ABSTRACT
KRAS mutations are common in non-small cell lung cancer (NSCLC) and are associated with patient prognosis; however, targeting KRAS has faced various difficulties. Currently, immunotherapy, chemotherapy, and chemoimmunotherapy play pivotal roles in the first-line treatment of KRAS-mutated NSCLC. Here, we summarize the current evidence on first-line therapies and compare the treatment outcomes and biomarkers for different regimens. KRAS inhibitors and other emerging alternative treatments are also discussed, as combining these drugs with immunotherapy may serve as a promising first-line treatment for KRAS-mutated NSCLC in the future. We hope that this review will assist in first-line treatment choices and shed light on the development of novel agents for KRAS-mutated NSCLC.
KEYWORDS: Kirsten rat sarcoma viral oncogene (KRAS), first-line, immune checkpoint inhibitors (ICIs), anti-vascular therapy, chemotherapy
1. Introduction
Lung cancer has high morbidity and mortality rates worldwide.1 The most common type of lung cancer is non-small cell lung cancer (NSCLC).2 Currently, first-line treatment options for unresectable NSCLC without driver mutations include chemotherapy, anti-PD-1/PD-L1 immunotherapy, and chemotherapy combined with immunotherapy.3 Advanced NSCLC with driver gene mutations, such as epidermal growth factor receptor (EGFR) mutations, are treated with targeted therapies, such as epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs).3
Kirsten rat sarcoma viral oncogene (KRAS) is a common driver oncogene mutation in NSCLC that plays a crucial role in cancer development and evolution.4,5 As a member of the GTPase family, it functions to catalyze GTP hydrolysis.6 KRAS mutations are common in NSCLC; the mutation sites include amino acids 12, 13, and 61, with the G12C mutation being the most common, followed by G12V, G12D, and G12A mutations.7,8 KRAS mutations reduce GTPase activity, leading to sustained KRAS activation and increased signaling in the downstream pathways. The mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)-protein kinase B (AKT) pathways are important downstream pathways of RAS signaling (Figure 1). The MAPK cascade plays an important role in cell proliferation and tumorigenesis.9 On the other hand, RAS also binds to and activates PI3K, leading to AKT phosphorylation and stimulation of the mammalian target of the rapamycin (mTOR) pathway.10 This contributes to cell proliferation and survival, which are essential for tumor development and maintenance.10 However, the efficacy and safety of the currently available drugs targeting KRAS remain unsatisfactory.11 Therefore, chemotherapy and immunotherapy still play important roles in the first-line treatment of NSCLC with KRAS mutations (KRASm).
Figure 1.
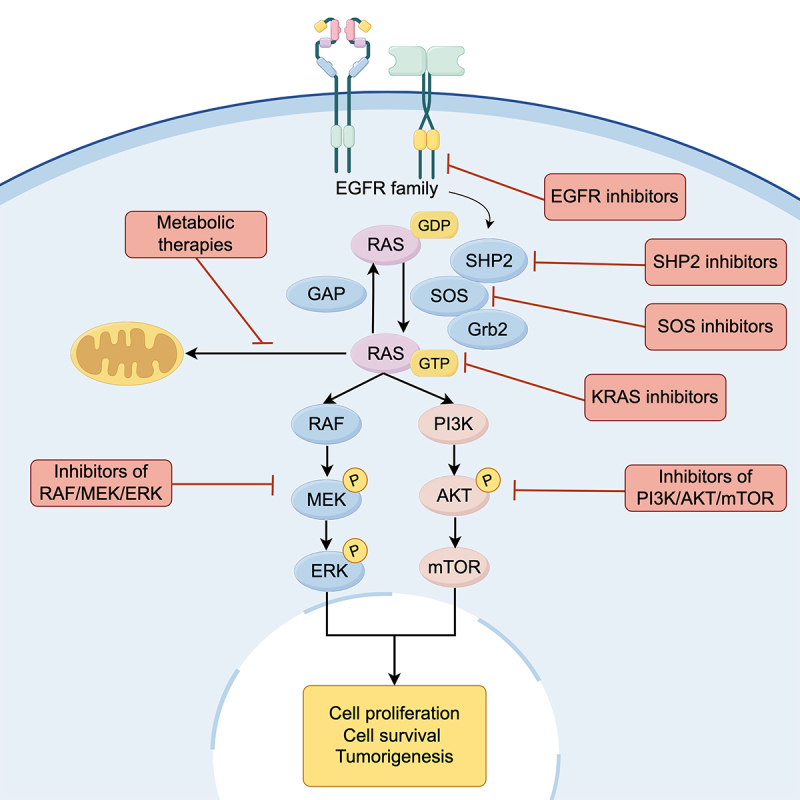
The KRAS signaling pathway.
Although numerous studies have focused on chemotherapy and immunotherapy for KRASm NSCLC, the results from previous studies are inconsistent owing to the heterogeneity of KRASm NSCLC. This review focuses on the best options for the first-line treatment of KRASm NSCLC by reviewing the current evidence from clinical studies.
2. Chemotherapy is the cornerstone of first-line treatment for KRASm NSCLC
First-line chemotherapy for KRASm NSCLC resulted in worse treatment outcomes than that for KRASwt NSCLC (Table 1). A retrospective study by Metro et al. showed that patients with KRASm NSCLC (n = 77) treated with first-line platinum-containing chemotherapy had a significantly lower objective response rate (ORR), disease control rate, progression-free survival (PFS), and overall survival (OS) than the KRASwt group (n = 127).12 Similarly, in a single-center retrospective study, Hames et al. compared the outcomes of patients with KRASm (n = 80) and driver gene-negative (n = 70) advanced NSCLC treated with first-line platinum-containing chemotherapy.13 The median PFS in the KRASm group was shorter than that of the driver gene-negative group by 1.2 months (4.5 vs. 5.7 months, p = .008), and the median OS was 4.7 months shorter (8.8 vs 13.5 months, p = .038), with subgroup analyses for adenocarcinoma and metastatic disease suggesting similar results.13 Eklund et al. also demonstrated that the OS of 104 patients with stage IV KRASm NSCLC treated with first-line chemotherapy was significantly shorter than that of 91 patients with KRASwt NSCLC (9 vs. 11 months, p = .018). Furthermore, the multivariate Cox analysis showed that the KRAS mutation was a risk factor for shorter OS (hazard ratio (HR): 1.564, p = .008).14 However, a retrospective study by Mellema et al. showed that the ORR of KRASm NSCLC (n = 60) for first-line platinum-containing chemotherapy was similar to that of KRASwt NSCLC (n = 101), with a median PFS (4.0 vs. 4.7 months, p = .12) and median OS (7.0 vs 9.3 months, p = .25) numerically reduced compared to KRASwt NSCLC, without statistical significance.15
Table 1.
First-line treatments for KRAS mutant NSCLC.
| References | First author | Year | Treatment regimen | Study design | Number of patients |
ORR, % |
Median PFS, months |
Median OS, months |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KRASm | KRASwt | KRASm | KRASwt | P value | KRASm | KRASwt | P value | KRASm | KRASwt | P value | |||||
| 12 | Metro | 2014 | Platinum-based chemotherapy | Retro | 77 | 127 | 27.3 | 42.5 | 0.04 | 5.4 | 6.8 | 0.05 | 14.7 | 23.4 | 0.21 |
| 13 | Hames | 2016 | Platinum-based chemotherapy | Retro | 80 | 70 | 4.5 | 5.7 | 0.008 | 8.8 | 13.5 | 0.038 | |||
| 14 | Eklund | 2022 | Platinum-based chemotherapy | Retro | 104 | 91 | 9 | 11 | 0.018 | ||||||
| Pembrolizumab | Retro | 20 | 17 | 23 | 6 | 0.006 | |||||||||
| 15 | Mellema | 2013 | Platinum-based chemotherapy | Retro | 60 | 101 | 16.7 | 21.8 | 4 | 4.7 | 0.12 | 7 | 9.3 | 0.25 | |
| 16 | Ghimessy | 2019 | Platinum-based chemotherapy + bevacizumab | Retro | 95 | 152 | 7.03 | 8.63 | 0.0255 | 14.23 | 21.57 | 0.0186 | |||
| Platinum-based chemotherapy | Retro | 75 | 179 | 10 | 11 | 0.6771 | |||||||||
| 17 | Peters | 2017 | Atezolizumab | Trial | 33 | 67 | 27 | 16 | 8.4 | 4.8 | NE | 20.1 | |||
| 18 | Liu | 2023 | ICI + (chemotherapy) | Retro | 20 | 49 | 10.1 | 9 | <0.05 | ||||||
| 19 | Sun | 2021 | ICI monotherapy | Retro | 363 | 342 | 21.1 | 13.6 | 0.03 | ||||||
| ICI + chemotherapy | Retro | 210 | 212 | 20 | 19.3 | 0.93 | |||||||||
| 20 | Frost | 2021 | Pembrolizumab | Retro | 62 | 57 | 50.9 | 46 | 0.62 | 13.3 | 6.2 | 0.05 | 23.4 | 26.1 | 0.74 |
| 21 | Li | 2022 | ICI + chemotherapy | Retro | 23 | 57 | 12.8 | 9.7 | <0.001 | 21 | 15.9 | 0.01 | |||
| 22 | Justeau | 2022 | Pembrolizumab | Retro | G12C vs non-G12C: 86 vs 141) | 454 | 47 vs 40 | 45 | 7 vs 4.8 | 8.5 | 0.2284 | 18.4 vs 20.6 | 27.1 | 0.5664 | |
| 23 | Noordhof | 2021 | Pembrolizumab | Retro | 338 | 257 | 19.2 | 16.8 | 0.86 | ||||||
| 24 | Kartolo | 2021 | ICI monotherapy | Retro | 30 | 29 | 37 | 26 | 0.268 | 6 | 5.4 | 0.416 | 12.9 | 19.3 | 0.87 |
| 25 | Gadgeel | 2019 | Pembrolizumab + chemotherapy | Trial | 59 | 145 | 40.7 | 47.6 | 9 | 9 | 21 | 23 | |||
| Chemotherapy | Trial | 30 | 55 | 26.7 | 10.9 | 5 | 5 | 14 | 9 | ||||||
| 26 | Nakajima | 2022 | ICI + chemotherapy | Pool | 219 | 313 | 46 | 51 | 22.4 | 18.7 | |||||
| ICI monotherapy | Pool | 135 | 240 | 37 | 33 | 16.2 | 16.4 | ||||||||
| Chemotherapy | Pool | 201 | 322 | 35 | 32 | 17.1 | 14.9 | ||||||||
| 27 | Alessi | 2023 | ICI + chemotherapy | Retro | 351 | 526 | 33 | 39 | 5.7 | 5.9 | 0.21 | 14.1 | 15 | 0.42 | |
| 28 | Veccia | 2023 | ICI + (chemotherapy) | Retro | 50 | 69 | 14.7 | 14.9 | 0.529 | ||||||
| 29 | Mok | 2023 | Pembrolizumab | Retro | 30 | 127 | 56.7 | 29.1 | 12.3 | 5.8 | 28.4 | 14.8 | |||
| Platinum-based chemotherapy | Retro | 39 | 105 | 18 | 21 | 6.2 | 6.3 | 11 | 12.1 | ||||||
| 30 | Liu | 2022 | ICI + (chemotherapy) | Retro | 50 | 44 | 11.7 | 23.8 | |||||||
| Chemotherapy + (AT) | Retro | 115 | 30.43 | 7 | 14.7 | ||||||||||
| 31 | Gu | 2023 | ICI + (chemotherapy) | Retro | 33 | 27.2 | 7.4 | 24.1 | |||||||
| Chemotherapy | Retro | 37 | 16.2 | 4.5 | 13.2 | ||||||||||
| 32 | Wang | 2023 | ICI + chemotherapy | Retro | 11 | 29 | 10.6 | 13.3 | 24.6 | NR | |||||
| Chemotherapy + (AT) | Retro | 10 | 23 | 7.2 | 6.9 | 13.3 | 21.1 | ||||||||
| 33 | Sun | 2022 | ICI + chemotherapy | Retro | 76 | 47.4 | 16.9 | 37.1 | |||||||
| Chemotherapy | Retro | 74 | 31.1 | 4.6 | 19.8 | ||||||||||
| Chemotherapy + AT | Retro | 33 | 21.2 | 7 | 20.7 | ||||||||||
| 34 | West | 2022 | Atezolizumab, bevacizumab, carboplatin, paclitaxel(ABCP) | Trial | 80 | 235 | 8.4 | 8.1 | 19.8 | 18.9 | |||||
| Atezolizumab, carboplatin, paclitaxel (ACP) | Trial | 74 | 234 | 6.8 | 4.8 | 11.7 | 19.5 | ||||||||
| Bevacizumab, carboplatin, paclitaxel (BCP) | Trial | 71 | 226 | 7 | 5.8 | 9.9 | 18.2 | ||||||||
*ORR(objective response rate), PFS(progression-free survival), OS(overall survival), ICI(immune checkpoint inhibitors), AT(antivascular therapy), KRASm(KRAS mutant), KRASwt(KRAS wild-type), Retro(retrospective), Trial(clinical trial analysis), Pool(pooled analysis), NR(not reach).
As a commonly used agent for non-squamous NSCLC, the reported efficacy of pemetrexed in KRASm NSCLC varies widely between studies. Several studies have suggested the survival benefit of pemetrexed-containing chemotherapy. A retrospective analysis of 115 patients with KRASm NSCLC treated with first-line chemotherapy by Liu et al. demonstrated that pemetrexed-containing regimens (n = 60) were associated with a longer PFS (10.1 vs 6.2 months, p < .001) and OS (16.4 vs 14.1 months, p = .112), although the OS benefit was not statistically significant.30 Similarly, Chen et al. compared the outcomes of three first-line chemotherapy regimens, pemetrexed/platinum (PP, n = 198), gemcitabine/platinum (GP, n = 64), and paclitaxel/platinum (TP, n = 38), in KRASm NSCLC.35 Although there was no significant difference in ORR and disease control rate (DCR) among the three regimens, in terms of PFS, the PP group (6.4 months) was significantly prolonged compared to the GP group (4.9 months, p = .033) and the TP group (5.6 months, p = .05); in terms of OS, the GP group (17.5 months) was significantly prolonged compared to the PP group (24.6 months, p = .03) and TP group (26.8 months, p < .001). As KRAS mutations are predominantly found in non-squamous NSCLC and pemetrexed is more effective in non-squamous cancers, pemetrexed-based chemotherapy regimens are more effective in treating KRASm NSCLC.36
However, a retrospective study by Ricciuti et al., which did not differentiate between the number of lines of treatment, suggested that treatment outcomes were worse with pemetrexed-containing chemotherapy regimens for advanced NSCLC.37 PP-based regimens (n = 81) were associated with a worse ORR (30.9% vs. 47.4%, p = .05), DCR (51.8% vs. 71.9%, p = .02), PFS (4.1 vs. 7.1 months, p = .03), and OS (9.7 vs. 26.9 months, p = .002) than non-PP-based regimens (n = 57).37
Therefore, as the cornerstone of first-line treatment, chemotherapy is less effective in KRASm-advanced NSCLC than in KRASwt NSCLC, and pemetrexed-based chemotherapy regimens may result in a better prognosis.
3. Chemotherapy combined with antivascular therapy prolongs survival in the first-line treatment of KRASm NSCLC
KRAS mutations are associated with overexpression of VEGF, which is involved in tumor angiogenesis and promotes lung cancer development and metastasis.38,39 Antivascular therapy inhibits the process of tumor angiogenesis by inhibiting the binding of VEGF to its receptor. Therefore, chemotherapy combined with antivascular therapy has the potential to serve as a first-line treatment for KRASm NSCLC.
Even with first-line chemotherapy combined with antivascular therapy, patients with KRASm NSCLC still had poorer treatment outcomes than those with KRASwt NSCLC. A study by Ghimessy et al. suggested that compared with KRASwt (n = 152), advanced lung adenocarcinoma patients with KRASm (n = 95) treated with first-line chemotherapy combined with bevacizumab had a worse OS (14.23 vs. 21.57 months, p = .0255) and PFS (7.03 vs 8.63 months, p = .0186).16 The association between KRAS mutations and OS was independent of age, sex, smoking status, Eastern Cooperative Oncology Group performance status (ECOG PS), and tumor stage.16
First-line chemotherapy combined with antivascular therapy improved survival in patients with KRASm NSCLC. A single-center retrospective study by Liu et al. demonstrated that first-line chemotherapy combined with antivascular therapy (n = 58) significantly prolonged PFS (10.0 vs. 6.5 months, p = .031) and OS (19.7 vs. 13.7 months, p = .004).30 Similarly, a retrospective study by Ghimessy et al. on advanced KRAS-mutant lung adenocarcinoma in stages IIIB – IV demonstrated that first-line platinum-containing chemotherapy in combination with bevacizumab (n = 95) had a significant OS benefit compared to platinum-containing chemotherapy alone (n = 75) (14.23 vs. 10 months, p = .0002).16
Regarding specific drug selection, Liu et al. showed that among 58 patients treated with chemotherapy combined with antivascular therapy, the ORR in the paclitaxel combined with antivascular drug group (59.09% vs. 30.56% vs. 12.5% vs. 26.92%, p = .032, p = .001, and p = .024) was significantly higher than that in the pemetrexed combined with antivascular drug group, the pemetrexed group, paclitaxel group, while PFS (14.0 vs. 4.0 vs. 8.0 vs. 5.0 months, p = .009, p = .008, and p < .001) and OS (25.0 vs. 10.0 vs. 19.0 vs. 11.0 months, p = .006, p = .508, and p < .001) in the pemetrexed-combined antivascular group were significantly longer than those in the pemetrexed group, the paclitaxel combined antivascular drug group, and the paclitaxel group.30 Similarly, a study by Mellema et al. showed that paclitaxel combined with antivascular therapy had the highest ORR (62%, n = 38) than paclitaxel (50%, n = 30), pemetrexed (21%, n = 334), and gemcitabine (25%, n = 62).40
Thus, first-line chemotherapy combined with antivascular therapy remains significantly less effective in patients with KRASm NSCLC than in those with KRASwt NSCLC but does improve survival in patients with KRASm NSCLC.
4. Immunotherapy is the mainstay of first-line treatment for KRASm NSCLC
4.1. The efficacy of immunotherapy in patients with KRASm NSCLC is not inferior to those with KRASwt NSCLC
Unlike chemotherapy or chemotherapy combined with antivascular therapy, the efficacy of first-line immunotherapy may be superior in KRASm NSCLC compared to KRASwt (Table 1). A meta-analysis integrating three trials (IMpower-150, Keynote-189, and Keynote-042) demonstrated that KRASm NSCLC had better survival with first-line immunotherapy than KRASwt NSCLC (χ2 = 6.26, p = .01).41 In the BIRCH trial, advanced NSCLC with PD-L1 expression ≥5% in tumor cells or tumor-infiltrating immune cells was treated with first-line atezolizumab in 33 patients that were KRASm patients and 67 patients that were KRASwt.17 The ORR (27% vs. 16%), PFS (8.4 vs. 4.8 months), and OS (NE vs. 20.1 months) were higher in patients who were KRASm than in those who were KRASwt.17 Similarly, a retrospective study by Liu et al. suggested that 20 patients with KRASm NSCLC treated with first-line anti-PD-1/PD-L1 therapy had a significantly longer PFS than 49 patients with KRASwt NSCLC.18 Sun et al. demonstrated that OS (21.1 vs. 13.6 months, p = .03) was significantly longer in KRASm NSCLC (n = 363) than in KRASwt NSCLC (n = 342) with first-line immunological monotherapy, and that the association between KRAS mutation status and OS remained significant in the multivariate Cox model (HR = 0.77).19 In addition, a study by Eklund et al. showed that stage IV NSCLC with KRASm (n = 20) had a significant OS (23 vs. 6 months, p = .006) benefit from first-line immunotherapy compared to KRASwt (n = 17) and that KRAS mutation was a favorable prognostic factor for OS in a multifactorial Cox regression (HR = 0.349, p = .016).14 In advanced adenocarcinomas with PD-L1 expression ≥50% treated with first-line pembrolizumab, KRASm NSCLC (n = 62) showed a significant PFS benefit compared to KRASwt (n = 57) (13.3 vs. 6.2 months, p = .05), with no significant difference in OS (23.4 vs. 26.1 months, p = .74).20 A retrospective study by Li et al. demonstrated that first-line pembrolizumab in combination with carboplatin, paclitaxel (for squamous cancers), or pemetrexed (for non-squamous cancers) for the treatment of KRASm NSCLC (n = 23) had a higher PFS (12.8 vs. 9.7 months, p < .05) and OS (21.4 vs. 26.1 months, p = .74) than KRASwt (n = 57), with KRAS mutation as a favorable factor for prolongation of OS (HR = 2.552, 95% confidence interval (CI): 1.141–5.708; p = .023).21
In addition, a retrospective study of long-term responders (LTRs) to first-line immunotherapy in NSCLC showed that KRAS mutations were more common in LTRs than in non-responders (39.4% vs. 28%, n = 13 vs. 7, p = .366).42 Similarly, a study by Notario et al. showed the enrichment of KRAS G12C mutations in LTRs (64%, p = .09).43 The response and efficacy of KRASm NSCLC cells to immunotherapy may be related to the immune microenvironment. A retrospective study by Liu et al., which did not limit the number of lines of immune checkpoint inhibitor (ICI) treatment, showed that an increased tumor mutational burden (TMB) was associated with increased immunogenicity and that KRASm NSCLC responded better to ICIs.44
However, other studies have suggested no significant difference between the efficacy of first-line immunotherapy for advanced KRASm and KRASwt NSCLC, either as ICI monotherapy or as immunochemotherapy. In terms of ICI monotherapy, the analysis of Justeau et al. based on a multicenter retrospective study (ESCKEYP) showed that among patients with non-squamous advanced NSCLC with PD-L1 ≥50% treated with first-line pembrolizumab, the KRAS G12C mutation group (n = 86), the KRAS non-G12C mutation group (n = 141), and the KRAS wild-type group (n = 454) were not significantly different regarding PFS (7 vs. 4.8 vs. 8.5 months, p = .2284) and OS (18.4 vs. 20.6 vs. 27.1 months, p = .5664).22 In addition, a retrospective study by Noordhof et al. showed that in patients with stage IV adenocarcinoma with PD-L1 expression ≥50% treated with first-line pembrolizumab, OS (19.2 vs. 16.8 months, p = .86) was not significantly different.23 Similarly, a study by Kartolo et al. demonstrated that advanced NSCLC with PD-L1 expression ≥50% treated with first-line anti-PD-1/PD-L1 monotherapy showed no significant difference in the ORR (37% vs. 26%, p = .268), PFS (6.0 vs. 5.4 months, p = .416), OS (12.9 vs. 19.3 months, p = .87) between the KRASm group (n = 30) and the KRASwt group (n = 29).24 In terms of immunochemotherapy, a KEYNOTE-189-based analysis by Gadgeel et al. showed that 59 cases of KRASm NSCLC treated with first-line pembrolizumab combined with chemotherapy had a similar ORR (40.7% vs. 47.6%), PFS (9 vs. 9 months), and OS (21 vs. 23 months) compared to 145 cases of KRASwt NSCLC.25 A study by Alessi et al. also found no significant differences in ORR, PFS, or OS between first-line immunochemotherapy in the KRASm group (n = 351) and the KRASwt group (n = 526) in advanced non-squamous NSCLC.27 Other studies that did not differentiate between ICI monotherapy and immunochemotherapy also suggested that the outcomes of first-line immunotherapy for KRASm and KRASwt NSCLC were not significantly different. Sun et al. showed that although OS in 363 cases of KRASm NSCLC treated with first-line immunologic monotherapy was significantly longer than in 342 KRASwt NSCLC (21.1 vs. 13.6 months, p = .03), OS in 210 KRASm NSCLC treated with first-line immune-combination chemotherapy was not significantly different from that in 212 cases of KRASwt NSCLC (20.0 vs 19.3 months, p = .93).19 A study by Veccia et al. showed no significant difference in OS (14.7 vs. 14.9 months, p = .529) between KRASm (n = 50) and KRASwt (n = 69) NSCLC treated with first-line immunochemotherapy or ICI monotherapy.28
Therefore, the results of several studies suggest that the efficacy of first-line immunotherapy for KRASm NSCLC may be superior or at least comparable to that for KRASwt NSCLC, especially in patients with positive PD-L1 expression.
4.2. ICI monotherapy improves treatment outcomes in KRASm NSCLC
First-line ICI monotherapy improves treatment outcomes in patients with KRASm NSCLC compared to chemotherapy. In the analysis by Mok et al., based on the KEYNOTE-042 trial, 30 cases of KRASm and 127 cases of KRASwt advanced NSCLC with PD-L1 expression ≥1% were treated with first-line pembrolizumab.29 Patients with KRASm showed significantly improved ORR (56.7% vs. 18%), PFS (12.3 vs. 6.2 months, HR = 0.51), and OS (28.4 vs. 11.0 months, HR = 0.42) compared to chemotherapy.29 Another single-center retrospective study by Liu et al. also showed that first-line single-agent immunotherapy (n = 50) significantly improved the ORR (44.00% vs. 30.43%), DCR (96.00% vs. 80.00%), PFS (11.7 vs. 7.0 months, p < .001), and OS (28.4 vs. 11.0 months, HR = 0.42) in KRASm NSCLC compared to chemotherapy (n = 115).30 Further subgroup analyses showed that in NSCLC with PD-L1 expression ≥1%, first-line immunotherapy was associated with a significantly higher PFS (12.9 vs. 9.0 months, p = .011) and a significantly lower risk of disease progression (HR = 0.377, p = .020) compared to chemotherapy.30
In patients with KRASm NSCLC with PD-L1 expression ≥50%, ICI monotherapy could serve as the first-line therapy. A real-world study by Velcheti et al. of advanced NSCLC with PD-L1 expression ≥50% treated with first-line pembrolizumab demonstrated that the median real-world time on treatment (rwToT) for first-line pembrolizumab in 164 patients with KRASm with ECOG PS scores of 1–2 was 7.6 months (95% CI: 6.3–10.6 months), and the median rwToT for 166 patients with KRASwt was 7.0 months (95% CI: 5.3–9.3 months).45 Thus, first-line ICI monotherapy showed a survival benefit in patients with PD-L1-overexpressing NSCLC, with or without KRAS mutations.
Therefore, first-line ICI monotherapy improves treatment outcomes in KRASm NSCLC compared to chemotherapy, especially in patients with PD-L1 expression ≥50%.
4.3. Immunochemotherapy is more effective than other treatments in KRASm NSCLC
First-line immunochemotherapy was more effective than chemotherapy in treating KRASm NSCLC. An analysis based on KEYNOTE-189 by Gadgeel et al. showed that in treating KRASm advanced NSCLC, first-line pembrolizumab in combination with platinum-containing chemotherapy (n = 59) had a significantly higher ORR (40.7% vs. 26.7%) and a trend toward a prolonged PFS (9 vs. 5 months; HR = 0.47, 95% CI: 0.29–0.77) and OS (21 vs. 14 months; HR = 0.79, 95% CI: 0.45–1.38) compared with the chemotherapy group (n = 30).25 Similar results have been obtained in multiple retrospective studies. A study by Gu et al. showed that first-line ICI combined with platinum-containing chemotherapy (n = 33) in KRASm NSCLC significantly increased PFS (7.4 vs. 4.5 months, p = .035) and OS (24.1 vs. 13.2 months, p = .007) compared to platinum-containing chemotherapy (n = 37).31 A retrospective study by Wang et al. also demonstrated that the OS (17 vs. 12 months, p = .11) was longer in the first-line immunochemotherapy group (n = 11) than in the non-immunotherapy group (n = 10, including chemotherapy and antivascular therapy) for KRASm NSCLC, but the difference was not significant.32 Similarly, a pooled analysis by Nakajima et al. showed that for first-line treatment of KRASm NSCLC, immunochemotherapy (n = 219) had ORR (46% vs. 35%) and OS (22.4 vs. 17.1 months) benefits over chemotherapy (n = 201).26
Compared to chemotherapy combined with antivascular therapy, KRASm NSCLC was better treated with first-line immunochemotherapy. Sun et al. showed that in advanced KRASm NSCLC (n = 76), first-line immunochemotherapy had a significant benefit in terms of ORR (47.4% vs. 31.1% vs. 21.2%), PFS (16.9 vs. 4.6 vs. 7.0 months), and OS (37.1 vs. 19.8 vs. 20.7 months) over chemotherapy (n = 74) or chemotherapy combined with antivascular therapy (n = 33).33
KRASm NSCLC was treated more effectively with first-line immunochemotherapy than with ICIs alone. Nakajima et al. demonstrated that compared with first-line ICIs alone, KRASm NSCLC with first-line immunochemotherapy (n = 219) resulted in an improved ORR (46% vs. 37%) and OS (22.4 vs. 16.2 months).26 However, in patients with KRASm NSCLC and high PD-L1 expression, first-line immunochemotherapy did not show an additional survival benefit compared with ICI monotherapy. Sun et al. demonstrated that among 573 patients with KRASm NSCLC and PD-L1 expression ≥50%, first-line immunochemotherapy (n = 210) and ICI monotherapy (n = 363) showed no significant differences in OS (20.0 vs. 21.1 months, p = .78).19
Thus, first-line immunochemotherapy is superior to other therapies, such as chemotherapy, chemotherapy combined with antivascular therapy, and ICI monotherapy, in patients with KRASm NSCLC. In patients with high PD-L1 expression, the efficacy of immunochemotherapy is similar to that of ICI monotherapy.
4.4. Immunotherapy combined with chemotherapy and antivascular therapy improves outcomes in the first-line treatment of KRASm NSCLC
Combining antivascular therapy with immunochemotherapy improves the outcomes of first-line treatment for KRASm NSCLC. Based on the IMpower150 trial, West et al. showed that in KRASm non-squamous NSCLC, first-line atezolizumab/bevacizumab/carboplatin/paclitaxel (ABCP) was more effective in prolonging PFS (8.1 vs. 5.8 vs. 4.8 months) and OS (19.8 vs. 9.9 vs. 11.7 months) compared to either the bevacizumab/carboplatin/paclitaxel (BCP) regimen (n = 71) or the atezolizumab/carboplatin/paclitaxel (ACP) regimen (n = 74).34 The ABCP regimen improved the OS (HR = 0.50; 95% CI: 0.34–0.72 vs. HR = 0.63; 95% CI: 0.43–0.91) and PFS (HR = 0.42; 95% CI: 0.29–0.61 vs. HR = 0.80; 95% CI: 0.56–1.80) more significantly than the ACP regimen. Thus, immunotherapy combined with chemotherapy and antivascular therapy may further improve the first-line treatment outcomes.
5. Impact of KRAS mutant subtypes on the efficacy of immunotherapy
In advanced KRASm NSCLC, G12C is the most common mutated subtype. Several studies have shown that first-line immunotherapy is more effective for patients with KRAS G12C mutations than for those with other KRAS mutations. For first-line immunotherapy in combination with chemotherapy, Elkrief et al. showed a significant PFS (6.8 vs. 5.4 months, p = .006) and OS (15 vs. 12 months, p = .12) benefit in the KRAS G12C group (n = 138) over the non-G12C group (n = 185).46 Cefalì et al. demonstrated that 11 of 44 patients with KRASm NSCLC with PD-L1 expression ≥50% treated with first-line ICI showed a significantly longer PFS in the KRAS G12C than in the non-G12C group (14.6 vs. 6.5 months, p = .03).47 Similarly, NSCLC with PD-L1 expression ≥50% treated with first-line pembrolizumab showed significant benefits in ORR (63.3% vs. 36.0%, p = .05) and PFS (19.8 vs. 5.8 months, p = .001) in 32 cases in the KRAS G12C group than in the non-G12C group, with a non-significant trend toward a longer OS (HR = 0.50, 95% CI: 0.25–1.01, p = .06).20 Attili et al. showed that for stage IV non-squamous NSCLC with PD-L1 expression <50%, the G12C mutation was significantly associated with PFS benefits with first-line immunochemotherapy (HR = 0.29, 95% CI: 0.10–0.91).48
However, other studies have shown no significant difference in the efficacy of first-line immunotherapy between KRAS G12C mutations and non-G12C mutations. Justeau et al. demonstrated that in advanced NSCLC patients with PD-L1 expression ≥50% treated with first-line pembrolizumab, the KRAS G12C mutation group (n = 86) and the KRAS non-G12C mutation group (n = 141) showed no significant difference in ORR (47% vs. 40%), PFS (7 vs. 4.8 months), and OS (18.4 vs. 20.6 months).22 A retrospective study by Arbour et al. showed that in NSCLC receiving first-line immunotherapy, patients with the KRAS G12C mutation (n = 352) had comparable PFS (3.7 vs. 3.3 months, p = .89) to the non-G12C mutation group (n = 418).49
The relationship between KRAS mutant subtypes and immunotherapy efficacy may be associated with PD-L1 expression levels. Several studies have suggested that the G12D mutation is associated with low PD-L1 expression, whereas the G12C mutation is associated with high PD-L1 expression.50,51 In vitro experiments suggested that ICIs combined with paclitaxel can recruit CD8+ tumor-infiltrating lymphocytes (TILs) by increasing CXCL10/CXCL11 levels and can inhibit tumor growth more effectively than ICIs alone in tumors with KRAS G12D mutations, suggesting that patients with KRAS G12D mutations can be treated with first-line ICI combined with paclitaxel therapy.51 For G12C, an analysis based on clinical genomic data from 10,023 patients with NSCLC showed that KRAS G12C-mutated NSCLC was associated with a high TMB and PD-L1 expression ≥50%.8,52
In summary, first-line immunotherapy for KRAS G12C mutations may have a better prognosis than that for other mutation subtypes, which may be related to PD-L1 expression levels.
6. Concurrent mutations are associated with the prognosis of KRASm NSCLC
Concurrent mutations in STK11, KEAP1, and TP53 were associated with the prognosis of KRAS-mutant NSCLC, and had implications on first-line treatment strategies.
The STK11 co-mutation was consistently associated with poor prognosis in KRASm NSCLC. Several retrospective studies showed that patients with KRAS and STK11 mutations have shorter overall survival (OS) and progression-free survival (PFS) than those without these co-mutations.53–56 Similarly, the KEAP1 co-mutation was also related to worse PFS and OS in KRASm NSCLC patients.34,54,55,57 The adverse prognostic roles of STK11 and KEAP1 co-mutations might be related to lower PD-L1 expression. Analysis of Negrao et al. suggested that negative PD-L1 expression (PD-L1 < 1%) was more common in KRASm patients with the STK11 or KEAP1 mutation and was related to decreased PFS and OS.58
On the other hand, the role of TP53 co-mutation in KRASm NSCLC was more complex. While some research posed that TP53 co-mutations were not related to survival outcomes, others suggested that TP53 co-mutations were related to survival benefits of KRASm NSCLC, especially in first-line immunotherapy.21,27,55,57 Additionally, Aredo et al. showed that TP53 co-mutations were more frequently found with high PD-L1 expression (≥50%).53 This association may explain the prolonged survival in KRASm patients with TP53 co-mutations receiving immunotherapy.
Co-mutations with STK11, KEAP1 and TP53 had substantial implications on first-line treatment strategies for KRASm NSCLC. For STK11 or KEAP1 co-mutations, first-line treatment options primarily include chemoimmunotherapy or the combination of chemotherapy and antivascular therapy. West et al. showed that first-line ABCP regimens had significant PFS (6.0 vs. 3.2 vs. 3.4 months) and OS (11.1 vs. 7.9 vs. 8.7 months) benefits over ACP or BCP regimens in KRASm NSCLC with STK11 or KEAP1 co-mutations.34 However, Sun et al. suggested that KRASm/STK11m NSCLC patients with first-line chemotherapy combined with bevacizumab had a PFS benefit (7.0 vs. 4.4 vs. 3.9 months, p = .043) compared with chemoimmunotherapy and chemotherapy groups.33
On the other hand, for KRASm NSCLC with TP53 co-mutation, first-line chemoimmunotherapy should be considered. Analyzing the IMpower 150 trial showed that PFS (14.3 vs. 4.6 vs. 4.2 months) and OS (30.6 vs. 11.7 vs. 9.5 months) in the first-line ABCP regimen were significantly longer than in ACP or BCP regimens.34 Consistently, research by Sun et al. indicated that PFS (18.7 vs. 6.1 vs. 6.8 months, p < .0001) of KRASm NSCLC with TP53 mutation were significantly longer in ICI combined with chemotherapy than those in the chemotherapy alone or chemotherapy combined with antivascular therapy.33
Therefore, concurrent mutations of STK11 or KEAP1 were negative prognostic factors for KRASm NSCLC, while TP53 seemed to be associated with improved survival outcomes. Combining immunotherapy and chemotherapy enhanced the outcomes in KRAS-mutant NSCLC patients with these concurrent mutations and could be applied in first-line treatment.
7. Kras-targeting therapies
Various new therapeutic approaches are available as first-line candidates for treating NSCLC with KRAS mutations. These therapies include KRAS-targeting therapies, metabolic therapies, and their combinations with existing first-line agents. Currently, these therapies are still being evaluated in clinical trials as second-line or higher treatment options.
7.1. KRAS(OFF) inhibitors
Drugs targeting the switch region of the KRAS G12C protein, including sotorasib (AMG 510) and adagrasib (MRTX849), have been developed (Table 2). Sotorasib (AMG 510), the first KRAS G12C inhibitor, binds to a cysteine residue in the switch II region and prevents activation of KRAS.59 Based on the results of phase 1 and single-arm phase 2 trials, sotorasib was first approved by the Food and Drug Administration (FDA) in 2021 for the treatment of advanced NSCLC in the second line and beyond.60–62 In the phase 3 trial, sotorasib had a significantly longer PFS than docetaxel (5.6 vs. 4.5 months, HR = 0.66, p = .0017), with fewer grade 3 or 4 adverse events.63 A first-line trial of sotorasib (NCT04933695) is currently underway. Thus, there is growing evidence that sotorasib is a promising candidate for treating NSCLC with KRAS G12C mutation.
Table 2.
Ongoing clinical trials for sotorasib and adagrasib in KRAS mutant NSCLC.
| Treatment | Regimen | Trial number |
|---|---|---|
| Sotorasib | ||
| Sotorasib monotherapy | Sotorasib | NCT03600883, NCT03600883, NCT04303780, NCT04625647, NCT05398094, NCT04933695, NCT05451056, NCT05273047, NCT05311709, NCT06127940, NCT06333678, NCT05400577, NCT05631249 |
| Sotorasib + Chemotherapy | Sotorasib + Platinum doublet | NCT05920356, NCT05118854 |
| Sotorasib + Antivascular therapy | Sotorasib + MVASI | NCT05180422 |
| Sotorasib + Aurora A kinase inhibitor | Sotorasib + VIC-1911 | NCT05374538 |
| Sotorasib + CXCL-8 inhibitor | Sotorasib + Ladarixin | NCT05815173, NCT05815186 |
| Sotorasib + Tyrosine kinase inhibitor | Sotorasib + Lenvatinib/Tarloxotinib | NCT06068153 |
| Sotorasib + HER2 inhibitor | Sotorasib + Tarloxotinib | NCT05313009 |
| Sotorasib + RAF/MEK inhibitor | Sotorasib + Avutometinib + (Defactinib) | NCT05074810 |
| Sotorasib + SHP2 inhibitor | Sotorasib + RMC-4630/BBP-398 | NCT05054725, NCT05480865 |
| Sotorasib + Proteasome inhibitor | Sotorasib + Carfilzomib | NCT06249282 |
| Adagrasib | ||
| Adagrasib monotherapy | Adagrasib | NCT03785249, NCT04685135, NCT05853575, NCT05673187 |
| Adagrasib + Immunotherapy | Adagrasib + Pembrolizumab/Nivolumab | NCT04613596, NCT05472623 |
| Adagrasib + RAF/MEK inhibitor | Adagrasib + Avutometinib | NCT05375994 |
| Adagrasib + mTOR inhibitor | Adagrasib + Nab-Sirolimus | NCT05840510 |
Adagrasib (MRTX8499) is a recently developed KRAS G12C inhibitor. Based on a phase I/IB study, NSCLC patients with KRAS G12C mutations treated with adagrasib had a median PFS of 11.1 months.64 In 2022, adagrasib was approved for patients with advanced NSCLC with KRAS G12C mutations who had previously received systemic therapy.65 In the phase 2 study, among 112 patients for whom baseline disease assessment was available, the ORR was 42.9%, the median PFS was 6.5 months, the median OS was 12.6 months, and the incidence of treatment-related adverse events of grade 3 or higher was 44.8%.66 Preliminary results from the phase 1/2 KRYSTAL-12 trial (NCT04685135) showed that in patients with KRAS G12C-mutated NSCLC who had previously received chemotherapy or immunotherapy, after 9.4 months of follow-up, the adagrasib group showed significant benefit in ORR (31.9% vs. 9.2%, p < .0001) and PFS (5.49 vs. 3.84 months, p < .0001) compared to the docetaxel group, with a similar incidence of grade 3 and higher TRAE (47.0% vs 45.7%).67 Further clinical trials are ongoing to explore the efficacy and safety of adagrasib monotherapy and combination therapy in advanced NSCLC.
Combination therapies for other KRAS inhibitors have also shown better efficacy. For combining immunotherapy, preliminary results from the phase 1/2 LOXO-RAS-20001 trial (NCT04956640) of the second-generation KRAS G12C inhibitor, olomorasib (LY3537982), in combination with pembrolizumab to treat advanced NSCLC, demonstrated an ORR of 63% in 50 patients at 6-month follow-up (95% CI: 44–80%), suggesting that KRAS inhibitors in combination with ICI may have superior efficacy.68 For combination chemotherapy, in the phase Ib CodeBreaK 101 (NCT04185883) study, 58 patients treated with first-line sotorasib in combination with pemetrexed and carboplatin had an ORR of 65% (95% CI: 46.5–80.3) and a median PFS of 10.8 months (95% CI: 5.4-NE months), with 30 patients (52%) experiencing grade 3–4 TRAE and 1 patient death.69 In terms of combining other targeted therapies, 27 patients with advanced NSCLC receiving the first-line KRAS G12C inhibitor fulzerasib (GFH925) in combination with the EGFR inhibitor cetuximab had an ORR of 80.0% (95% CI: 56.3–94.3%) in the phase II KROCUS study (NCT05756153), with a DCR of 100% (95% CI: 83.2–100.0%), with 5 patients experiencing grade 3 or higher TRAE.70 Thus, combination therapies with KRAS inhibitors are expected to be a future first-line treatment option.
Several ongoing trials are evaluating the outcomes of sotorasib and adagrasib monotherapies and in combination with other therapies (Table 2).
7.2. KRAS(ON) inhibitors
Sotorasib and adagrasib are classified as KRAS(OFF) inhibitors, targeting the KRAS protein in its inactive state. In contrast, KRAS(ON) inhibitors specifically target the active, GTP-bound KRAS to combat KRAS-driven cancers.71 Several KRAS(ON) inhibitors have been developed and tested in preclinical and clinical studies, including RMC-6236, RMC-4998, and RMC-7977.
RMC-6236 combined cyclophilin A (CYPA) to target KRAS in an active state, forming a tri-complex that inhibited downstream signal transduction.72 Preclinical results suggested that RMC-6236 down-regulated RAS signaling, leading to tumor regression in the mouse xenograft model.73 Clinical trials are ongoing to evaluate RMC-6236 as a monotherapy and in combination with immune checkpoint inhibitors (NCT05379985, NCT06162221). Similarly, tri-complex inhibitor RMC-4998 could overcome resistance to sotorasib both in vitro and in vivo.74 Combining RMC-4998 and sotorasib inhibited cell proliferation and downstream signal transduction with increased efficacy on KRAS-mutant tumors.74 Currently, a phase 1/2 clinical trial is ongoing to test RMC-6291 in KRAS-mutant tumors (NCT05462717). In addition, RMC-7977 was a tri-complex RAS inhibitor targeting KRAS, NRAS, and HRAS. In preclinical studies, RMC-7977 led to the regression of KRAS-mutant tumors and showed substantial efficacy in tumor models with KRAS exon 12 alterations.75 Recently, RMC-7977 has been assessed in patients with KRAS-mutant solid tumors (NCT05379985).
Further research was needed to evaluate the efficacy and safety of KRAS(ON) inhibitors before application in the clinic.
In addition to the drugs mentioned above, several KRAS G12C inhibitors (LY3499446, GDC-6036, D-1553, JDQ443, BI 1,823,911, LY3537982, JAB-21822, YL-15293, and RMC-6291) and KRAS G12D inhibitors (KRpep-2d, KS-58, and MRTX1133) have been used in preclinical and clinical trials.76–79 Further evidence is needed for KRAS-targeted therapies as the first-line treatment of KRAS-mutant NSCLC.
8. Conclusion and future perspectives
KRAS mutations are common in NSCLC. Clinical evidence has shown that first-line chemotherapy or chemotherapy combined with antivascular therapy for KRASm NSCLC is not as effective as for KRASwt NSCLC, but first-line immunotherapy is better than or at least comparable to KRASwt NSCLC. Chemotherapy combined with immunotherapy is the preferred first-line treatment for KRASm NSCLC, with better efficacy when combined with antivascular therapy. ICI monotherapy was also an option for patients with a PD-L1 tumor proportion score ≥50%. In addition to chemotherapy, antivascular therapies, and immunotherapy, a variety of emerging treatments are expected to become first-line therapies in the future, and KRAS inhibitors, such as sotorasib and adagrasib, may gradually become first-line treatments for KRASm NSCLC.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
The study is supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-054).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–12. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):358–376. doi: 10.1016/j.annonc.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Parikh K, Banna G, Liu SV, Friedlaender A, Desai A, Subbiah V, Addeo ADK. Drugging KRAS: current perspectives and state-of-art review. J Hematol Oncol. 2022;15(1):152. doi: 10.1186/s13045-022-01375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokota J, Kohno T. Molecular footprints of human lung cancer progression. Cancer Sci. 2004;95(3):197–204. doi: 10.1111/j.1349-7006.2004.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin G, Huang J, Petela J, Jiang H, Zhang Y, Gong S, Wu J, Liu B, Shi J, Gao Y. Targeting small GTPases: emerging grasps on previously untamable targets, pioneered by KRAS. Signal Transduct Target Ther. 2023;8(1):212. doi: 10.1038/s41392-023-01441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 2022;12(4):924–937. doi: 10.1158/2159-8290.CD-21-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamiya Y, Matsumoto S, Zenke Y, Yoh K, Ikeda T, Shibata Y, Kato T, Nishino K, Nakamura A, Furuya N, et al. Large-scale clinico-genomic profile of non-small cell lung cancer with KRAS G12C: results from LC-SCRUM-Asia study. Lung Cancer. 2023;176:103–111. doi: 10.1016/j.lungcan.2022.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8(8):1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krygowska AA, Castellano E. PI3K: a crucial piece in the RAS signaling puzzle. Cold Spring Harb Perspect Med. 2018;8(6):a031450. doi: 10.1101/cshperspect.a031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick F. KRAS as a therapeutic target. Clin Cancer Res. 2015;21(8):1797–1801. doi: 10.1158/1078-0432.CCR-14-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metro G, Chiari R, Bennati C, Cenci M, Ricciuti B, Puma F, Flacco A, Rebonato A, Giannarelli D, Ludovini V, et al. Clinical outcome with platinum-based chemotherapy in patients with advanced nonsquamous EGFR wild-type non–small-Cell lung cancer segregated according to KRAS mutation status. Clin Lung Cancer. 2014;15(1):86–92. doi: 10.1016/j.cllc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hames ML, Chen H, Iams W, Aston J, Lovly CM, Horn L. Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer☆. Lung Cancer. 2016;92:29–34. doi: 10.1016/j.lungcan.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eklund EA, Wiel C, Fagman H, Akyürek LM, Raghavan S, Nyman J, Hallqvist A, Sayin VI. KRAS mutations impact clinical outcome in metastatic non-small cell lung cancer. Cancers (Basel). 2022;14(9):2063. doi: 10.3390/cancers14092063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellema WW, Dingemans AM, Thunnissen E, Snijders PJ, Derks J, Heideman DA, Van Suylen R, Smit EF. KRAS mutations in advanced nonsquamous non–small-cell lung cancer patients treated with first-line platinum-based chemotherapy have No predictive value. J Thorac Oncol. 2013;8(9):1190–1195. doi: 10.1097/JTO.0b013e318298764e. [DOI] [PubMed] [Google Scholar]
- 16.Ghimessy AK, Gellert A, Schlegl E, Hegedus B, Raso E, Barbai T, Timar J, Ostoros G, Megyesfalvi Z, Gieszer B, et al. KRAS mutations predict response and outcome in advanced lung adenocarcinoma patients receiving first-line bevacizumab and platinum-based chemotherapy. Cancers (Basel). 2019;11(10):1514. doi: 10.3390/cancers11101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE, Carcereny Costa E, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1–selected advanced non–small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Li F, Zhao J, Zhuo X, Lai J, Wang J, Jiang F, Xu W, Luan F, Lin X, et al. The real-world therapeutic analysis of first-line immunotherapy in Chinese patients with drive gene positive for advanced non-small cell lung cancer. J Cancer. 2023;14(6):952–965. doi: 10.7150/jca.77199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L, Hsu M, Cohen RB, Langer CJ, Mamtani R, Aggarwal C. Association between KRAS variant status and outcomes with first-line immune checkpoint inhibitor–based therapy in patients with advanced non–small-Cell lung cancer. JAMA Oncol. 2021;7(6):937–939. doi: 10.1001/jamaoncol.2021.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost N, Kollmeier J, Vollbrecht C, Grah C, Matthes B, Pultermann D, von Laffert M, Lüders H, Olive E, Raspe M, et al. KRASG12C/TP53 co-mutations identify long-term responders to first line palliative treatment with pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma. Transl Lung Cancer Res. 2021;10(2):737–752. doi: 10.21037/tlcr-20-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Zhou Q, Zhao S, Wu P, Shi P, Zeng J, Xiong X, Chen H, Kittaneh M, Bravaccini S, et al. KRAS mutation predict response and outcome in advanced non-small cell lung carcinoma without driver alterations receiving PD-1 blockade immunotherapy combined with platinum-based chemotherapy: a retrospective cohort study from China. Transl Lung Cancer Res. 2022;11(10):2136–2147. doi: 10.21037/tlcr-22-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justeau G, Huchot E, Simonneau Y, Roa M, Le Treut J, Le Garff G, Bylicki O, Schott R, Bravard A-S, Tiercin M, et al. Impact of KRAS G12C mutation in patients with advanced non-squamous non-small cell lung cancer treated with first-line pembrolizumab monotherapy. Lung Cancer. 2022;174:45–49. doi: 10.1016/j.lungcan.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Noordhof AL, Damhuis RAM, Hendriks LEL, de Langen Aj, Timens W, Venmans BJW, van Geffen WH, de Langen AJ. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer. 2021;155:163–169. doi: 10.1016/j.lungcan.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Kartolo A, Feilotter H, Hopman W, Fung AS, Robinson A. A single institution study evaluating outcomes of PD-L1 high kras-mutant advanced non-small cell lung cancer (NSCLC) patients treated with first line immune checkpoint inhibitors. Cancer Treat Res Commun. 2021;27:100330. doi: 10.1016/j.ctarc.2021.100330. [DOI] [PubMed] [Google Scholar]
- 25.Gadgeel S, Rodriguez-Abreu D, Felip E, Esteban E, Speranza G, Reck M, Hui R, Boyer M, Garon EB, Horinouchi H, et al. KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Of Oncol. 2019;30:xi64–xi65. doi: 10.1093/annonc/mdz453.002. [DOI] [Google Scholar]
- 26.Nakajima EC, Ren Y, Vallejo JJ, Akinboro O, Mishra-Kalyani PS, Larkins EA, Drezner NL, Tang S, Pazdur R, Beaver JA, et al. Outcomes of first-line immune checkpoint inhibitors with or without chemotherapy according to KRAS mutational status and PD-L1 expression in patients with advanced NSCLC: FDA pooled analysis. J Clin Oncol. 2022;40(16_suppl):9001–. doi: 10.1200/JCO.2022.40.16_suppl.9001. [DOI] [Google Scholar]
- 27.Alessi JV, Elkrief A, Ricciuti B, Wang X, Cortellini A, Vaz VR, Lamberti G, Frias RL, Venkatraman D, Fulgenzi CAM, et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced NSCLC. J Thorac Oncol. 2023;18(6):731–743. doi: 10.1016/j.jtho.2023.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veccia A, Dipasquale M, Kinspergher S, Monteverdi S, Girlando S, Barbareschi M, Caffo O. Impact of KRAS mutations on clinical outcomes of patients with advanced non-squamous non-small cell lung cancer receiving anti-PD-1/PD-L1 therapy. Target Oncol. 2023;18(1):129–138. doi: 10.1007/s11523-022-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok TSK, Lopes G, Cho BC, Kowalski DM, Kasahara K, Wu YL, de Castro G, Turna HZ, Cristescu R, Aurora-Garg D, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann Oncol. 2023;34(4):377–388. doi: 10.1016/j.annonc.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Gao Y, Wang Y, Zhao C, Zhang Z, Li B, Zhang T. A single center analysis of first-line treatment in advanced KRAS mutant non-small cell lung cancer: real-world practice. BMC Cancer. 2022;22(1):1175. doi: 10.1186/s12885-022-10236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu X, Si J, Guan Y, Xu Y, Shao L, Zhang Y, Xu C, Pan W, Lu Y, Song Z, et al. Efficacy of immune checkpoint inhibitors in patients with kras-mutant advanced non-small cell lung cancer: a retrospective analysis. Open Med (Wars). 2023;18(1):20230653. doi: 10.1515/med-2023-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang MM, Zhang Y, Wu S, Zhang SY, Shan HL, Yang XM, Xu X, Song L-Q, Qu S-Y. Clinical outcomes of kras-mutant non-small cell lung cancer under untargeted therapeutic regimes in the real world: a retrospective observational study. Transl Lung Cancer Res. 2023;12(10):2030–2039. doi: 10.21037/tlcr-23-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Li Z, Jian H, Xia L, Lu S. Impact of KRAS mutation subtypes and Co-occurring mutations on response and outcome in advanced NSCLC patients following first-line treatment. J Clin Med. 2022;11(14):4003. doi: 10.3390/jcm11144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West HJ, McCleland M, Cappuzzo F, Reck M, Mok TS, Jotte RM, Nishio M, Kim E, Morris S, Zou W, et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in kras-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J Immunother Cancer. 2022;10(2):e003027. doi: 10.1136/jitc-2021-003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Huang D, Lin G, Yang X, Zhuo M, Chi Y, Zhai X, Jia B, Wang J, Wang Y, et al. The prevalence and real-world therapeutic analysis of Chinese patients with KRAS-mutant non-small cell lung cancer. Cancer Med. 2022;11(19):3581–3592. doi: 10.1002/cam4.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in Chemotherapy-Naive patients with advanced-stage non–small-Cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 37.Ricciuti B, Brambilla M, Cortellini A, De Giglio A, Ficorella C, Sidoni A, Bellezza G, Crinò L, Ludovini V, Baglivo S, et al. Clinical outcomes to pemetrexed-based versus non-pemetrexed-based platinum doublets in patients with KRAS-mutant advanced non-squamous non-small cell lung cancer. Clin Transl Oncol. 2020;22(5):708–716. doi: 10.1007/s12094-019-02175-y. [DOI] [PubMed] [Google Scholar]
- 38.Konishi T, Huang CL, Adachi M, Taki T, Inufusa H, Kodama K, Kohno N, Miyake M. The K-ras gene regulates vascular endothelial growth factor gene expression in non-small cell lung cancers. Int J Oncol. 2000;16(3):501–511. doi: 10.3892/ijo.16.3.501. [DOI] [PubMed] [Google Scholar]
- 39.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55(20):4575–4580. [PubMed] [Google Scholar]
- 40.Mellema WW, Masen-Poos L, Smit EF, Hendriks LE, Aerts JG, Termeer A, Goosens MJ, Smit HJM, van den Heuvel MM, van der Wekken AJ, et al. Comparison of clinical outcome after first-line platinum-based chemotherapy in different types of KRAS mutated advanced non-small-cell lung cancer. Lung Cancer. 2015;90(2):249–254. doi: 10.1016/j.lungcan.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Landre T, Justeau G, Assié JB, Chouahnia K, Davoine C, Taleb C, Chouaïd C, Duchemann B. Anti-pd-(L)1 for kras-mutant advanced non-small–cell lung cancers: a meta-analysis of randomized–controlled trials. Cancer Immunol Immunother. 2022;71(3):719–726. doi: 10.1007/s00262-021-03031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghanem P, Murray JC, Hsu M, Guo MZ, Ettinger DS, Feliciano J, Forde P, Hann CL, Lam VK, Levy B, et al. Clinical and genomic characterization of long-term responders receiving immune checkpoint blockade for metastatic non–small-cell lung cancer. Clin Lung Cancer. 2023;25(2):109–118. doi: 10.1016/j.cllc.2023.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Notario L, Cucurull M, Cerdà G, Sanz C, Carcereny E, Muñoz-Mármol A, Hernández A, Domènech M, Morán T, Sánchez-Céspedes M, et al. Characterization of a cohort of metastatic lung cancer patients harboring KRAS mutations treated with immunotherapy: differences according to KRAS G12C vs. non-G12C. Front Oncol. 2023;13:1239000. doi: 10.3389/fonc.2023.1239000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, Xu H, Lu Z, Huang J, Lei Y, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in kras-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. doi: 10.1016/j.canlet.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 45.Velcheti V, Hu X, Li Y, El-Osta H, Pietanza MC, Burke T. Real-world Time on treatment with first-line pembrolizumab monotherapy for advanced NSCLC with PD-L1 expression ≥ 50%: 3-year follow-up data. Cancers (Basel). 2022;14(4):1041. doi: 10.3390/cancers14041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkrief A, Riccuiti B, Alessi JV, Fei T, Kalvin HL, Egger JV, Rizvi H, Thummalapalli R, Lamberti G, Plodkowski A, et al. Outcomes of combination platinum-doublet chemotherapy and anti-pd(l)-1 blockade in KRASG12C-mutant non-small cell lung cancer. Oncologist. 2023;28(11):978–985. doi: 10.1093/oncolo/oyad197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cefalì M, Epistolio S, Ramelli G, Mangan D, Molinari F, Martin V, Freguia S, Mazzucchelli L, Froesch P, Frattini M, et al. Correlation of KRAS G12C mutation and high PD-L1 expression with clinical outcome in NSCLC patients treated with anti-PD1 immunotherapy. J Clin Med. 2022;11(6):1627. doi: 10.3390/jcm11061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attili I, Valenza C, Santoro C, Antonarelli G, Trillo Aliaga P, Del Signore E, Catania C, Spitaleri G, Passaro A, de Marinis F. Comparison of real-world data (RWD) analysis on efficacy and post-progression outcomes with pembrolizumab plus chemo vs chemo alone in metastatic non-squamous non-small cell lung cancer with PD-L1 <>. Front Oncol. 2022;12:980765. doi: 10.3389/fonc.2022.980765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arbour KC, Rizvi H, Plodkowski AJ, Hellmann MD, Knezevic A, Heller G, Yu HA, Ladanyi M, Kris MG, Arcila ME, et al. Treatment outcomes and clinical characteristics of patients with KRAS-G12C–mutant non–small cell lung cancer. Clin Cancer Res. 2021;27(8):2209–2215. doi: 10.1158/1078-0432.CCR-20-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricciuti B, Alessi JV, Elkrief A, Wang X, Cortellini A, Li YY, Vaz VR, Gupta H, Pecci F, Barrichello A, et al. Dissecting the clinicopathologic, genomic, and immunophenotypic correlates of KRAS(G12D)-mutated non-small-cell lung cancer. Ann Oncol. 2022;33(10):1029–1040. doi: 10.1016/j.annonc.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C, Zheng S, Wang Z, Wang S, Wang X, Yang L, Xu H, Cao Z, Feng X, Xue Q, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun (Lond). 2022;42(9):828–847. doi: 10.1002/cac2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frost MG, Jensen KJ, Gotfredsen DR, Sørensen AMS, Ankarfeldt MZ, Louie KS, Sroczynski N, Jakobsen E, Andersen JL, Jimenez-Solem E, et al. KRAS G12C mutated advanced non-small cell lung cancer (NSCLC): characteristics, treatment patterns and overall survival from a Danish nationwide observational register study. Lung Cancer. 2023;178:172–182. doi: 10.1016/j.lungcan.2023.02.021. [DOI] [PubMed] [Google Scholar]
- 53.Aredo JV, Padda SK, Kunder CA, Han SS, Neal JW, Shrager JB, Wakelee HA. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer. 2019;133:144–150. doi: 10.1016/j.lungcan.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan H, Judd J, Xiu J, Ullah A, Raval GG, Ma PC, Nieva JJ, Radovich M, Oberley MJ, Kim SY, et al. Co-mutational status and PD-L1 expression in KRAS mutant non-small cell lung cancer (NSCLC): role in treatment selection and association with clinical outcomes. J Clin Oncol. 2023;41(16_suppl):9038–. doi: 10.1200/JCO.2023.41.16_suppl.9038. [DOI] [Google Scholar]
- 55.Proulx-Rocray F, Routy B, Nassabein R, Belkaid W, Tran-Thanh D, Malo J, Tonneau M, Ouarzadi OE, Florescu M, Tehfe M, et al. The prognostic impact of KRAS, TP53, STK11 and KEAP1 mutations and their influence on the NLR in NSCLC patients treated with immunotherapy. Cancer Treat Res Commun. 2023;37:100767. doi: 10.1016/j.ctarc.2023.100767. [DOI] [PubMed] [Google Scholar]
- 56.La Fleur L, Falk-Sörqvist E, Smeds P, Berglund A, Sundström M, Mattsson JSM, Brandén E, Koyi H, Isaksson J, Brunnström H, et al. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Cancer. 2019;130:50–58. doi: 10.1016/j.lungcan.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, Lito P, Berger M, Solit DB, Hellmann M, et al. Effects of Co-occurring genomic alterations on outcomes in patients with KRAS -mutant non–small cell lung cancer. Clin Cancer Res. 2018;24(2):334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Negrao MV, Wu W-H, Lindsay CR, Caparica R, Prêtre V, Kang Y, Caro N, Farago A, Ye F, Castro GD. Abstract 918: real-world clinical characteristics and treatment (tx) outcomes by co-mutation status in patients (pts) with KRAS G12C-mutated non-small cell lung cancer (NSCLC). Cancer Res. 2023;83(7_Supplement):918–. doi: 10.1158/1538-7445.AM2023-918. [DOI] [Google Scholar]
- 59.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 60.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, et al. KRAS G12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.FDA approves first KRAS inhibitor: sotorasib. Cancer Discov. 2021;11(8):OF4–OF4. doi: 10.1158/2159-8290.CD-NB2021-0362. [DOI] [PubMed] [Google Scholar]
- 63.de Langen Aj, Johnson ML, Mazieres J, Dingemans AMC, Mountzios AC, Pless M, de Langen AJ, Wolf J, Schuler M, Lena H, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401(10378):733–746. doi: 10.1016/S0140-6736(23)00221-0. [DOI] [PubMed] [Google Scholar]
- 64.Ou SI, Jänne PA, Leal TA, Rybkin S II, Barve JK, Barve, Ma MA, Bazhenova L, Johnson ML, Velastegui KL, et al. First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRAS G12C solid tumors (KRYSTAL-1). J Clin Oncol. 2022;40(23):2530–2538. doi: 10.1200/JCO.21.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhillon S. Adagrasib: first approval. Drugs. 2023;83(3):275–285. doi: 10.1007/s40265-023-01839-y. [DOI] [PubMed] [Google Scholar]
- 66.Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, et al. Adagrasib in non–small-Cell lung cancer harboring a KRAS G12C mutation. N Engl J Med. 2022;387(2):120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 67.Mok TSK, Yao W, Duruisseaux M, Doucet L, Azkárate Martínez A, Gregorc V, Juan-Vidal O, Lu S, De Bondt C, de Marinis F, et al. KRYSTAL-12: phase 3 study of adagrasib versus docetaxel in patients with previously treated advanced/metastatic non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. J Clin Oncol. 2024;42(17_suppl):LBA8509–LBA. doi: 10.1200/JCO.2024.42.17_suppl.LBA8509. [DOI] [Google Scholar]
- 68.Burns TF, Dragnev KH, Fujiwara Y, Murciano-Goroff YR, Lee DH, Hollebecque A, Koyama T, Cassier PA, Italiano A, Heist RS, et al. Efficacy and safety of olomorasib (LY3537982), a second-generation KRAS G12C inhibitor (G12Ci), in combination with pembrolizumab in patients with KRAS G12C-mutant advanced NSCLC. J Clin Oncol. 2024;42(16_suppl):8510–. doi: 10.1200/JCO.2024.42.16_suppl.8510. [DOI] [Google Scholar]
- 69.Li BT, Clarke JM, Felip E, Ruffinelli JC, Garrido P, Zugazagoitia J, Goldberg SB, Ramalingam SS, Victoria I, Puri S, et al. Sotorasib plus carboplatin and pemetrexed in KRAS G12C advanced NSCLC: updated analysis from the international CodeBreaK 101 trial. J Clin Oncol. 2024;42(16_suppl):8512–. doi: 10.1200/JCO.2024.42.16_suppl.8512. [DOI] [Google Scholar]
- 70.Gregorc V, González-Cao M, Salvagni S, Koumarianou A, Gil-Bazo I, Maio M, Viteri S, Majem M, Gutiérrez V, Bernabe Caro R, et al. KROCUS: a phase II study investigating the efficacy and safety of fulzerasib (GFH925) in combination with cetuximab in patients with previously untreated advanced KRAS G12C mutated NSCLC. J Clin Oncol. 2024;42(17_suppl):LBA8511–LBA. doi: 10.1200/JCO.2024.42.17_suppl.LBA8511. [DOI] [Google Scholar]
- 71.Liguori L, Salomone F, Viggiano A, Sabbatino F, Pepe S, Formisano L, Bianco R, Servetto A. KRAS mutations in advanced non-small cell lung cancer: from biology to novel therapeutic strategies. Crit Rev Oncol Hematol. 2024;205:104554. doi: 10.1016/j.critrevonc.2024.104554. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Shokat KM. Bifunctional small-molecule ligands of K-Ras induce its association with immunophilin proteins. Angew Chem Int Ed Engl. 2019;58(45):16314–16319. doi: 10.1002/anie.201910124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang J, Jiang L, Maldonato BJ, Wang Y, Holderfield M, Aronchik I, Winters IP, Salman Z, Blaj C, Menard M, et al. Translational and therapeutic evaluation of RAS-GTP inhibition by RMC-6236 in RAS-Driven cancers. Cancer Discov. 2024;14(6):994–1017. doi: 10.1158/2159-8290.CD-24-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nokin MJ, Mira A, Patrucco E, Ricciuti B, Cousin S, Soubeyran I, San José S, Peirone S, Caizzi L, Vietti Michelina S, et al. RAS-ON inhibition overcomes clinical resistance to KRAS G12C-OFF covalent blockade. Nat Commun. 2024;15(1):7554. doi: 10.1038/s41467-024-51828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Araujo HA, Pechuan-Jorge X, Zhou T, Do MT, Hu X, Rojas Alvarez FR, Salvatierra ME, Ibarguen HP, Lee R, Raghulan R, et al. Mechanisms of response and tolerance to active RAS inhibition in KRAS -mutant non–small cell lung cancer. Cancer Discov. 2024;14(11):2183–2208. doi: 10.1158/2159-8290.CD-24-0421. [DOI] [PubMed] [Google Scholar]
- 76.O’Sullivan É, Keogh A, Henderson B, Finn SP, Gray SG, Gately K. Treatment strategies for KRAS-Mutated non-small-cell lung cancer. Cancers (Basel). 2023;15(6):1635. doi: 10.3390/cancers15061635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakamoto K, Kamada Y, Sameshima T, Yaguchi M, Niida A, Sasaki S, Miwa M, Ohkubo S, Sakamoto J-I, Kamaura M, et al. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem Biophys Res Commun. 2017;484(3):605–611. doi: 10.1016/j.bbrc.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 78.Sakamoto K, Masutani T, Hirokawa T. Generation of KS-58 as the first K-Ras(G12D)-inhibitory peptide presenting anti-cancer activity in vivo. Sci Rep. 2020;10(1):21671. doi: 10.1038/s41598-020-78712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hallin J, Bowcut V, Calinisan A, Briere DM, Hargis L, Engstrom LD, Laguer J, Medwid J, Vanderpool D, Lifset E, et al. Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat Med. 2022;28(10):2171–2182. doi: 10.1038/s41591-022-02007-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


