviral illnesses, recent research into the gut microbiome offers a pivotal opportunity for advancements in the field of diagnostics. The distinct alterations in gut microbiome profiles observed in Long COVID patients present a promising potential for the development of diagnostic biomarkers [11,107]. However, the creation of reliable biomarkers is not without challenges. It requires extensive validation through large-scale, longitudinal studies, and there remains some skepticism about whether these biomarkers can be consistently accurate and reproducible across ethnicities and geographical locations. Additionally, the standardization of microbiome analysis techniques is essential, but currently lacks uniformity, raising further concerns about the feasibility and reliability of these potential diagnostic tools.
This uncertainty surrounding the gut microbiome also extends to therapeutic approaches. Given the proposed role of the gut in Long COVID pathophysiology, gut-targeted therapies have been suggested as a potential area of exploration. However, as of now, there is no substantial evidence supporting the efficacy of probiotics, prebiotics, or dietary interventions in the treatment of Long COVID [158,159]. Therefore, any intervention in this regard remains experimental. In cases with symptoms resembling IBS, exclusion or FODMAP diets could be theoretically considered, though their effectiveness in Long COVID remains unclear, and the restrictive nature of these diets may carry risks, such as the potential development of disordered eating behaviors [160]. This lack of rigorous data necessitates a cautious approach, particularly to avoid encouraging Long COVID patients to invest in these interventions without clear therapeutic benefit. While placebo effects cannot be entirely dismissed [161], the promotion of such treatments should be carefully considered in the absence of definitive scientific support.
Similarly, FMT, which aims to restore microbial diversity, remains highly experimental, with its application in Long COVID yet to be substantiated. A recent study involving 60 patients suggested that FMT may be effective and safe for alleviating long-COVID-related insomnia, but these findings are preliminary and require validation in larger trials [162]. Vagus nerve stimulation, which has shown some promise in modulating autonomic nervous system function and reducing inflammation in other conditions [163,164] may hold potential, but rigorous studies evaluating its impact on Long COVID are lacking. While these therapies are under consideration, further research is necessary before their clinical relevance can be confirmed.
Therapies for Long COVID require a multifaceted approach that addresses the complex and varied symptoms of the condition [117]. Key strategies include lifestyle modifications, exercise, psychological support to manage mental health challenges, and physical rehabilitation for those experiencing fatigue and physical deconditioning [165]. In addition to these supportive measures, several promising pharmaceutical interventions are being explored, including SSRIs [153] and antiviral agents like Nirmatrelvir-Ritonavir [106]. Considerations regarding the use of these therapies, including their potential benefits and limitations, are discussed in detail elsewhere in this review.
More research is needed to fully understand the role of pharmaceutical interventions in Long COVID, particularly in addressing the diverse underlying mechanisms of the condition. Clinical trials specifically evaluating the efficacy of these treatments in Long COVID patients could provide valuable insights into their potential as therapeutic options. Additionally, research into personalized medicine approaches—tailoring treatments based on individual patient profiles, including gut microbiome composition, serotonin levels and specific symptomatology—could enhance therapeutic outcomes for Long COVID patients.
Limitations and Challenges
While the evidence supporting the gut’s role in Long COVID is compelling, several limitations must be acknowledged. Much of the current research is observational, and causality between gut dysbiosis and Long COVID symptoms remains to be firmly established. The complexity of the gut microbiome and its interactions with the immune system and other bodily systems presents significant challenges in deciphering the exact mechanisms by which the gut contributes to Long COVID.
Another limitation is the variability in methodologies used across studies, including differences in microbiome sampling, sequencing techniques, and data analysis. This variability can lead to inconsistencies in findings and makes it difficult to draw definitive conclusions. Furthermore, the gut microbiome is highly individualized and influenced by a multitude of factors, including diet, medications, and environmental exposures, adding another layer of complexity to research in this area.
To overcome these challenges, there is a critical need for more longitudinal studies that track changes in the gut microbiome over time in individuals recovering from COVID-19. Such studies would provide valuable insights into the temporal dynamics of the gut microbiome and its relationship with Long COVID development and progression. Longitudinal data could also help identify specific microbial changes that precede the onset of Long COVID symptoms, offering potential targets for early intervention.
Moreover, future research should focus on integrating multi-omics approaches, combining microbiome analysis with other data types, such as metabolomics, proteomics, and transcriptomics, to gain a more comprehensive understanding of the gut’s role in Long COVID. This integrative approach could uncover novel biomarkers and therapeutic targets by revealing the complex interactions between the gut microbiome, host metabolism, and immune responses.
While significant progress has been made in understanding the gut’s role in Long COVID, much work remains to translate these findings into clinical practice. Addressing the limitations of current research and pursuing more robust and integrative studies will be essential for developing effective diagnostic and therapeutic strategies that can improve the outcomes for individuals affected by Long COVID.
Future Directions
Despite the growing body of evidence suggesting a critical role for the gut in the pathophysiology of Long COVID, significant gaps remain in our understanding. One major gap is the lack of comprehensive, longitudinal data that tracks changes in the gut microbiome and gut-related biomarkers over the course of COVID-19 and its aftermath. While cross-sectional studies have provided insights into microbiome alterations associated with Long COVID, they do not capture the dynamic changes that may occur over time or how these changes relate to the development and persistence of symptoms.
Another critical gap is the mechanistic understanding of how specific gut microbial alterations contribute to the systemic inflammation, immune dysregulation, and neuropsychiatric symptoms observed in Long COVID. The causal relationships between gut dysbiosis, gut barrier dysfunction, and the broader pathophysiology of Long COVID are not yet fully elucidated, making it challenging to develop targeted interventions.
Additionally, there is a need for more research on the interactions between the gut microbiome and other body systems in Long COVID, particularly the immune system and the CNS. Understanding how gut-derived signals, such as metabolites and cytokines, influence these systems could provide deeper insights into the multi-organ nature of Long COVID and identify potential therapeutic targets.
As our understanding of the gut’s role in Long COVID evolves, several emerging areas of research hold promise for advancing diagnosis and treatment summarized in Table 1.
Table 1. Advancing Diagnosis and Treatment.
| Personalized Microbiome Therapies | The recognition that the gut microbiome varies significantly between individuals has led to the exploration of personalized microbiome therapies. These therapies could involve tailored probiotic or prebiotic interventions, designed based on an individual’s specific microbiome profile, to restore microbial balance and alleviate Long COVID symptoms. Personalized diets that target specific microbial communities might also be developed to support gut health and reduce inflammation. |
| Advanced Omics Technologies | Integrating advanced omics technologies, such as metagenomics, metabolomics, and transcriptomics, into Long COVID research offers a powerful approach to understanding the complex interactions between the gut microbiome and the host. Metagenomics can provide detailed insights into the composition and functional potential of the gut microbiome, while metabolomics can reveal the metabolic products of microbial activity and their effects on host physiology. Transcriptomics can help uncover how gut-derived signals influence gene expression in different tissues, including the immune system and the brain. |
| Microbiome-Immune Interactions | Emerging research is also focusing on the intricate interactions between the gut microbiome and the immune system in Long COVID. Understanding how specific gut microbes modulate immune responses could lead to novel immunomodulatory therapies that target the gut to treat systemic inflammation and autoimmunity associated with Long COVID. |
| Gut-Brain Axis Studies | The gut-brain axis is another area of intense interest, particularly in relation to the neuropsychiatric symptoms of Long COVID. Research is increasingly focusing on how gut microbiome alterations influence brain function and behavior through serotonin production, vagal nerve signaling, and inflammatory pathways. Studies in this area could lead to new treatments that address both gastrointestinal and neurological symptoms of Long COVID. |
| Longitudinal Cohort Studies | To address the existing research gaps, future studies should include large, well-characterized longitudinal cohorts of COVID-19 patients. These studies would ideally track participants from the acute phase of infection through recovery and beyond, capturing detailed data on microbiome composition, immune function, and clinical outcomes. Such research could identify early indicators of Long COVID, reveal potential therapeutic targets, and inform the development of prevention strategies. |
In conclusion, while significant strides have been made in understanding the gut’s role in Long COVID, there is still much to learn. Addressing the current research gaps through innovative approaches and emerging areas of study will be crucial for developing effective diagnostic tools and treatments for Long COVID. By leveraging personalized therapies and advanced omics technologies, we can move closer to unraveling the complexities of Long COVID and improving outcomes for those affected by this condition.
Conclusion
The exploration of the gut’s role in Long COVID has unveiled significant insights, yet it has also highlighted the complexity and multifactorial nature of this condition. The gut microbiome, with its intricate connections to the immune system and the gut-brain axis, emerges as a central player in the pathophysiology of Long COVID. Alterations in the gut microbiome during and after acute COVID-19 may contribute to the persistence of symptoms, influencing not only gastrointestinal health but also systemic inflammation, immune dysregulation, and neuropsychiatric manifestations.
Despite the promising potential for gut-targeted therapies and the development of diagnostic biomarkers, the current evidence remains inconclusive. The challenges of establishing causality, standardizing methodologies, and interpreting highly individualized microbiome data underscore the need for further research. The variability in findings across studies, coupled with the lack of longitudinal data, makes it difficult to draw definitive conclusions about the gut’s role in Long COVID and the efficacy of potential interventions.
Looking forward, the integration of advanced technologies, personalized medicine approaches, and large-scale longitudinal studies will be crucial. These strategies could provide deeper insights into the dynamic changes in the gut microbiome and its interactions with other body systems, paving the way for more targeted and effective treatments. However, while these approaches are promising, the feasibility of such ambitious research endeavors may be compromised by waning public and institutional focus, potentially hindering the continuity and scale of necessary investigations. Addressing the limitations of current research through robust and integrative studies will be essential for translating these findings into clinical practice.
In summary, while significant progress has been made in understanding the gut’s involvement in Long COVID, much work remains to fully elucidate its role and harness this knowledge to improve patient outcomes. As the research evolves, it will be critical to remain cautious yet optimistic about the potential for gut-targeted therapies and personalized interventions in the management of Long COVID.
Final Thoughts
The relationship between the gut and Long COVID represents a complex and multifaceted area of research with significant clinical implications. While the focus on COVID-19 has diminished in the global healthcare conversation, understanding the gut’s role in Long COVID remains crucial for developing effective interventions for those still affected. The potential for gut-targeted therapies to alleviate symptoms and improve the quality of life for Long COVID patients is promising, but it requires further validation through rigorous research.
Continued investigation into the gut microbiome, its interactions with the immune system, and its influence on systemic and neuropsychiatric health is essential. By addressing the current research gaps and embracing innovative approaches, we can move closer to unlocking the complexities of Long COVID and providing solutions for those dealing with its lingering effects. The integration of personalized medicine and advanced technologies will be key to achieving this goal, ultimately leading to more targeted and effective treatments that reflect the unique needs of each patient.
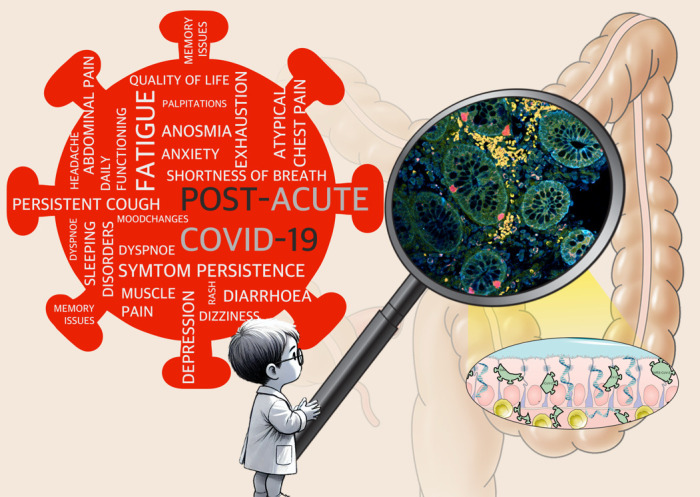
Graphical abstract
Glossary
- ACE-2
angiotensin-converting enzyme 2
- CFS
Chronic Fatigue Syndrome
- CNS
Central Nervous System
- FMT
Fecal Microbiota Transplantation
- GALT
Gut-Associated Lymphoid Tissue
- IBS
Irritable Bowel Syndrome
- LPS
Lipopolysaccharides
- MCAS
Mast Cell Activation Syndrome
- PASC
Post-Acute Sequelae of SARS-CoV-2 infection
- POTS
Postural Orthostatic Tachycardia Syndrome
- SIBO
Small Intestinal Bacterial Overgrowth
Data Transparency Statement
Data will be made available after publication.
References
- Ely EW, Brown LM, Fineberg HV, National Academies of Sciences, Engineering, and Medicine Committee on Examining the Working Definition for Long Covid . Long Covid Defined. N Engl J Med. 2024. Nov;391(18):1746–53. 10.1056/NEJMsb2408466 [DOI] [PubMed] [Google Scholar]
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021. Aug;38:101019. 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballering AV, van Zon SK, Olde Hartman TC, Rosmalen JG, Lifelines Corona Research Initiative . Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022. Aug;400(10350):452–61. 10.1016/S0140-6736(22)01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahanic S, Tymoszuk P, Ausserhofer D, Rass V, Pizzini A, Nordmeyer G, et al. Phenotyping of Acute and Persistent Coronavirus Disease 2019 Features in the Outpatient Setting: Exploratory Analysis of an International Cross-sectional Online Survey. Clin Infect Dis. 2022. Aug;75(1):e418–31. 10.1093/cid/ciab978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023. Mar;21(3):133–46. 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Devason AS, Umana IC, Cox TO, Dohnalová L, Litichevskiy L, et al. Serotonin reduction in post-acute sequelae of viral infection. Cell. 2023;186(22):4851-67.e20. Epub 20231016. https://doi.org/ 10.1016/j.cell.2023.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Wick N, et al. Clearance of Gut Mucosal SARS-CoV-2 Antigens and Postacute COVID-19 After 2 Years in Patients With Inflammatory Bowel Disease. Gastroenterology. 2024;167(3):604-7.e8. Epub 20240416. https://doi.org/ 10.1053/j.gastro.2024.04.008. [DOI] [PubMed] [Google Scholar]
- Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rössler A, et al. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology. 2022;163(2):495-506.e8. Epub 20220501. https://doi.org/ 10.1053/j.gastro.2022.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011. Jul;12(8):453–66. 10.1038/nrn3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011. Oct;10(4):311–23. 10.1016/j.chom.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698-706. Epub 20210111. https://doi.org/ 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71(3):544-52. Epub 20220126. https://doi.org/ 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451-63. Epub 20131205. https://doi.org/ 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016. Dec;167(6):1469–1480.e12. 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskov H, Burcharth J, Pommergaard HC, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7(5):365-83. Epub 20160729. https://doi.org/ 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie CE, Lowe DJ, McAuley A, Winter AJ, Mills NL, Black C, et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. 2022;13(1):5663. Epub 20221012. https://doi.org/ 10.1038/s41467-022-33415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012. Jun;486(7402):207–14. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010. Mar;464(7285):59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011. Jun;474(7351):327–36. 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480-4. Epub 20081130. https://doi.org/ 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174-80. Epub 20110420. https://doi.org/ 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. Epub 20120926. https://doi.org/ 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006. Dec;444(7122):1022–3. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012. Sep;489(7415):220–30. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014. Mar;157(1):121–41. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4-14. Epub 20120101. https://doi.org/ 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Harrison OJ. Homeostatic Immunity and the Microbiota. Immunity. 2017. Apr;46(4):562–76. 10.1016/j.immuni.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016. Nov;167(4):915–32. 10.1016/j.cell.2016.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145-55. Epub 20170116. https://doi.org/ 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144(1):36-49. Epub 20121012. https://doi.org/ 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9. [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015. Apr;161(2):264–76. 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016. Jun;165(6):1332–45. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Wick N, et al. Clearance of Gut Mucosal SARS-CoV-2 Antigens and Postacute COVID-19 After 2 Years in Patients With Inflammatory Bowel Disease. Gastroenterology. 2024. Aug;167(3):604–607.e8. 10.1053/j.gastro.2024.04.008 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268-73. Epub 20120606. https://doi.org/ 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015. May;17(5):565–76. 10.1016/j.chom.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Detrimental effects of COVID-19 in the brain and therapeutic options for long COVID: The role of Epstein-Barr virus and the gut-brain axis. Mol Psychiatry. 2023;28(12):4968-76. Epub 20230704. https://doi.org/ 10.1038/s41380-023-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020. Jul;369(6499):50–4. 10.1126/science.abc1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020. Aug;69(8):1543–4. 10.1136/gutjnl-2020-321388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831-3.e3. Epub 20200303. https://doi.org/ 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159(1):81-95. Epub 20200403. https://doi.org/ 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434-5. Epub 20200320. https://doi.org/ 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020. May;115(5):766–73. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology. 2020;159(2):765-7.e2. Epub 20200422. https://doi.org/ 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner A, Watschinger C, Rössler A, Farcet MR, Penner A, Böhm V, et al. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine. 2021. Aug;70:103539. 10.1016/j.ebiom.2021.103539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Hsu MT, Lee MY, Chou CK. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses. 2022;14(6). Epub 20220530. https://doi.org/ 10.3390/v14061188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70(2):276-84. Epub 20200720. https://doi.org/ 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Lu R, Zhang T, Wu Q, Cai W, Han X, et al. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun Biol. 2021;4(1):240. Epub 20210218. https://doi.org/ 10.1038/s42003-021-01796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021;18(4):269-83. Epub 20210215. https://doi.org/ 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444-8. Epub 20200304. https://doi.org/ 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Integrative Human Microbiome Project . Nature. 2019;569(7758):641-8. Epub 20190529. https://doi.org/ 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestad B, Ueland T, Lerum TV, Dahl TB, Holm K, Barratt-Due A, et al. Respiratory dysfunction three months after severe COVID-19 is associated with gut microbiota alterations. J Intern Med. 2022;291(6):801-12. Epub 20220317. https://doi.org/ 10.1111/joim.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Lau RI, Liu Q, Su Q, Chan FKL, Ng SC. Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications. Nat Rev Gastroenterol Hepatol. 2023;20(5):323-37. Epub 20221021. https://doi.org/ 10.1038/s41575-022-00698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159(3):944-55.e8. Epub 20200520. https://doi.org/ 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottein F, Sokol H. Potential Causes and Consequences of Gastrointestinal Disorders during a SARS-CoV-2 Infection. Cell Rep. 2020;32(3):107915. Epub 20200703. https://doi.org/ 10.1016/j.celrep.2020.107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang Y, Li C, Chang W, Zhang L. The relationship between gut microbiota and COVID-19 progression: new insights into immunopathogenesis and treatment. Front Immunol. 2023;14:1180336. Epub 20230502. https://doi.org/ 10.3389/fimmu.2023.1180336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Bao L, Song Z, Zhang L, Yu P, Xu Y, et al. Infection with SARS-CoV-2 can cause pancreatic impairment. Signal Transduct Target Ther. 2024. Apr;9(1):98. 10.1038/s41392-024-01796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14(6):2211-7. Epub 20201117. https://doi.org/ 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-Onset Diabetes in Covid-19. N Engl J Med. 383. United States2020. p. 789-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HK, Makker J, Alemam A, Chilimuri S. Diarrhea due to SARS-CoV-2-Related Exocrine Pancreatic Insufficiency. Case Rep Gastrointest Med. 2021;2021:9920981. Epub 20210521. https://doi.org/ 10.1155/2021/9920981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149-65. Epub 20210203. https://doi.org/ 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F, et al. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022. Apr;7(1):143. 10.1038/s41392-022-00986-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Canoll PD, Klein RS. How COVID-19 Affects the Brain. JAMA Psychiatry. 2021. Jun;78(6):682–3. 10.1001/jamapsychiatry.2021.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185(14):2452-68.e16. Epub 20220613. https://doi.org/ 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Deitchman AN, Torres L, Iyer NS, Munter SE, Nixon CC, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021;36(6):109518. Epub 20210806. https://doi.org/ 10.1016/j.celrep.2021.109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C, Wang Z, Lorenzi JC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021. Mar;591(7851):639–44. 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508-12. Epub 20140831. https://doi.org/ 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022. May;20(5):270–84. 10.1038/s41579-022-00713-0 [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008. Apr;3(4):213–23. 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-4. Epub 20200316. https://doi.org/ 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-62. Epub 20200311. https://doi.org/ 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010. Mar;140(6):859–70. 10.1016/j.cell.2010.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667-85. Epub 20140919. https://doi.org/ 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020. Dec;71(10):2669–78. 10.1093/cid/ciaa709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337-42. Epub 20200322. https://doi.org/ 10.55563/clinexprheumatol/xcdary. [DOI] [PubMed] [Google Scholar]
- Woodruff MC, Bonham KS, Anam FA, Walker TA, Faliti CE, Ishii Y, et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat Commun. 2023. Jul;14(1):4201. 10.1038/s41467-023-40012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Lu S, Tang AF, Durstenfeld MS, Ho HE, Goldberg SA, et al. Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis. 2021. Dec;224(11):1839–48. 10.1093/infdis/jiab490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golla R, Vuyyuru S, Kante B, Kumar P, Thomas DM, Makharia G, et al. Long-term Gastrointestinal Sequelae Following COVID-19: A Prospective Follow-up Cohort Study. Clin Gastroenterol Hepatol. 2023;21(3):789-96.e1. Epub 20221021. https://doi.org/ 10.1016/j.cgh.2022.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin LB, Weinstock LB, Molderings GJ. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327-32. Epub 20200910. https://doi.org/ 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G, et al. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin Infect Dis. 2023. Feb;76(3):e487–90. 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. NIH COVID-19 Autopsy Consortium . SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022. Dec;612(7941):758–63. 10.1038/s41586-022-05542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A, Zlitni S, Brooks EF, Vance SE, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med. 2022;3(6):371-87.e9. Epub 20220413. https://doi.org/ 10.1016/j.medj.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, He D, Liang C, Du S, Hua Z, Nie Q, et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: a cross-sectional cohort study in China. Lancet Infect Dis. 2024;24(8):845-55. Epub 20240422. https://doi.org/ 10.1016/S1473-3099(24)00171-3. [DOI] [PubMed] [Google Scholar]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID. Nature. 2020;581(7809):465-9. Epub. 2019;20200401: https://doi.org/ 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Swank ZN, Goldberg SA, Lu S, Dalhuisen T, Borberg E, et al. Plasma-based antigen persistence in the post-acute phase of COVID-19. Lancet Infect Dis. 2024;24(6):e345-e7. Epub 20240408. https://doi.org/ 10.1016/S1473-3099(24)00211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Julg B, Mohandas S, Bradfute SB. Viral persistence, reactivation, and mechanisms of long COVID. Elife. 2023;12. Epub 20230504. https://doi.org/ 10.7554/eLife.86015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181(5):1016-35.e19. Epub 20200427. https://doi.org/ 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer EM, Babiker A, Adelman MW, Allman B, Key A, Kleinhenz JM, et al. SARS-CoV-2 Evolution and Immune Escape in Immunocompromised Patients. N Engl J Med. 386. 2022. p. 2436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinicci M, Mazzoni A, Borchi B, Graziani L, Mazzetti M, Bartalesi F, et al. AIDS patient with severe T cell depletion achieved control but not clearance of SARS-CoV-2 infection. Eur J Immunol. 2022;52(2):352-5. Epub 20211204. https://doi.org/ 10.1002/eji.202149574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann PJ, Minor NR, Haddock Iii LA, Maddox R, Moreno GK, Braun KM, et al. Evolution of a globally unique SARS-CoV-2 Spike E484T monoclonal antibody escape mutation in a persistently infected, immunocompromised individual. Virus Evol. 2023;9(2):veac104. Epub 20221105. https://doi.org/ 10.1093/ve/veac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari S, Tahor M, Rutsinsky N, Meijer S, Miller D, Henig O, et al. Drivers of adaptive evolution during chronic SARS-CoV-2 infections. Nat Med. 2022;28(7):1501-8. Epub 20220620. https://doi.org/ 10.1038/s41591-022-01882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, Zovi A, Ferrara F. Association between microbiota and immune response to Sars-CoV-2 infection. Infect Dis Now. 2023;53(4):104646. Epub 20230113. https://doi.org/ 10.1016/j.idnow.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinti I, Mortari EP, Fernandez Salinas A, Milito C, Carsetti R. IgA Antibodies and IgA Deficiency in SARS-CoV-2 Infection. Front Cell Infect Microbiol. 2021;11:655896. Epub 20210406. https://doi.org/ 10.3389/fcimb.2021.655896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52(6):910-41. Epub 20200506. https://doi.org/ 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210-6. Epub 20220113. https://doi.org/ 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- Buonsenso D, Martino L, Morello R, Mariani F, Fearnley K, Valentini P. Viral persistence in children infected with SARS-CoV-2: current evidence and future research strategies. Lancet Microbe. 2023;4(9):e745-e56. Epub 20230626. https://doi.org/ 10.1016/S2666-5247(23)00115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsteen S, Files JK, Fram T, Erdmann N. The role of immune activation and antigen persistence in acute and long COVID. J Investig Med. 2023;71(5):545-62. Epub 20230306. https://doi.org/ 10.1177/10815589231158041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bharathi V, Dokoshi T, de Anda J, Ursery LT, Kulkarni NN, et al. Viral afterlife: SARS-CoV-2 as a reservoir of immunomimetic peptides that reassemble into proinflammatory supramolecular complexes. Proc Natl Acad Sci U S A. 2024;121(6):e2300644120. Epub 20240202. https://doi.org/ 10.1073/pnas.2300644120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KL, Waickman AT. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infection. Curr Opin Immunol. 2022;77:102228. Epub 20220524. https://doi.org/ 10.1016/j.coi.2022.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020. Aug;324(6):603–5. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623(7985):139-48. Epub 20230925. https://doi.org/ 10.1038/s41586-023-06651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JWY, Chan FKL, Ng SC. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020;5(7):644-5. Epub 20200425. https://doi.org/ 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail A, Cheema HA, Mithani MS, Shahid A, Nawaz A, Hermis AH, et al. Probiotics for the prevention and treatment of COVID-19: a rapid systematic review and meta-analysis. Front Nutr. 2023;10:1274122. Epub 20231027. https://doi.org/ 10.3389/fnut.2023.1274122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biazzo M, Deidda G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J Clin Med. 2022;11(14). Epub 20220715. https://doi.org/ 10.3390/jcm11144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wei Y, Hung CT, Lin G, Jiang X, Li C, et al. Association of nirmatrelvir-ritonavir with post-acute sequelae and mortality in patients admitted to hospital with COVID-19: a retrospective cohort study. Lancet Infect Dis. 2024;24(10):1130-40. Epub 20240503. https://doi.org/ 10.1016/S1473-3099(24)00217-2. [DOI] [PubMed] [Google Scholar]
- Geng LN, Bonilla H, Hedlin H, Jacobson KB, Tian L, Jagannathan P, et al. Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial. JAMA Intern Med. 2024. Sep;184(9):1024–34. 10.1001/jamainternmed.2024.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-31. Epub 20210310. https://doi.org/ 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021. Apr;27(4):601–15. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021. Apr;27(4):626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. Covid-19: Increased risk of some neurological and psychiatric disorders remains two years after infection, study finds. BMJ. 2022;378:o2048. Epub 20220817. https://doi.org/ 10.1136/bmj.o2048. [DOI] [PubMed] [Google Scholar]
- Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815-27. Epub 20220817. https://doi.org/ 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275-87. Epub 20211007. https://doi.org/ 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020. Aug;324(6):603–5. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021. Jun;594(7862):259–64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-14. Epub 20220725. https://doi.org/ 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2):e210830. Epub 20210201. https://doi.org/ 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. Epub 20200811. https://doi.org/ 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- Ghoshal UC, Shukla R, Ghoshal U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver. 2017. Mar;11(2):196–208. 10.5009/gnl16126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015. Mar;313(9):949–58. 10.1001/jama.2015.0954 [DOI] [PubMed] [Google Scholar]
- Nasserie T, Hittle M, Goodman SN. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw Open. 2021;4(5):e2111417. Epub 20210503. https://doi.org/ 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Feltz-Cornelis CM, Moriarty AS, Strain WD. Neurological Dysfunction in Long COVID Should Not Be Labelled as Functional Neurological Disorder. Viruses. 2023;15(3). Epub 20230318. https://doi.org/ 10.3390/v15030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JT, Fonseca Neto O. Post-COVID-19 irritable bowel syndrome: an integrative review. Rev Col Bras Cir. 2023;50:e20233618. Epub 20231120. doi: https://doi.org/ 10.1590/0100-6991e-20233618-en [DOI] [PMC free article] [PubMed]
- Pick A. Is Long Covid a Functional Disorder? Adv Rehabil Sci Pract. 2023. Jan;12:11795727221141193. 10.1177/11795727221141193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut S. “Long COVID-19” and viral “fibromyalgia-ness”: Suggesting a mechanistic role for fascial myofibroblasts (Nineveh, the shadow is in the fascia). Front Med (Lausanne). 2023;10:952278. Epub 20230406. https://doi.org/ 10.3389/fmed.2023.952278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed E, Rydberg L, Ambrose AF, Bhavaraju-Sanka R, Fine JS, Fleming TK, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of neurologic sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. 2023;15(5):640-62. Epub 20230509. https://doi.org/ 10.1002/pmrj.12976. [DOI] [PubMed] [Google Scholar]
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. Nov;5(11):1265–73. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Peluso MJ, Luo X, Thomas R, Shin MG, Neidleman J, et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat Immunol. 2024. Feb;25(2):218–25. 10.1038/s41590-023-01724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022. Jan;13(1):446. 10.1038/s41467-021-27797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020. Dec;21(12):1506–16. 10.1038/s41590-020-00814-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28(5):911-23. Epub 20220518. https://doi.org/ 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]
- Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20(4):102792. Epub 20210219. https://doi.org/ 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204-18. Epub 20171214. https://doi.org/ 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036-45.e9. Epub 20200515. https://doi.org/ 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonsenso D, Piazza M, Boner AL, Bellanti JA. Long COVID: A proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 2022. May;43(3):187–93. 10.2500/aap.2022.43.220018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Long-Haul COVID. Neurology. 95. 2020. p. 559-60. [DOI] [PubMed] [Google Scholar]
- Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-95.e20. Epub 20220125. https://doi.org/ 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-8. Epub 20200521. https://doi.org/ 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-8. Epub 20200421. https://doi.org/ 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022. Oct;22(10):639–49. 10.1038/s41577-022-00762-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020. Sep;41(32):3038–44. 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. 2024. Jan;15(1):17. 10.1038/s41467-023-44432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205-11. Epub 20210330. https://doi.org/ 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res. 2020;30(4):299-315. Epub 20200722. https://doi.org/ 10.1007/s10286-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nat Rev Neurosci. 2021. Nov;22(11):685–702. 10.1038/s41583-021-00523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan AC, Ebinger JE, Wei J, Le CN, Oft JR, Zabner R, et al. Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. Nat Cardiovasc Res. 2022. Dec;1(12):1187–94. 10.1038/s44161-022-00177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaccia G, Ricci F, Recce V, Serio A, Iannetti G, Chahal AA, et al. Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? J Cardiovasc Dev Dis. 2021;8(11). Epub 20211115. https://doi.org/ 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. Epub 20180817. https://doi.org/ 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007. Jan;132(1):397–414. 10.1053/j.gastro.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000. Oct;95(10):2698–709. 10.1111/j.1572-0241.2000.03177.x [DOI] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21(6):738-48. Epub 20160419. https://doi.org/ 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-27. Epub 20200518. https://doi.org/ 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-27. Epub 20210406. https://doi.org/ 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus CP, de Vries BE, de Vries IE, Nutma I, Kooij JJ. Treatment of 95 post-Covid patients with SSRIs. Sci Rep. 2023. Nov;13(1):18599. 10.1038/s41598-023-45072-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. SSRI differentiation: Pharmacology and pharmacokinetics. Hum Psychopharmacol. 1995;10 Suppl 3(S3):S149-s58. https://doi.org/ 10.1002/hup.470100903. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26(3):469-79. Epub 20120111. https://doi.org/ 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue. 2020;8(2):61–9. 10.1080/21641846.2020.1778227 [DOI] [Google Scholar]
- Gonzalez AE, Suzuki E. The impacts of long COVID across OECD countries. 2024. https://doi.org/ 10.1787/18152015. [DOI] [Google Scholar]
- Naidu AS, Wang C-K, Rao P, Mancini F, Clemens RA, Wirakartakusumah A, et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. NPJ Science of Food. 2024;8(1):19. https://doi.org/ 10.1038/s41538-024-00261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman B, Ramasamy MN. Synbiotics in post-acute COVID-19 syndrome-a potential new treatment framework? Lancet Infect Dis. 2024;24(3):219-21. Epub 20231207. https://doi.org/ 10.1016/S1473-3099(23)00735-1. [DOI] [PubMed] [Google Scholar]
- Chan WW, Grover M. The COVID-19 Pandemic and Post-Infection Irritable Bowel Syndrome: What Lies Ahead for Gastroenterologists. Clin Gastroenterol Hepatol. 2022;20(10):2195-7. Epub 20220806. https://doi.org/ 10.1016/j.cgh.2022.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KS, Mogensen TH, Agergaard J, Schiøttz-Christensen B, Østergaard L, Vibholm LK, et al. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur. 2023;24:100539. Epub 20221102. https://doi.org/ 10.1016/j.lanepe.2022.100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau RI, Su Q, Ching JY, Lui RN, Chan TT, Wong MT, et al. Fecal Microbiota Transplantation for Sleep Disturbance in Post-acute COVID-19 Syndrome. Clin Gastroenterol Hepatol. 2024. Jun;20240620:S1542-3565(24)00542-1. https://doi.org/ 10.1016/j.cgh.2024.06.004. [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Toschi-Dias E, Appratto de Souza L, Rabello Casali K, Vicenzi M, Sandrone G, et al. Cardiac and Peripheral Autonomic Responses to Orthostatic Stress During Transcutaneous Vagus Nerve Stimulation in Healthy Subjects. J Clin Med. 2019;8(4). Epub 20190411. https://doi.org/ 10.3390/jcm8040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2(6):692-9. Epub 20090922. https://doi.org/ 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- Guidelines WHO, Approved by the Guidelines Review Committee . Clinical management of COVID-19: Living guideline. Geneva: World Health Organization 2021;2022. [PubMed] [Google Scholar]


