Abstract
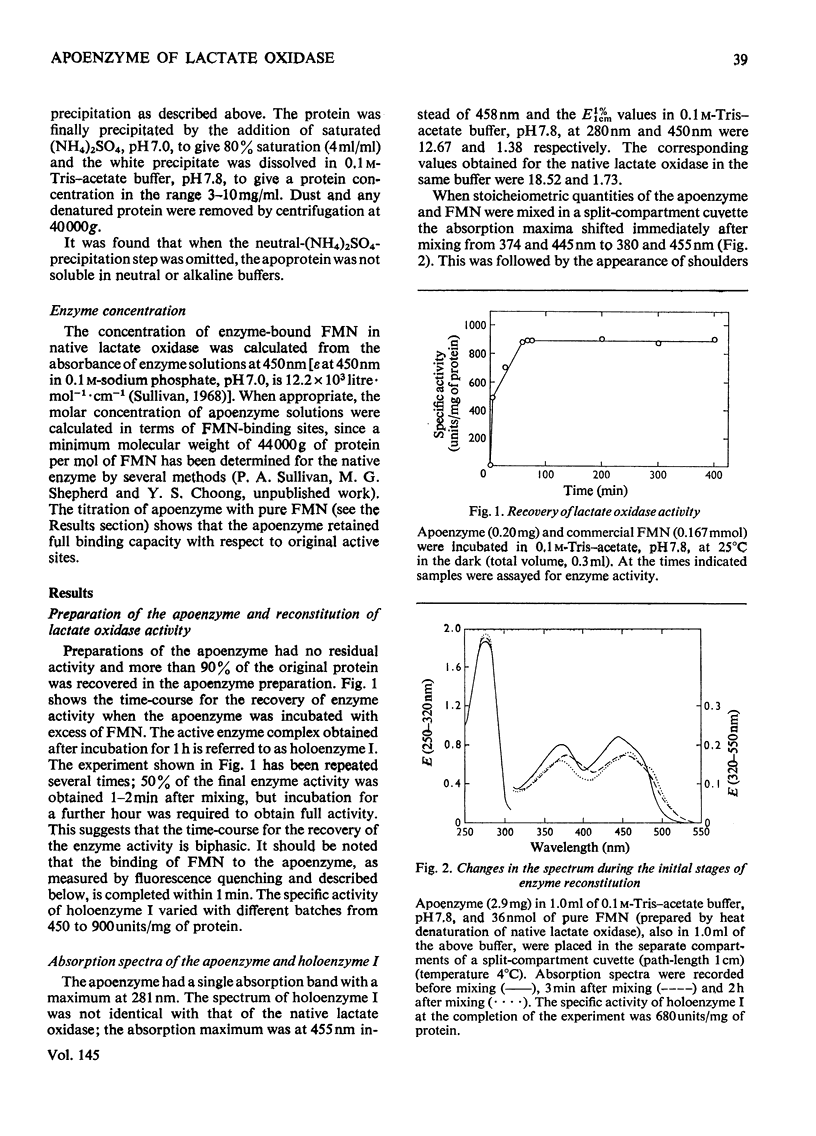
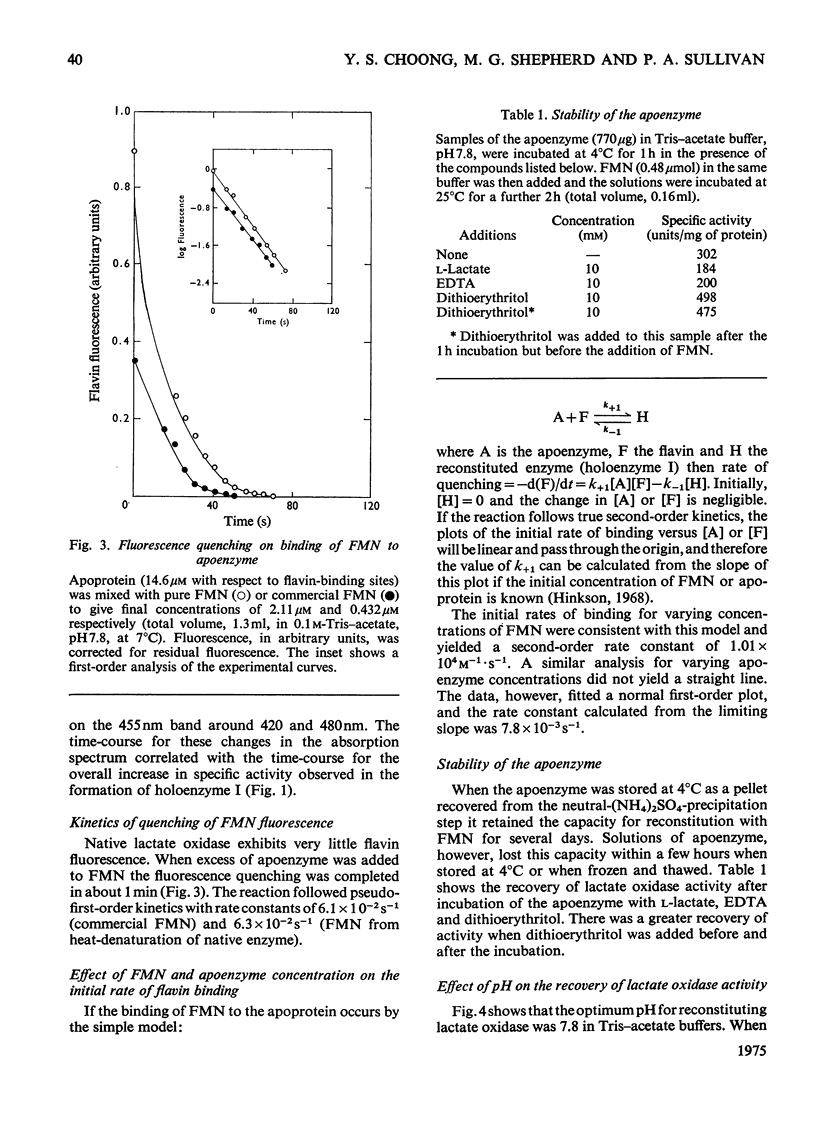
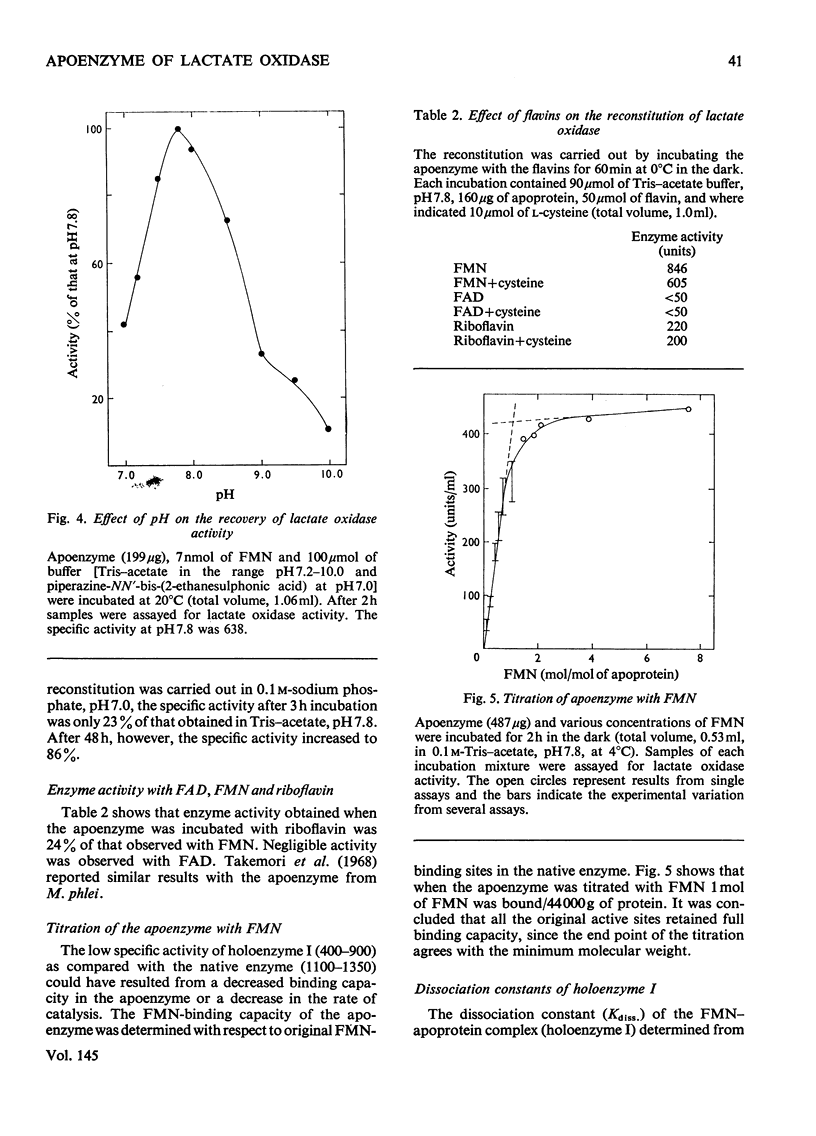
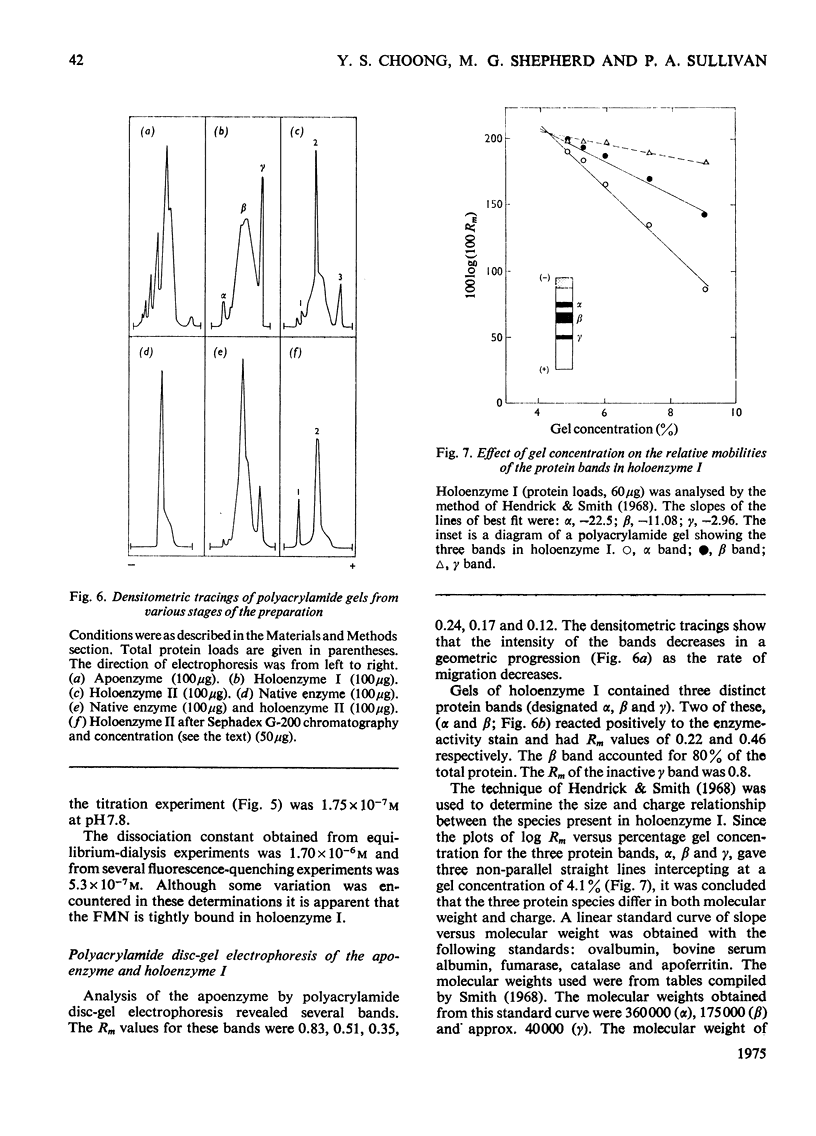
1. Lactate oxidase from Mycobacterium smegmatis is completely resolved into free flavin and apoenzyme by treatment with acid (NH4)2SO4. 2. Reconstitution involves rapid binding of FMN, but the recovery of enzyme activity was slower and appeared to be biphasic. 3. The preparation of the holoenzyme obtained differs from the native enzyme in specific activity, extinction coefficients and mobility on disc-gel electrophoresis. 4. Dialysis of this reconstituted enzyme in 0.1 M-sodium phosphate buffer, pH 7.0, at 0 degrees C for 1 week yields a preparation which closely resembles the native enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hinkson J. W. Azotobacter free-radical flavoprotein. Preparation and properties of the apoprotein. Biochemistry. 1968 Jul;7(7):2666–2672. doi: 10.1021/bi00847a033. [DOI] [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lockridge O., Massey V., Sullivan P. A. Mechanism of action of the flavoenzyme lactate oxidase. J Biol Chem. 1972 Dec 25;247(24):8097–8106. [PubMed] [Google Scholar]
- Mayhew S. G. Studies on flavin binding in flavodoxins. Biochim Biophys Acta. 1971 May 12;235(2):289–302. doi: 10.1016/0005-2744(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Möhler H., Brühmüller M., Decker K. Covalently bound flavin in D-6-hydroxynicotine oxidase from Arthrobacter oxidans. Identification of the 8 -(N-3-histidyl)-riboflavin-linkage between FAD and apoenzyme. Eur J Biochem. 1972 Aug 18;29(1):152–155. doi: 10.1111/j.1432-1033.1972.tb01969.x. [DOI] [PubMed] [Google Scholar]
- PEEL J. L. THE CATALYSIS OF THE AUTO-OXIDATION OF 2-MERCAPTOETHANOL AND OTHER THIOLS BY VITAMIN B12 DERIVATIVES. POLAROGRAPHIC AND OTHER INVESTIGATIONS. Biochem J. 1963 Aug;88:296–308. doi: 10.1042/bj0880296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON W. B. Sulfhydryl and prosthetic groups of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1955 Oct;216(2):749–761. [PubMed] [Google Scholar]
- Sullivan P. A. Crystallization and properties of L-lactate oxidase from Mycobacterium smegmatis. Biochem J. 1968 Nov;110(2):363–371. doi: 10.1042/bj1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda B. E. The relationship between molecular conformation and the binding of flavin-adenine dinucleotide in glucose oxidase. Biochim Biophys Acta. 1969 Mar;175(2):365–379. doi: 10.1016/0005-2795(69)90014-2. [DOI] [PubMed] [Google Scholar]
- Takemori S., Nakazawa K., Nakai Y., Suzuki K., Katagiri M. A lactate oxygenase from Mycobacterium phlei. Improved purification and some properties of the enzyme. J Biol Chem. 1968 Jan 25;243(2):313–319. [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]


