Abstract
Introduction
We demonstrated Toll-like receptor (TLR) 4 in the pathogenesis of angiotensin II (AngII)-mediated abdominal aortic aneurysm (AAA) formation. Here, we study TLR2 in the AAA formation.
Methods
Male ApoE−/− and ApoE−/−TLR2−/− mice were treated with AngII. Mice were injected with the TLR2 agonist Pam3CSK4. The incidence and severity of AAA were determined. MCP-1, MCP-5, RANTES, CXCL10, CCR5, and CXCR3 were analyzed. M1 and M2 macrophages in the aorta were detected by flow cytometry.
Results
These studies demonstrated an increase in AAA formation in TLR2−/− mice and a decrease by Pam3CSK4. Pam3CSK4 decreased the ratio of M1/M2 and the levels of RANTES, CXCL10, CCR5, and CXCR3. Furthermore, Pam3CSK4 treatment 1 week following AngII retarded the progression of AAA.
Conclusion
These data demonstrated a protective effect of TLR2 signaling on AAA in association with a decrease in the ratio of M1 to M2 macrophages and the expression of chemokines and their receptors. Furthermore, the treatment of Pam3CSK4 after AngII demonstrated a marked retardation of lesion progression. Given the fact that most AAA patients are detected late in the disease process, these findings suggest that TLR2 stimulation may play a therapeutic role in retarding disease progression.
Keywords: Abdominal aortic aneurysm, Toll-like receptor 2, Pam3CSK4, M1/M2 macrophage ratio, CCR5/CXCR3 inhibitor TAK-779
Introduction
Abdominal aortic aneurysm (AAA) is a potentially life-threatening degenerative disease of the aortic wall that primarily affects 6–9% of men over the age of 65 years, with an annual mortality of more than 15,000 [1]. Surgical repair and/or endovascular repair with stenting are the only effective means of AAA treatment. Both are associated with significant morbidity and mortality and significant cost [2]. Although several animal models of AAA have been developed in the mouse, the one which most closely resembles the human disease involves infusion of ApoE−/− mice with angiotensin II (AngII) via subcutaneous osmotic minipumps [3] which results in secretion of MCP-1 and RANTES [4, 5], macrophage infiltration of the suprarenal aorta with secretion of metalloproteinases, disruption of the media, rupture of the elastic layer, clot formation, neovascularization, and rupture of the aortic wall often followed by sudden death [6]. Although multiple pathways have been implicated in AAA formation, no molecular pathway has emerged that might serve as a therapeutic target for treatment of AAA.
Toll-like receptors (TLRs) are a class of pattern recognition receptors that mediate the innate immune response, resulting in the recruitment of inflammatory cells to the site. The innate immune response is the first line of defense against microbial infections. TLR4 plays a role in response to Gram-negative infection and is stimulated by lipopolysaccharide, whereas TLR2 plays a role in the response to Gram-positive bacteria and is stimulated by peptidoglycans, lipoteichoic acid, and bacterial lipoproteins [7]. A balance between the stimulation of pro-inflammatory and anti-inflammatory cells and molecules assures the response of the organism to an infectious insult while protecting it against the development of autoimmunity. Pretreatments of rats with the TLR4 ligand lipopolysaccharide and mice with the TLR2 agonist Pam3CSK4 have been shown to increase myocardial survival following ischemia reperfusion [8]. TLR2 has been shown to attenuate the eosinophilic response in a mouse model for allergic conjunctivitis [9] and in the cutaneous inflammatory response in TPA-treated D6-deficient mice [10] and in immune tolerance in an allergic airway model for inflammation [11]. TLR2 has also been shown to control the expansion and function of regulatory T cells, which play a central role in the suppression of immune reactions and prevention of autoimmune responses [12]. Furthermore, recent studies have identified subsets of macrophages: pro-inflammatory “classically activated” M1 and “alternatively activated” healing M2 macrophages [13, 14], which play a role in establishing the pro-inflammatory and anti-inflammatory balance. TLRs have been implicated in establishing this balance.
Macrophage infiltration of the abdominal aorta in the pathogenesis of AAA involves the recruitment of monocytes to the aortic wall followed by the conversion of monocytes to macrophages. Monocyte recruitment to the endothelial cell surface involves cell surface adhesion molecules such as ICAM and VCAM and the binding of chemokines such as RANTES and CXCL10 to CCR5 and CXCR3 receptors on the surface of monocytes. AngII has been shown to increase the expression of adhesion molecules and chemokines and cytokines that promote the recruitment of pro-inflammatory cells. We and others have demonstrated that AngII-mediated AAA formation is caused in part via a TLR4-dependent pathway [15, 16], and we have further demonstrated that this effect is dependent on the activation of STAT3 [15]. The suppressor of cytokine signaling (SOCS) protein family (SOCS1-7) is a key regulator of cellular responses to cytokines. Specifically, SOCS proteins are inhibitors of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways, which serve as important feedback of the cytokine stimulation pathway. While SOCS1 expression in atherosclerotic lesions has been shown to be a key regulator of vascular and immune responses [17, 18], no role in the pathogenesis of AAA has been reported. Here, we present data supporting a protective role of TLR2 signaling in AngII-mediated AAA formation and downstream pathways that might serve as therapeutic targets in the treatment of AAA.
Methods
Mice
Male, 4-month-old ApoE−/− mice on C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, Me) and bred in a pathogen-free environment. Male ApoE−/−TLR2−/− mice were generated by interbreeding ApoE−/− and TLR2−/− mice [19]. Age-matched male mouse groups were used for all experiments. Only male mice were used in this study. All mice were maintained on a normal mouse chow diet. Animal care and the experimental procedures were carried out in accordance with the National Institute of Health and approved by the Institutional Animal Care Committee at Tufts University.
Drug Treatment
Alzet osmotic minipumps (Model 2004, Durect, CA, USA) were implanted into male ApoE−/− or ApoE−/−TLR2−/− mice at 4 months age to deliver AngII subcutaneously at a dose of 650 ng/kg/min or saline vehicle for 28 days, as described previously [15, 20]. AngII-treated mice were injected subcutaneously with either Pam3CSK4 (50 µg/mouse, once a week, InvivoGen) [21, 22], TAK-779 (250 µg, every other day, Takeda, kindly provided by NIH) [23, 24], or vehicle 1 day prior to minipump implantation, and the treatment continued for 28 days.
Determination of Blood Pressure and Lipids
Systolic blood pressures were measured in trained, prewarmed conscious mice prior to implantation of the minipump and after the drug treatment by using a tail cuff apparatus coupled to a PC-based data acquisition system (Kent Scientific Corporation). To avoid procedure-induced anxiety, mice were initially acclimated to the instrument for 3 consecutive days before the actual measurement. On the fourth day, pressures were measured in both the morning and afternoon. Moreover, the first 5 of 30 blood pressure values at each session were disregarded, and the remaining 25 values were averaged and used for analysis. Serum samples were obtained from individual mice at the time of sacrifice for analysis of cholesterol enzymatically (IDEXX Laboratories).
Tissue Harvesting
Mice were anesthetized with 2.5% isoflurane in 100% oxygen and perfused from the left ventricles with cold saline followed by perfusion with 4% paraformaldehyde for histologic studies, with cold saline followed by RNAlater solution (Ambion) for RNA analysis or with cold saline buffer for Western blot analysis. Aneurismal segments of the aortas were harvested, snap-frozen in liquid nitrogen, and then stored at −80°C pending further processing.
Aortic Diameter Measurement
After animals were perfused with 4% paraformaldehyde, the abdominal aorta was exposed under a dissecting microscope, and the periadventitial tissue carefully removed from the aortic wall. The outer diameter of the suprarenal aorta was measured with a caliper. Aneurysmal severity was rated from type I to IV according to the method of Daugherty et al. [25]: type I, dilated lumen without thrombus; type II, remodeled aneurysmal tissue with thrombus; type III, a pronounced bulbous form of type II with thrombus; and type IV, multiple often overlapping aneurysms containing thrombus.
Histology
Histological staining after harvesting was performed in the same region of the abdominal aorta and imaged to visualize morphologic changes. Abdominal aortic tissues perfusion-fixed with 4% paraformaldehyde were embedded in paraffin, cut in cross-section (5∼10 μm), and stained with hematoxylin and eosin and Verhoeff-Van Gieson for elastin.
Macrophage Infiltration and Ratio of M1/M2 Macrophages in Aneurismal Tissue
Flow cytometric analysis was used to determine the total number of macrophages in aneurismal tissue and the ratio of M1 and M2 macrophages by using anti-CD11b (M1/70, BD Pharmingen), anti-CD11c HL3 (BD Pharmingen), anti-CD206 (CO68C2 Biolegend), anti-MHC class II (MS114.15.2, eBioscience), anti-F4/80 (BM8 eBioscience), anti-CD90 (30H12, eBioscience), anti-DX5 (BD Pharmingen), anti-NK1.1 (BD Pharmingen), anti-B220 (RA3-6B2, BD Pharmingen), and anti-Ly6G (1A8, Biolegend). Briefly, aortas were harvested after treatment and digested by the method of Galkina et al. [26]. Each aorta was digested in 125 U/mL collagenase type XI, 60 U/mL hyaluronidase type I-S, 60 U/mL DNase I, and 450 U/mL collagenase type 1 (Sigma-Aldrich) in PBS containing 20 mm HEPES at 37oC for 1 h. Cells were filtered through a 70-µm strainer prior to analysis. Cells were analyzed on the FACSCanto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (BD, Ashland OR). The gating strategy was showed in Supplemental Figure S1 (for all online suppl. material, see https://doi.org/10.1159/000541651).
RNA Quantification
Total RNA was isolated from abdominal aortic tissue using the RNeasy mini kit (QIAGEN). RNA was quantified by NanoDrop (Agilent Technologies) and only used for analysis when the 260/280 ratio was ∼2.0. cDNA was synthesized from 500 ng of total RNA using an RT2 First Strand Kit (QIAGEN) according to the manufacturer’s instruction. Quantitative real-time PCR was then performed using RT2 SYBR Green qPCR Mastermix and predesigned primer pairs (MilliporeSigma) based on the instructions. Relative expression of the genes was calculated using the 2−ΔΔCt method.
ELISA
Native proteins from the suprarenal aorta were isolated using the Total Protein Extraction Kit (Biochain Institute, Inc.). The protein levels of RANTES, MCP-1, MCP-5, and CXCL10 in the aortic lysate were determined using commercially available ELISA kits (BD Biosciences) according to manufacturer’s instructions.
Western Blotting
Protein extracts were obtained from the corresponding regions of aneurismal tissues. Western blot analysis was performed as previously described [27]. Briefly, about 50 μg of total protein were separated by electrophoresis on a 4–12% SDS-polyacrylamide NuPAGE gradient gel (Invitrogen) and transferred to a PVDF membrane followed by probing with primary antibodies. The CCR5 and CXCR3 antibodies were obtained from Santa Cruz Biotechnology; the phospho-STAT3, total STAT3, and SOCS1 antibodies were obtained from Cell Signaling Technology. The protein bands were visualized by using chemiluminescent substrates (Pierce) and analyzed by ImageJ.
Statistical Analysis
The data are expressed as mean ± S.E. Statistical analysis was performed using Student’s t test. Probability (p) values of <0.05 were considered to be significant. The χ2 test was used to determine the difference between groups. Further, ordinal logistic regression was employed to evaluate the association between treatment groups. The quality of the data for ordinal logistic regression analysis was demonstrated by a high p value in the model of goodness of fit (IBM SPSS Statistics, version 29).
Results
AngII Treatment Increases the Expression of TLR2 in Abdominal Aortic Aneurysms in ApoE−/− Mice
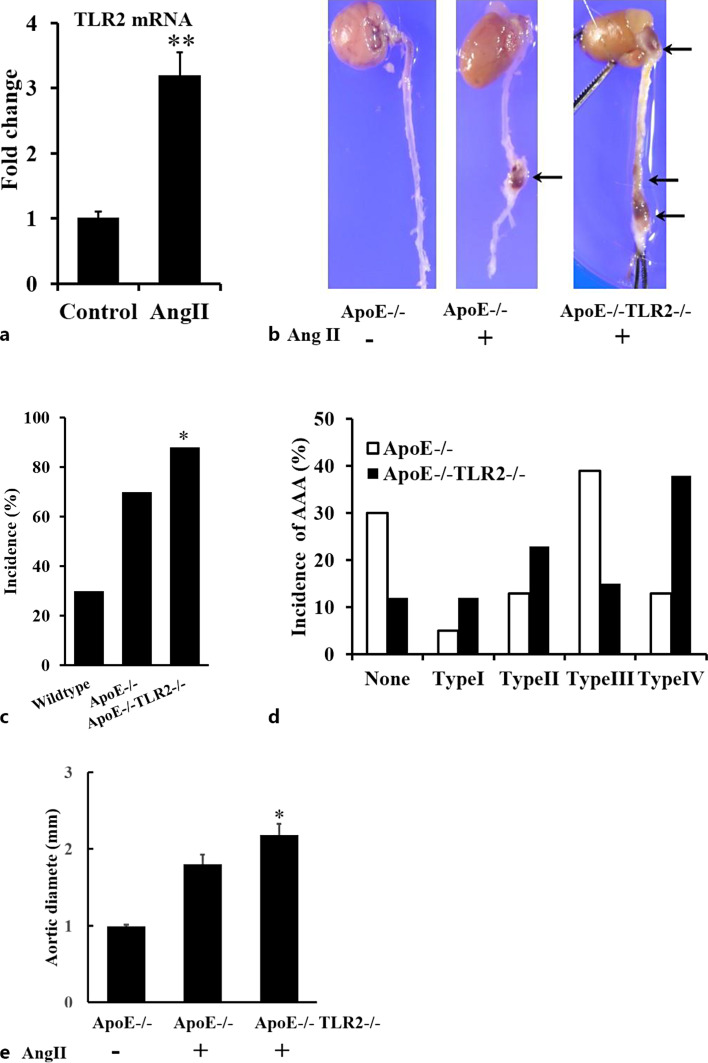
In order to study the effect of AngII on expression of TLR2, real-time RT-PCR analysis was used to determine the RNA level of TLR2 in the suprarenal aorta of ApoE−/− mice. AngII increased TLR2 mRNA level 3.21 ± 0.34-fold (Fig. 1a).
Fig. 1.
Role of TLR2 in AngII-induced AAA formation in ApoE−/− mice. a AngII increased the mRNA level of TLR2 in aneurismal lesions determined by qPCR (**p < 0.01, n = 4/group). b Representative aortas. c–e Solid arrows indicate abdominal and thoracic aortic aneurysms. Deficiency of TLR2 significantly increased the incidence (c) and severity (d, e) of AAA formation in ApoE−/− mice (*p < 0.05 vs. ApoE−/−TLR2+/+, n = 54, 52). Classification of aneurysms as described by Daugherty. The maximal aortic diameter was also significantly increased by TLR2 deficiency (*p < 0.05, n = 54, 52).
Knockout of the TLR2 Increases the Incidence and Severity of AngII-Induced AAA Formation
In order to elucidate the role of TLR2 in AngII-mediated AAA formation, male ApoE−/−TLR2+/+ or ApoE−/−TLR2−/− mice were infused with either placebo or AngII. Typical aortas from each group are shown in Figure 1b. ApoE−/−TLR2−/− mice showed increased incidence and severity of AAAs after AngII treatment. The incidence of AAA increased from 70% to 88%, while the external aortic diameters increased from 1.80 ± 0.13 mm to 2.18 ± 0.14 mm (p < 0.05) in ApoE−/−TLR2+/+ (n = 54) and ApoE−/−TLR2−/− (n = 52), respectively (Fig. 1c, d). Furthermore, based on the classification system of Daugherty [25], the distribution of aneurysms was significantly different between AngII-treated ApoE−/−TLR2+/+ and ApoE−/−TLR2−/− mice (Fig. 1e), as determined by Chi-square analysis (χ2 = 18.92, degrees of freedom = 4, p = 0.00082, Prism GraphPad), indicating that the groups were different. Furthermore, ordinal regression analysis demonstrated a significant difference in aneurysm severity between the two groups (p < 0.001). Specifically, 20 out of 52 ApoE−/−TLR2−/− mice developed more severe multiple compound aneurysms and intramural clots, compared with 7 out of 54 ApoE−/−TLR2+/+ mice (Daugherty, type IV, online suppl. Table 1). Interestingly, 13 out of 52 ApoE−/−TLR2−/− mice developed aneurysms which involved the thoracic aorta and aortic arch, while none of ApoE−/−TLR2+/+ mice developed these types of aneurysms. TLR2 deficiency had no effect on plasma cholesterol concentration, systolic blood pressure, or body weight (online suppl. Table 2). These data suggest that TLR2 might exert a protective role in AngII-induced aneurysm formation and that knock out of TLR2 in ApoE−/− mice results in increased incidence and severity of AAA formation.
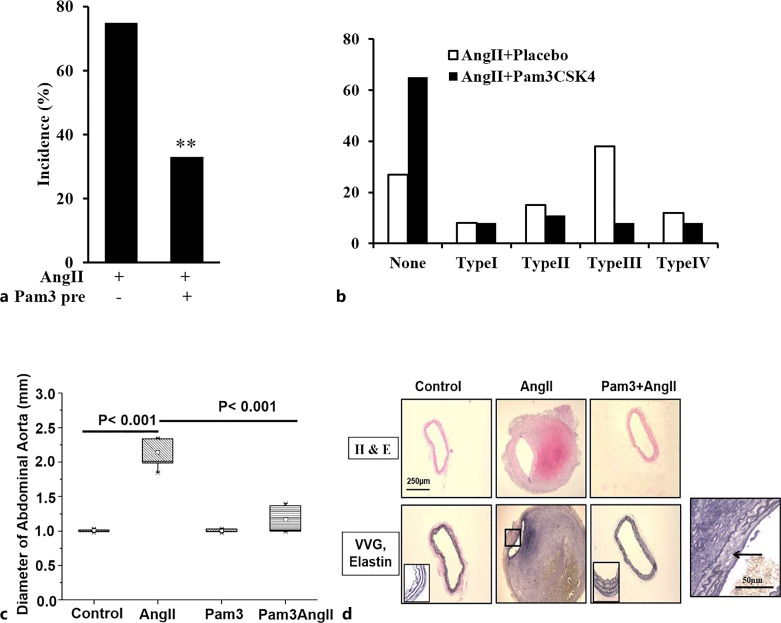
Stimulation of TLR2 Signaling Attenuates the Incidence and Severity of AngII-Induced AAA Formation and Decreases Remodeling of the Aortic Wall in Response to AngII
If TLR2 signaling plays a protective role in the pathogenesis of AAAs, then treatment of ApoE−/− with the TLR2 agonist Pam3CSK4 should attenuate AngII-induced AAA formation. ApoE−/− mice were injected with Pam3CSK4/mouse i.p. one day prior to implantation of Alzet osmotic minipumps. IP injections were continued weekly for 4 weeks. Effect on aneurysm formation was determined based on the incidence of AAA formation and the diameter of the suprarenal aorta. Pam3CSK4 pretreatment markedly attenuated AngII-induced increases in external diameters of suprarenal aortas and AAA incidence. Specifically, the incidence of AAA in response to AngII decreased from 73% in placebo-treated mice to 35% in Pam3CSK4-pretreated mice (n = 26/group, p < 0.05, Fig. 2a). The aortic diameter increased from 0.99 ± 0.02 mm in control mice to 1.88 ± 0.17 mm in AngII-treated mice, while Pam3CSK4 pretreatment decreased the aortic diameter to 1.14 ± 0.04 mm (n = 26, p < 0.001). Pam3CSK4 treatment alone had no effect on aortic diameter (Fig. 2b). Based on the classification of Daugherty et al. [25], the distribution of aneurysms between none and type IV was significantly different between placebo- and Pam3CSK4-treated groups as demonstrated by Chi-square (χ2 = 9.843, degrees of freedom = 4, p = 0.0432). Furthermore, ordinal regression analysis demonstrated a significant difference in aneurysm severity between the two groups (p = 0.04, Fig. 2c, online suppl. Table 1). Furthermore, Pam3CSK4 treatment had no effect on systolic blood pressure but a modest effect on plasma cholesterol (369 ± 49 versus 267 ± 25 mg/dL) (online suppl. Table 2). Importantly, Pam3CKS4 had no obvious effect on the extent of atherosclerotic lesions in aortas from AngII-treated mice compared with placebo (online suppl. Fig. S2).
Fig. 2.
Pretreatment of the TLR2 ligand Pam3CSK4 decreases the incidence and severity of AAA formation. Treatment of Pam3CSK4 significantly reduced the incidence (a) and severity (b) of AAA formation (**p < 0.01, n = 26/group). c The external diameter of the aorta was also significantly decreased by Pam3CSK4 pretreatment (p < 0.001 vs. placebo, n = 26). d Pam3CSK4 pretreatment attenuates AngII-induced remodeling of the aortic wall. Area defined by the square in VVG staining shows elastin fibers; the square at lower right from AngII treatment alone demonstrates the elastin degradation. VVG, Verhoeff-Van Gieson.
Hematoxylin and eosin staining of cross sections taken from the suprarenal region of the aorta demonstrated that AngII treatment resulted in a thickening of the abdominal aortic wall, disruption of the intima with thrombus formation, and destruction of the media and adventitia. Verhoeff-Van Gieson-stained sections demonstrated the delicate organization of elastin fibers in control aortas and the disruption of the elastin fibers in the region of apparent discontinuity in the aortic wall in response to AngII (see insert, Fig. 2d). These histological changes were dramatically less marked in mice treated with AngII plus Pam3CSK4 (Fig. 2d).
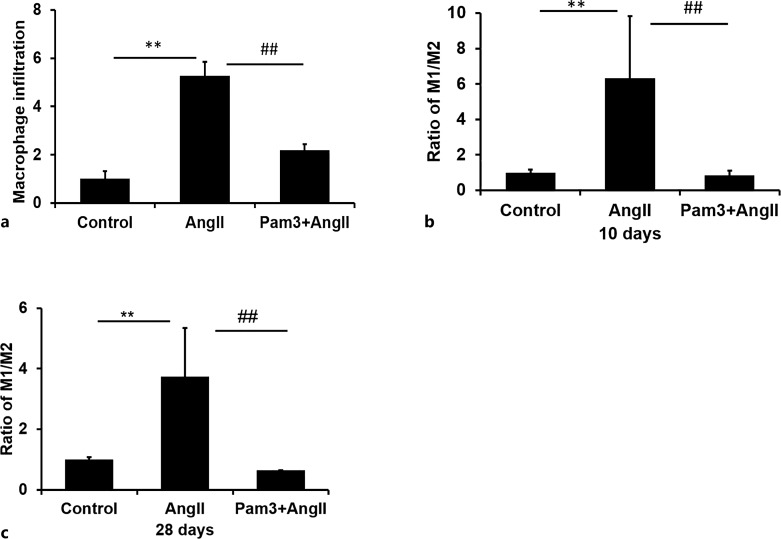
AngII-Mediated Increases in Macrophage Infiltration and the Ratio of M1/M2 Macrophages in Aneurismal Lesions Is Decreased by Pam3CSK4 Pretreatment
In order to determine whether the decrease in the incidence and severity of AAAs was associated with an effect on macrophage infiltration, we carried out flow cytometric analysis of F4/80-positive macrophage in cells from aneurismal lesions at day 28 of Ang II treatment. Total infiltration of CD11b+F4/80+ macrophages was increased 5.28 ± 0.57-fold in mice treated with AngII compared with placebo-treated mice, indicating a significant increase in macrophage infiltration (p < 0.05). In contrast, when mice were treated with both AngII plus Pam3CSK4, infiltration of CD11b+F4/80+ macrophages increased 2.18 ± 0.26-fold, which was significantly less than observed with AngII treatment alone (Fig. 3a).
Fig. 3.
In vivo administration of Pam3CSK4 attenuates AngII-induced effects of macrophage infiltration and M1/M2 polarization. a Groups of mice were treated with AngII, a combination of AngII and Pam3CSK4 (Pam3+AngII) or placebo (control). Flow cytometric analysis demonstrated a significant increase in total macrophage (CD11b+Lin−F4/80+) infiltration in AngII-treated mice relative to controls. The treatment of mice with Pam3CSK4 significantly decreased AngII-induced total macrophage infiltration in the abdominal aortas (n = 5/group). b, c CD11b+Lin−F4/80+ cells were analyzed for the expression of CD11c (M1) or CD206 (M2). The ratio of expression of M1-associated markers relative to M2-associated markers was significantly increased by AngII treatment. Pam3CSK4 treatment markedly decreased the M1/M2 ratio at (panel b) day 10 (n = 6/group) and (panel c) day 28 (n = 5/group). **p < 0.01 versus control, ##p < 0.01 versus AngII alone.
Macrophages are a highly heterogeneous cell type that are commonly divided into two broad subtypes, M1 and M2. M1 macrophages are classically activated, produce pro-inflammatory cytokines, are phagocytotic, and are involved in the immune responses to pathogens. Here, we used the markers CD11c and CD206 as proxies for the identification of M1 and M2 subsets. Flow cytometric analysis of infiltrating cells present in the suprarenal aorta at 10 days post AngII treatment demonstrated that the fraction of Lin-F4/80+CD11b+CD11c+ M1 macrophages relative to the Lin-F4/80+CD11b+CD206+ M2 macrophage phenotype increased significantly (6.33 ± 3.49-fold) in the presence of AngII compared to placebo-treated controls (Fig. 3b). In contrast, concurrent treatment with Pam3CSK4 in addition to AngII for 10 days decreased the ratio of M1/M2 to levels seen in control cells (Fig. 3b). At 28 days treatment, the patterns of macrophage polarization were similar to those at 10 days, with a ratio of M1/M2 of 3.73 ± 1.61-fold above control which was decreased to control levels by Pam3CKS4 treatment (Fig. 3c).
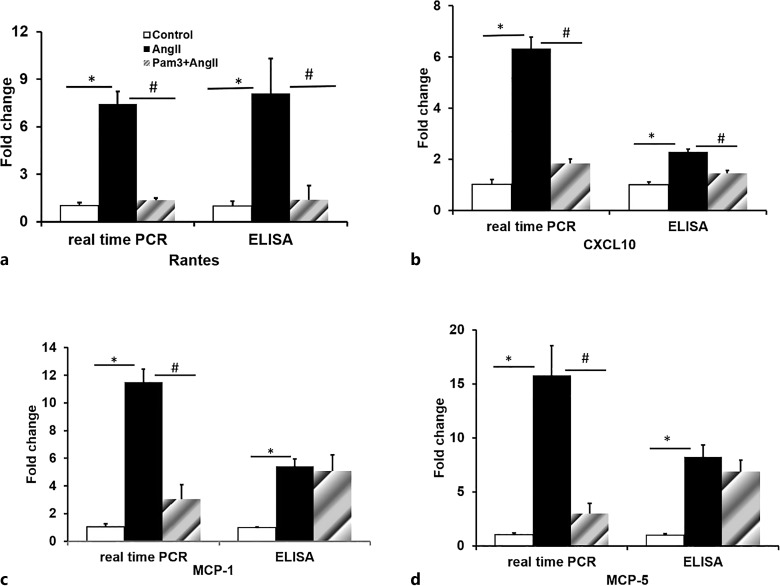
Pam3CSK4 Pretreatment Attenuates the Expression of Chemokines: RANTES, CXCL10, MCP-1, and MCP-5 in AngII-Induced AAA
AngII has been shown to recruit inflammatory cells by stimulating the expression of cell adhesion molecules, chemokines, and chemokine receptors. We have demonstrated that Pam3CSK4 pretreatment initiated 1 day prior to the start of AngII infusion decreased AngII-mediated recruitment of macrophages to the abdominal aorta. In order to determine whether this decrease in macrophage recruitment was due to an effect of Pam3CSK4 on AngII-stimulated expression of chemokines, levels of expression of RANTES, CXCL10, MCP-1, and MCP-5 were measured by RT-PCR (SABioscienses) and ELISA assays. Data summarized in Fig 4a–d demonstrated that treatment with AngII for 28 days markedly stimulated levels of mRNA coding for RANTES, CXCL10, MCP-1, and MCP-5 in the suprarenal aorta by 7.44 ± 0.71-, 6.32 ± 0.85-, 11.50 ± 1.45-, and 15.77 ± 1.97-fold, respectively, while Pam3CSK4 treatment reversed the AngII effect on these mRNA levels. Furthermore, levels of these proteins measured by ELISA demonstrated that while RANTES and CXCL10 protein were also increased by AngII and decreased by Pam3CSK4 treatment (Fig. 4a, b), protein levels of MCP-1 and MCP-5 were increased by AngII, while Pam3CSK4 pretreatment had no effect on protein levels of MCP-1 and MCP-5 (Fig. 4c, d). These data indicate that AngII-stimulated recruitment of monocytes and macrophages to the abdominal aorta in the development of AAAs is due, at least in part, by an effect on expression of chemokines and that the protective effect of Pam3CSK4 treatment is at least in part related to Pam3CSK4 inhibition of AngII-mediated expression of chemokines and the resultant decrease in recruitment of macrophages to the suprarenal aorta.
Fig. 4.
Pam3CSK4 attenuates AngII-induced gene expression of inflammatory cytokines (RANTES (a), CXCL10 (b), MCP-1 (c), MCP-5 (d)) in the aneurismal lesions measured by real-time PCR and ELISA (n = 4/group). *p < 0.05 versus control, #p < 0.05 versus AngII alone.
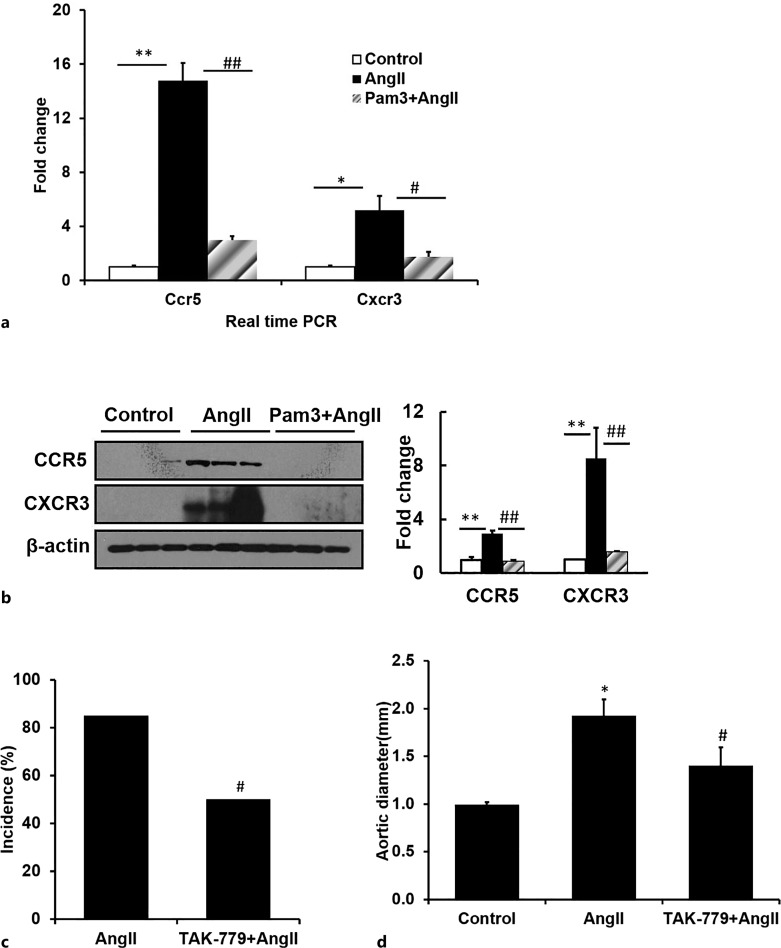
Pam3CSK4 Attenuates the AngII-Mediated Increase in Expression of RANTES and CXCL10 Receptors CCR5 and CXCR3, Respectively
Real-time qRT-PCR demonstrated that AngII markedly stimulated the RNA levels of CCR5 and CXCR3, the receptors for RANTES and CXCL10, respectively, while Pam3CSK4 pretreatment reversed the AngII effect on receptor expression (Fig. 5a). Western blot analysis further confirmed a similar effect on protein levels (Fig. 5b, n = 3). These data suggest that TLR2 stimulation by Pam3CSK4 markedly decreased AngII-stimulated expression of RANTES, CXCL10, and their receptors CCR5, CXCR3 in parallel with a marked decrease in macrophage infiltration, aortic diameter, and the incidence of AAA.
Fig. 5.
Pam3CSK4 attenuates AngII-induced gene expression of CCR5 and CXCR3 in aneurismal lesions measured by real-time PCR (a) and Western blot (b) (n = 4/group). TAK-779, a CCR5 antagonist, decreases the incidence and severity of AAA formation measured by incidence (c) and aortic diameter (d). *p < 0.05 versus control, #p < 0.05 versus AngII alone, **p < 0.01 versus control, ##p < 0.01 versus AngII alone.
Inhibition of CCR5 and CXCR3 Attenuates AngII-Induced AAA Formation
In order to determine whether the inhibition of RANTES/CCR5 and CXCL10/CXCR3 in response to the TLR2 agonist could have played a role in the protection from AngII-mediated AAA formation, ApoE−/− mice were treated with AngII (1,000 ng/kg/min) and either placebo or the CCR5/CXCR3 inhibitor TAK-779 [28] for 28 days. Treatment with TAK-779 markedly reduced incidence and severity of AAA formation: incidence decreased from 85% to 50% (n = 12, p < 0.05) in TAK-779-treated mice compared with placebo and decreased aortic diameter from 1.93 ± 0.17 mm to 1.40 ± 0.19 mm (n = 12, p < 0.05, Fig. 5c, d). These data strongly support the conclusion that TLR2 stimulation attenuated AAA formation via a decrease in the expression of chemokines and their receptors involved in the recruitment of macrophages and T cells to the abdominal aorta.
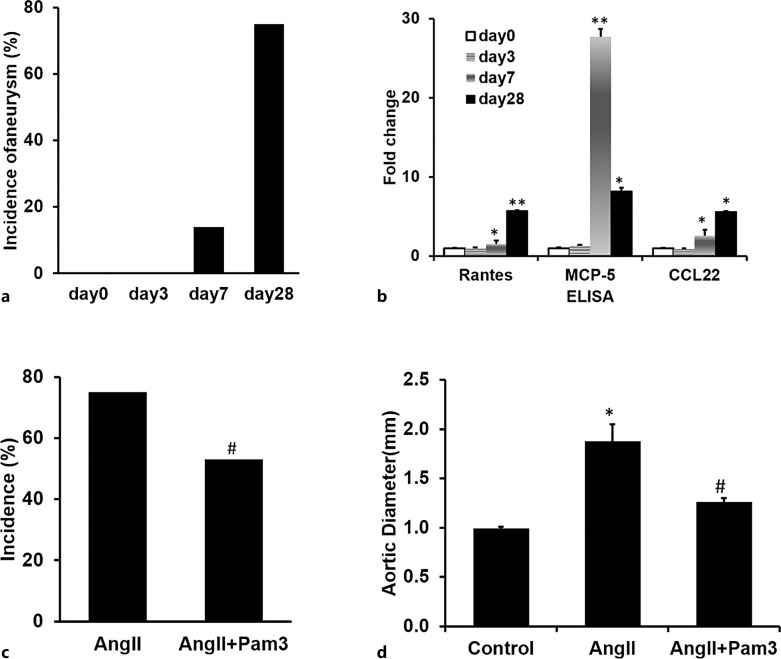
Pam3CSK4 Administered following AngII Treatment Attenuated the Progression of AngII-Mediated AAA Formation
Since in most patients AAAs are asymptomatic and hence discovered as an incidental finding either on routine physical examination or radiographic or ultrasound examination of the abdomen, it would be critical to determine whether Pam3CSK4 treatment is capable of altering the natural history of a preexisting aneurysm. In order to test the effect of Pam3CSK4 treatment on a preexisting AAA, we first studied the time course of the effect of AngII on AAA formation and on expression of chemokines at days 3, 7, and 28 following the initiation of AngII infusion. At day 3, there is no aneurysm formation; at day 7, 14% of mice developed aneurysm; and at day 28, about 75% of mice developed aneurysm, as shown previously (Fig. 6a). However, as early as day 3, qRT-PCR arrays demonstrated increases in mRNA levels coding for multiple chemokines in a time-dependent manner (online suppl. Table 3). ELISA analysis further confirmed that the protein levels of RANTES, MCP-5, and CCL22 increased significantly as early as day 7 and remained elevated at day 28 (Fig. 6b). These data suggest that increased expression of chemokines and chemokine receptors precede AAA formation in response to AngII. To determine the effect of Pam3CSK4 on the progression of preexisting AAA, we chose 7 days after AngII minipump implantation to initiate Pam3CSK4 treatment and determined the effect of Pam3CSK4 on the incidence and severity of AAAs at day 28 compared to placebo-treated mice. The drug was administered once a week for 3 weeks, and at day 28, aortas were harvested. The treatment of Pam3CSK4 starting from day 7 decreased AngII-mediated AAA formation at day 28 decreasing incidence from 75% (n = 24) to 53% (n = 24) and decreased the diameter of suprarenal aortas from 1.88 ± 0.17 mm (n = 24) to 1.26 ± 0.10 mm (n = 24) (Fig. 6c, d). These data suggest that Pam3CSK4 treatment not only prevents AAA formation but also attenuates the progression of preexisting AAA, which supports the conclusion that activation of TLR2 signaling may serve as a potential therapeutic approach for this life-threatening disease.
Fig. 6.
Time course of AngII effects shows on AAA formation and pro-inflammatory cytokines effect of PAM3CSK4 treatment initiated at day 7 of AngII infusion. Aneurysms first appear at day 7 following AngII treatment (a) (n = 10/group), gene expression of some inflammatory cytokines also increases at day 7 (b) (n = 4/group). Both increased over time. The treatment of Pam3CSK4 after 7 days of AngII infusion decreases the incidence and severity of AAA formation as measured by incidence (c) and aortic diameter (d) (n = 24/group). *p < 0.05 versus day 0, **p < 0.01 versus day 0, #p < 0.05 versus AngII alone.
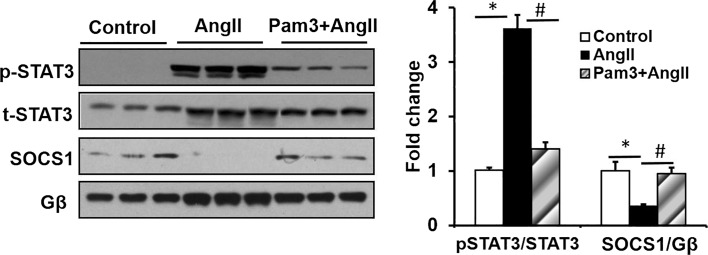
Pam3CSK4 Pretreatment Attenuates STAT3 Phosphorylation and Increases the Level of SOCS1 in AngII-Induced AAA
We previously demonstrated that AngII-mediated AAA formation in ApoE−/− mice was mediated at least in part via the JAK/STAT pathway. Specifically, we showed that inhibition of STAT3 markedly attenuated the incidence and severity of AAA formation [15]. The SOCS protein functions in a negative-feedback loop to restrain inflammatory responses, including STAT activation via STAT phosphorylation [29]. In order to determine whether the attenuation of AAA formation by Pam3CSK4 is mediated via an effect on STAT activation, the levels of pSTAT3 were measured by Western blot analysis of extracts from suprarenal aortic tissues. While the basal abundance of p-STAT3 was low, treatment of AngII markedly increased STAT3 phosphorylation. However, pretreatment with Pam3CSK4 significantly decreased AngII-induced STAT3 phosphorylation. In contrast, SOCS1 expression was robustly decreased by AngII treatment and increased by pretreatment with Pam3CSK4 in combination with AngII (Fig. 7a, b). Taken together, these findings implicate Pam3CSK4 stimulation of SOCS1 in attenuating pSTAT3 stimulated AAA formation.
Fig. 7.
Pam3CSK4 decreases AngII-induced pSTAT3 via activation of SOCS1 in aneurismal lesions determined by Western blot analysis. *p < 0.05 versus control, #p < 0.05 versus AngII alone (n = 3/group).
Discussion
Inflammation is a protective response to a foreign organism that involves a delicate balance between an acute pro-inflammatory phase and a healing phase. The balance between the 2 phases is characteristic of the responding system. AngII-stimulated AAA formation in an ApoE−/− mouse has proven to be an excellent model for the study of the pathogenesis of the human disease both in terms of localization, cellular infiltration, clot formation, and penetration of the aortic wall. Using measurements of effects of ANG II infusion on the incidence, severity, and progression of lesion formation as well as levels of chemokines and chemokine receptors, macrophage infiltration, and polarization, here, we demonstrate a potent protective effect of TLR2 signaling on the formation and progression of AAAs. Importantly, the protective effect of TLR2 signaling is demonstrated by a marked increase in the incidence of AAAs and the progression of severity of the lesions in AngII-treated ApoE−/−TLR2−/− mice. Specifically, the effects of TLR2 KO are measured by an increase in the preponderance of type IV over type III lesions with clot formation and increased aortic dilatation as described by Daugherty et al. [25]. Conversely, the role of TLR2 signaling was further supported by the findings that treatment of ApoE−/− mice with the TLR2 agonist Pam3CKS3 markedly decreased the incidence and severity of AngII-mediated AAAs. These findings are consistent with studies demonstrating a marked increase in the severity of mycobacterial infections in TLR2−/− mice [30]. Furthermore, TLR2 signaling has been shown to have a protective effect in autoimmune disease, improving disease outcomes in murine lupus [31].
Of note, several studies have suggested that TLR2 signaling contributes to the pathogenesis of both AAA and atherosclerosis [22]. A study by Yan et al. [32] concluded that antagonism of TLR2 signaling attenuated the formation and progression of AAAs. However, this study generated AAA in response to perivascular application of 0.5 mol/L CaCl2 to the infrarenal aorta of the mouse to create AAAs. Whether CaCl2 induces AAA via a mechanism similar to that of AngII is unclear. In another study, Owens et al. [16] demonstrated that TLR2 deficiency had no effect on AngII-stimulated AAA formation in LDLR−/−TLR2−/− mice fed with a saturated fat-enriched diet. Owens’ study utilized a dose of AngII of 1,000 ng/kg/min. We had found a similar lack of effect on AAA formation at this dose of AngII in ApoE−/−TLR2−/−mice. We reasoned that at this dose of AngII (1,000 ng/kg/min), AAA formation had almost reached maximal capacity and the exacerbating effect of TLR2 deficiency might not be detectable. For this reason, we carried out our studies using 650 ng/kg/min of AngII.
The wall of the suprarenal abdominal aorta in the AngII-treated ApoE−/− mouse demonstrated aneurysm formation in association with infiltration of macrophages and increased expression of chemokines and their receptors. Studies of the mechanism of the TLR2 protective effect demonstrated a marked decrease in AngII-mediated macrophage infiltration in the aortic wall in association with a decrease in AngII-stimulated increases in expression of chemokines RANTES and CXCL10 measured at both the level of RNA and protein. Interestingly, while Pam3CSK4 treatment decreased mRNA levels of both MCP-1 and MCP-5 in AngII-treated mice, protein levels of MCP-1 and MCP-5 measured by ELISA were unchanged in response to Pam3CSK4. Chemokine receptors CCR5 and CXCR3 were both markedly elevated in AngII-treated mice, while their levels were significantly reduced following Pam3CSK4 treatment. These data taken together with the finding that the CCR5 inhibitor TAK-779 decreased both the incidence and severity of AngII-mediated AAA formation in ApoE−/− mice strongly support the conclusion that TLR2 attenuates AAA formation via a direct effect on chemokine recruitment and macrophage infiltration to the abdominal aorta. Data discussed below further support the conclusion that TLR2 interferes with M1/M2 polarization once macrophages have penetrated the endothelium.
The dynamic balance between pro-inflammatory and healing states is also determined by the balance between pro-inflammatory M1 macrophage and healing M2 macrophage. The role of TLR2 signaling in regulating this balance remains controversial. Monocytes from patients with lupus have been shown to differentiate into M2 macrophages when treated with Pam3CSK4 [31]. However, in a study of the effects of TLR2 stimulation on M2 macrophages from patients with rheumatoid arthritis, the anti-inflammatory activity of the M2 macrophages was decreased by Pam3CSK4 treatment, generating a chimeric M1/M2 phenotype [33]. Here, we demonstrated that the ratio of M1/M2 macrophages in the abdominal aorta of ApoE−/− mice is markedly increased by AngII and that Pam3CSK4 treatment reversed the M1/M2 ratio. Importantly, this increase in the M1/M2 ratio is observed as early as 10 days following the initiation of AngII infusion at a time when only 14% of mice demonstrated aneurysm formation. Furthermore, a similar effect on the M1/M2 ratio is observed in mice after 4 weeks of treatment. Interestingly, however, the M1/M2 ratio decreased from 6-fold at day 10 to 3-fold at day 28, suggestive of a possible increase of the anti-inflammatory response over time. Interestingly, a detailed study of the time course of disease progression in response to AngII infusion demonstrated that increased expression of chemokines and their receptors in the wall of the abdominal aorta preceded detectable lesion formation and could be observed as early as day 3 of AngII infusion consistent with macrophage recruitment as an expected early event in lesion formation.
Finally, we had previously demonstrated that S31-201, a small molecule inhibitor of STAT3, decreased the incidence and severity of AngII-induced AAA formation [15]. Here, we determined whether TLR2 attenuation of AAA formation might be mediated via an effect on the inhibition of STAT3 activation. We demonstrated that Pam3CSK4 treatment markedly increased SOCS1 levels and decreased levels of phosphorylation of STAT3. Given that SOCS1 regulates STAT3 activity via a feedback inhibition of STAT3 phosphorylation, and given that STAT3 has been shown to play a role in the regulation of chemokine expression [34], these data were consistent with the a potential role of TLR2 regulation of macrophage recruitment via a SOCS1/STAT3 regulation of chemokine expression.
The diagnosis of AAA is almost always a coincidental finding on routine physical examination of the abdomen, an abdominal ultrasound or radiographic examination. Hence, for the attenuation of the progression of AAA formation via these TLR2-dependent pathways to offer therapeutic potential, they must not only be capable of preventing the development of AAAs but of arresting the progression of preexisting disease. Here, we demonstrate that between 7 and 10 days following initiation of AngII infusion, when only 14% of mice demonstrate any identifiable aortic lesions, the levels of chemokines, their receptors, and the ratio of M1/M2 macrophages are already significantly elevated. We further demonstrated that initiation of Pam3CSK4 therapy at this stage had a profound effect on the progression and severity of disease. Although the incidence and severity of AAA formation at day 7 may only in part recapitulate detectable disease in the patient population, the finding that PAM3CSK4 arrests disease progression at a point where disease is not only detectable via lesion formation but also at the level of pro-inflammatory markers and macrophage polarization strongly supports a therapeutic effect of TLR2 signaling in the prevention of progression of preexisting disease. Thus, these pathways offer potential therapeutic targets for the prevention of AAA progression.
Statement of Ethics
The animal study protocol was reviewed and approved by the Tufts University Institutional Animal Care and Use Committee (IACUC), Approval No. B2012-123.
Conflict of Interest Statement
The authors have no disclosures to declare. The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by Grant 5R01HL074876 and 5R01HL153433 from the National Heart, Lung, and Blood Institute.
Author Contributions
Yali Zhang, Ho-Jin Park, Andrew Lichtman, Debbie Beasley, and Jonas B. Galper conceived of and designed the research. Yali Zhang, Jessamyn Bagley, and Elena Maganto-Garcia conducted the experiments. Yali Zhang and Xuehong Cao analyzed the data. Yali Zhang and Jonas B. Galper wrote the manuscript. Xuehong Cao and Debbie Beasley edited the manuscript. All the authors read and approved the manuscript.
Funding Statement
This work was supported by Grant 5R01HL074876 and 5R01HL153433 from the National Heart, Lung, and Blood Institute.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Daugherty A, Cassis LA. Mechanisms of abdominal aortic aneurysm formation. Curr Atheroscler Rep. 2002;4(3):222–7. [DOI] [PubMed] [Google Scholar]
- 2. Cowan JA Jr, Dimick JB, Henke PK, Rectenwald J, Stanley JC, Upchurch GR Jr. Epidemiology of aortic aneurysm repair in the United States from 1993 to 2003. Ann N Y Acad Sci. 2006;1085:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malle E, Sodin-Semrl S, Kovacevic A. Serum amyloid A: an acute-phase protein involved in tumour pathogenesis. Cell Mol Life Sci. 2009;66(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kashiwagi M, Masutani K, Shinozaki M, Hirakata H. MCP-1 and RANTES are expressed in renal cortex of rats chronically treated with nitric oxide synthase inhibitor. Involvement in macrophage and monocyte recruitment. Nephron. 2002;92(1):165–73. [DOI] [PubMed] [Google Scholar]
- 6. Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24(3):429–34. [DOI] [PubMed] [Google Scholar]
- 7. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. [DOI] [PubMed] [Google Scholar]
- 8. Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, et al. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78(3):546–53. [DOI] [PubMed] [Google Scholar]
- 9. Fukushima A, Yamaguchi T, Ishida W, Fukata K, Ueno H. TLR2 agonist ameliorates murine experimental allergic conjunctivitis by inducing CD4 positive T-cell apoptosis rather than by affecting the Th1/Th2 balance. Biochem Biophys Res Commun. 2006;339(4):1048–55. [DOI] [PubMed] [Google Scholar]
- 10. McKimmie CS, Moore M, Fraser AR, Jamieson T, Xu D, Burt C, et al. A TLR2 ligand suppresses inflammation by modulation of chemokine receptors and redirection of leukocyte migration. Blood. 2009;113(18):4224–31. [DOI] [PubMed] [Google Scholar]
- 11. Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, et al. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol. 2005;174(12):7558–63. [DOI] [PubMed] [Google Scholar]
- 12. Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116(2):485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–39. [DOI] [PubMed] [Google Scholar]
- 14. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin Z, Bagley J, Sukhova G, Baur WE, Park HJ, Beasley D, et al. Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J Mol Cell Cardiol. 2015;87:160–70. [DOI] [PubMed] [Google Scholar]
- 16. Owens AP 3rd, Rateri DL, Howatt DA, Moore KJ, Tobias PS, Curtiss LK, et al. MyD88 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation independent of signaling through Toll-like receptors 2 and 4. Arterioscler Thromb Vasc Biol. 2011;31(12):2813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortiz-Munoz G, Martin-Ventura JL, Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O, et al. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(4):525–31. [DOI] [PubMed] [Google Scholar]
- 18. Recio C, Oguiza A, Lazaro I, Mallavia B, Egido J, Gomez-Guerrero C. Suppressor of cytokine signaling 1-derived peptide inhibits Janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice. Arterioscler Thromb Vasc Biol. 2014;34(9):1953–60. [DOI] [PubMed] [Google Scholar]
- 19. Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(1):50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Naggar JC, Welzig CM, Beasley D, Moulton KS, Park HJ, et al. Simvastatin inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice: possible role of ERK. Arterioscler Thromb Vasc Biol. 2009;29(11):1764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169(1):10–4. [DOI] [PubMed] [Google Scholar]
- 22. Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115(11):3149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y, et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31(4):783–9. [DOI] [PubMed] [Google Scholar]
- 24. Takami S, Minami M, Katayama T, Nagata I, Namura S, Satoh M. TAK-779, a nonpeptide CC chemokine receptor antagonist, protects the brain against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2002;22(7):780–4. [DOI] [PubMed] [Google Scholar]
- 25. Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134(4):865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203(5):1273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang D, Park HJ, Georgescu SP, Sebti SM, Hamilton AD, Galper JB. Simvastatin potentiates tumor necrosis factor alpha-mediated apoptosis of human vascular endothelial cells via the inhibition of the geranylgeranylation of RhoA. Life Sci. 2006;79(15):1484–92. [DOI] [PubMed] [Google Scholar]
- 28. Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A. 1999;96(10):5698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trengove MC, Ward AC. SOCS proteins in development and disease. Am J Clin Exp Immunol. 2013;2(1):1–29. [PMC free article] [PubMed] [Google Scholar]
- 30. Hu W, Spaink HP. The role of TLR2 in infectious diseases caused by mycobacteria: from cell biology to therapeutic target. Biology. 2022;11(2):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horuluoglu B, Bayik D, Kayraklioglu N, Goguet E, Kaplan MJ, Klinman DM. PAM3 supports the generation of M2-like macrophages from lupus patient monocytes and improves disease outcome in murine lupus. J Autoimmun. 2019;99:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan H, Cui B, Zhang X, Fu X, Yan J, Wang X, et al. Antagonism of toll-like receptor 2 attenuates the formation and progression of abdominal aortic aneurysm. Acta Pharm Sin B. 2015;5(3):176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res Ther. 2017;19(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu M, Tan L, Lin Z, Lui VCH, Tam PKH, Lamb JR, et al. Down-regulation of STAT3 enhanced chemokine expression and neutrophil recruitment in biliary atresia. Clin Sci. 2021;135(7):865–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.