Abstract
Juvenile hormone (JH) is one of the most essential hormones controlling insect metamorphosis and physiology. While it is well known that JH affects many tissues throughout the insect life cycle, the difference in JH responsiveness and the repertoire of JH-inducible genes among different tissues has not been fully investigated. In this study, we monitored JH responsiveness in vivo using transgenic Drosophila melanogaster flies carrying a JH response element-GFP (JHRE-GFP) construct. Our data highlight the high responsiveness of the epithelial cells within the seminal vesicle, a component of the male reproductive tract, to JH. Specifically, we observe an elevation in the JHRE-GFP signal within the seminal vesicle epithelium upon JH analogue administration, while suppression occurs upon knockdown of a gene encoding the intracellular JH receptor, germ cell-expressed. Starting from published transcriptomic and proteomics datasets, we next identified Lactate dehydrogenase as a JH-response gene expressed in the seminal vesicle epithelium, suggesting insect seminal vesicles undergo metabolic regulation by JH. Together, this study sheds new light on the biology of the insect reproductive regulatory system.
Keywords: Drosophila melanogaster, juvenile hormone, juvenile hormone response element-GFP, lactate dehydrogenase, seminal vesicle
1. Introduction
Juvenile hormone (JH) was initially discovered in the 1930s as an insect metamorphosis inhibition factor [1–4]. JH is synthesized in the corpora allata (CA) and regulates many aspects of insect physiology throughout the life cycle [5–8]. JH signalling is mediated through intracellular JH receptors, Methoprene-tolerant (Met) and its orthologues, which belong to the basic helix-loop-helix (bHLH)-Per-Arnt-Sim (PAS) family of transcriptional factors [9–12]. Met and its orthologous transcription factors bind to JH with high affinity [10,13]. Upon JH binding, these intracellular receptors associate with specific JH response elements (JHREs), containing a C-box sequence (CACGCG, an E-box-like motif) or a canonical E-box sequence (CACGTG) [13], followed by the transcriptional induction of target genes, such as Krüppel-homolog 1 (Kr-h1) [13–18].
In the last decade, the fruit fly Drosophila melanogaster has contributed to elucidating molecular mechanisms of JH-responsiveness [19]. Two intracellular JH receptors have been identified in D. melanogaster, known as Met and Germ cell-expressed (Gce). Single loss-of-function of either Met and gce is adult viable, while double mutants of Met and gce result in developmental arrest during pupation, like CA-ablated flies [9,20], suggesting that Met and Gce act redundantly to regulate JH-responsive gene expression [10]. A recent study using GAL4- and LexA-based reporters showed that Met and gce are both broadly expressed in many, but not all, tissues throughout D. melanogaster development [21], suggesting many tissues have the potential to transcriptionally respond to JH. Yet, whether all tissues that express JH receptors have active JH transcriptional signalling is unknown.
To approach this problem, we conducted a study using a D. melanogaster strain carrying a JH response element-GFP (JHRE-GFP) [22]. The JHRE-GFP construct contains eight tandem copies of a JHRE, originally identified from the early trypsin gene of Aedes aegypti [16,17,23]. It has also been confirmed that JHRE is responsive to JH analogues (JHAs) in D. melanogaster S2 cultured cells [10]. In addition, a recent study has shown that GFP signals of JHRE-GFP transgenic flies can monitor JH-responsiveness in D. melanogaster embryos [22].
In this study, we show that JHRE-GFP signal is found in epithelial cells of the adult seminal vesicle, which is a part of the male reproductive tract in D. melanogaster. The JHRE-GFP signal in the seminal vesicle epithelium is elevated upon administration of the JHA, methoprene and conversely suppressed in animals depleted of gce by RNAi. We also show that JHRE-GFP in the seminal vesicle epithelium is elevated after mating, consistent with a previous hypothesis that mating elevates JH titer in male adults [24,25]. Furthermore, we identified Lactate dehydrogenase (Ldh) as a JH-response gene expressed in the seminal vesicle epithelium. Our study demonstrates the seminal vesicle as a novel JH-responsive tissue in D. melanogaster.
2. Results
2.1. The seminal vesicle in male D. melanogaster is a JH-responsive tissue
In previous studies, while the functions of JH during development and its effects on the reproductive system of adult females have been extensively studied [1–8,19], its functions in adult males have received less investigation. Therefore, we investigated which cells/tissues are responsive to JH in the adult males using JHRE-GFP transgenic flies. Whereas JHRE-GFP strain has been used for monitoring JH-responsive cells during embryogenesis [22], it has not been used for adult males. Therefore, we first examined JHRE-GFP fluorescence signals in whole male adult bodies. We used two strains in this study, namely JHREWild-type (WT)-GFP males with JHREMutated (Mut)-GFP males [22]. JHREWT-GFP strain carries a wild-type JHRE, while JHREMut-GFP strain carries a mutated JHRE in which Met and Gce binding sites are disrupted [10,22]. We observed strong GFP signals in the scattered hemocytes and some tissues in the abdominal region of JHREWT-GFP, but not JHREMut-GFP flies (electronic supplementary material, figure S1a). We also orally administrated methoprene to these animals and found that the GFP signals in the abdomen were particularly elevated in JHREWT-GFP, but not JHREMut-GFP flies (electronic supplementary material, figure S1a, arrowhead). Based on the data, we further anatomically characterized where JHREWT-GFP was expressed in abdominal tissues.
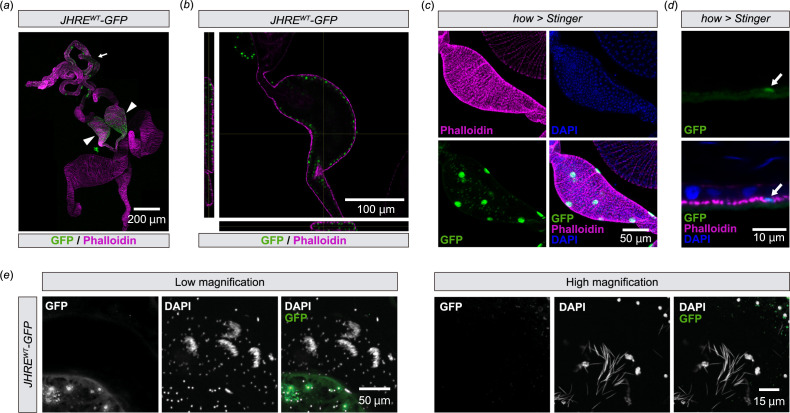
Dissection of JHREWT-GFP male abdomens revealed that the JHRE-GFP signal was present in a part of the male reproductive tract, including the testes and seminal vesicles (figure 1a), which is known to store sperm produced in the testis [26]. As the seminal vesicle showed the most remarkable JHRE-GFP signal in the male reproductive tract, we decided to focus on this tissue for the rest of this study. Within the seminal vesicles, JHRE-GFP was active in cells located on the lumen side compared with the muscle layer surrounding the seminal vesicle labelled with fluorescence-conjugated phalloidin (figure 1b). We assume that these luminal side cells were not muscle cells but epithelial cells, as GFP driven by the muscle driver how-GAL4 [27] was expressed in fewer cells than JHRE-GFP-positive cells in the seminal vesicles (figure 1c) and embedded in the phalloidin-positive muscle layer (figure 1d). In addition, we found that the JHRE-GFP signal was not observed in extracted sperm (figure 1e). Together, these results suggest that JHRE-GFP is expressed in the seminal vesicle epithelial cells.
Figure 1.
JHRE-GFP is expressed in seminal vesicle epithelial cells (a,b) Immunostaining with anti-GFP (green) and phalloidin (magenta) of JHREWT-GFP adult male. (a) Image of the male reproductive tract. The arrowhead and the arrow indicate the seminal vesicles and the testis, respectively. (b) Cross-section image of the seminal vesicle. Left and bottom images indicate horizontal and vertical cross-sectional views, respectively. (c,d) Transgenic visualization of muscles by nuclear GFP (Stinger) driven by how-GAL4. Samples were immunostained with anti-GFP antibody (green), phalloidin (magenta) and DAPI (blue). Samples were derived from virgin males 2 days after eclosion. (c) Image of the seminal vesicle. (d) Magnified view of the seminal vesicle epithelial cells. The arrow indicates a cell with GFP signal. (e) Immunostaining of sperm with anti-GFP (green) and DAPI (white) in JHREWT-GFP adult male.
We also examined whether these cells were labelled with another JH reporter strain, JH response region (JHRR)-LacZ. JHRR-LacZ is a LacZ reporter fused with the JHRR of the D. melanogaster Kr-h1 promoter, which is responsive to JH via Met and Gce [14]. We found that JHRR-LacZ was also expressed in the seminal vesicle cells, some cells in the testes just anterior to the seminal vesicle and some secondary cells of the male accessory gland (electronic supplementary material, figure S1b). Similar to JHRE-GFP, JHRR-LacZ also labelled the epithelial cells of the seminal vesicles (electronic supplementary material, figure S1c,d).
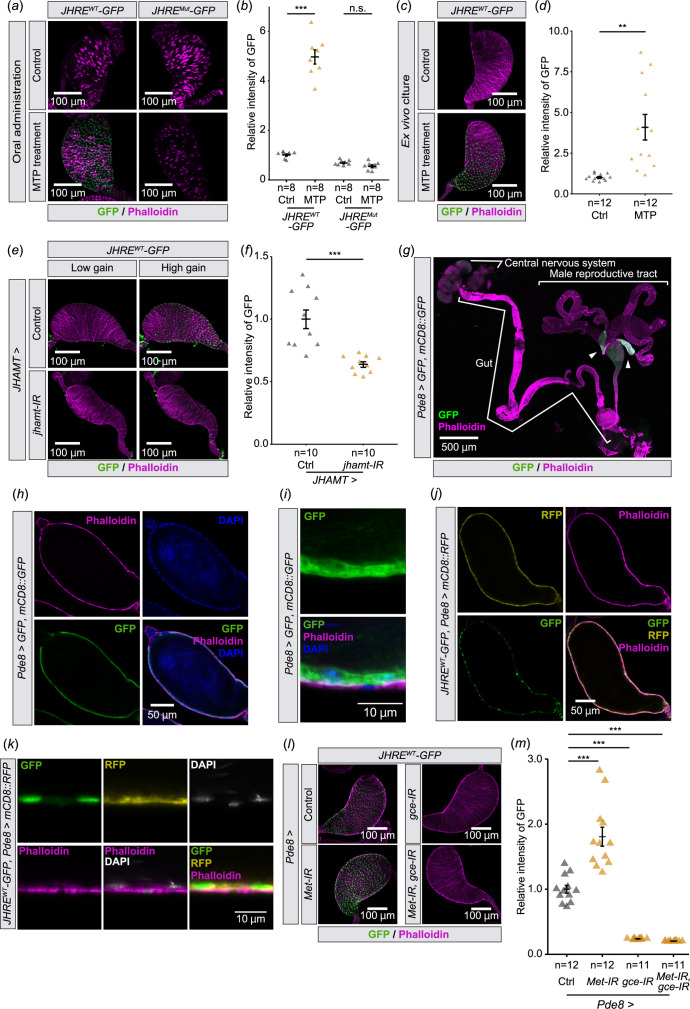
We next examined whether seminal vesicle cells respond to the JH signalling. We found that the oral administration of methoprene increased JHRE-GFP signal in the seminal vesicles of virgin males carrying the JHREWT-GFP, but not JHREMut-GFP, transgene (figure 2a,b). In addition, JHRE-GFP signal was elevated in ex vivo cultured seminal vesicles 16 h after incubation with methoprene (figure 2c,d), suggesting that the seminal vesicle itself responds to JH. Conversely, when JH biosynthesis was blocked by knocking down JH acid O-methyltransferase (jhamt), a rate-limiting enzyme for JH biosynthesis in the CA [28,29], JHRE-GFP signal in the seminal vesicle decreased (figure 2e,f). These results suggest that the seminal vesicle epithelial cells respond to changes in circulating JH.
Figure 2.
JHRE-GFP signal in the seminal vesicle changes depending on JH signalling. (a,b) JHRE-GFP signal in the seminal vesicle of JHREWT-GFP and JHREMut-GFP males 7 days after eclosion with or without oral administration of methoprene (MTP). (a) Representative images of the seminal vesicles immunostained with GFP and phalloidin (F-actin) shown in green and magenta, respectively. (b) Quantification of JHRE-GFP signals in the seminal vesicles of control (Ctrl) and MTP-administered males. (c,d) JHRE-GFP signals in the seminal vesicle of JHREWT-GFP males 4 days after eclosion. Male reproductive tracts without male accessory glands are cultured ex vivo with or without MTP. (c) Representative images of the seminal vesicles immunostained for GFP (green) and phalloidin (magenta). (d) Quantification of JHRE-GFP signal in the seminal vesicles. (e,f) JHRE-GFP signal in the seminal vesicle of control and JHAMT-GAL4-driven jhamt RNAi males 4 days after eclosion. Control RNAi was achieved with a VDRC KK control line. GFP and phalloidin (F-actin) signals are shown in green and magenta, respectively. (e) Representative images of the seminal vesicles. ‘Low gain’ GFP signals were captured with the same gain as shown in (c). ‘High gain’ GFP signals were captured with 1.23-fold gain setting compared with ‘Low gain’ (800vs650). GFP and phalloidin (F-actin) signals are shown in green and magenta, respectively. (f) Quantification of JHRE-GFP signal in the seminal vesicle. (g,i) Immunostaining with anti-GFP (Green), phalloidin (Magenta), and DAPI (Blue) of males carrying Pde8-GAL4 along with both UAS-GFP and UAS-mCD8::GFP 4 days after eclosion. (g) Image of the central nervous system, gut and male reproductive tract. Arrowheads indicate the seminal vesicles. (h) Cross-section image of the seminal vesicle. (i) Magnified view of the seminal vesicle epithelial cells. (j,k) Immunostaining with anti-GFP (Green), anti-RFP (Magenta), phalloidin (Blue) and DAPI (White) of JHRE-GFPWT, Pde8-GAL4 UAS-mCD8::RFP males 4 days after eclosion. (j) Cross-section image of the seminal vesicle. (k) Magnified view of the seminal vesicle epithelial cells. (l,m) JHRE-GFP signal in the seminal vesicle of control males and Pde8-GAL4-driven Met and/or gce RNAi males 7 days after eclosion. Note that this experiment was conducted with food supplemented with MTP, as the MTP administration allowed us to see more drastic difference in JHRE-GFP signals between control and RNAi. Control flies were obtained by crossing w1118 with Pde8-GAL4 driver. (l) Representative images of the seminal vesicles. (m) Quantification of JHRE-GFP signal in the seminal vesicle. Values in b,d,f and m are presented as mean ± s.e. Statistical analysis: Student’s t‐test for b,d,f. Wilcoxon rank sum exact test with Bonferroni’s correction for m.**p < 0.01 ***p < 0.001. n.s. not significant.
We also examined whether JHRR-LacZ expressed in seminal vesicles is upregulated by methoprene feeding. However, anti-LacZ immunostaining signal in the seminal vesicle was not increased by methoprene feeding in JHRR-LacZ flies (electronic supplementary material, figure S1e,f). Since the JH dependence of JHRR-LacZ expression in seminal vesicles was unclear, we used JHRE-GFP as a JH-responsive marker in subsequent analyses.
2.2. JH signalling in the seminal vesicle requires Met and Gce
We next confirmed that JHRE-GFP expression in the seminal vesicle was mediated by intracellular JH receptors, Met and Gce [13–18]. However, since Met and gce double mutant flies die during the larval-pupal transition [9], we conducted transgenic RNAi to knockdown Met and gce with a GAL4 driver that labels the seminal vesicle epithelial cells. After our GAL4 driver screen (see §4 for details), we found that Pde8-GAL4 driver drives gene expression in the seminal vesicles (figure 2g). Our further detailed analysis confirmed that Pde8-GAL4 labels the seminal vesicle epithelial cells (figure 2h,i). In addition, cells labelled with JHREWT-GFP colocalized with Pde8>mCD8::RFP seminal vesicle epithelial cells (figure 2j,k). Using this GAL4 driver, we found that JHRE-GFP signal in the seminal vesicle epithelial cells was decreased by Met and gce double knockdown (figure 2l,m). Furthermore, a reduction in JHRE-GFP signalling was also seen in gce knockdown flies, but not in Met knockdown flies (figure 2l,m). These results suggest that JH is received mainly by Gce in the seminal vesicle epithelial cells.
2.3. Mating activates JH signalling in the seminal vesicle
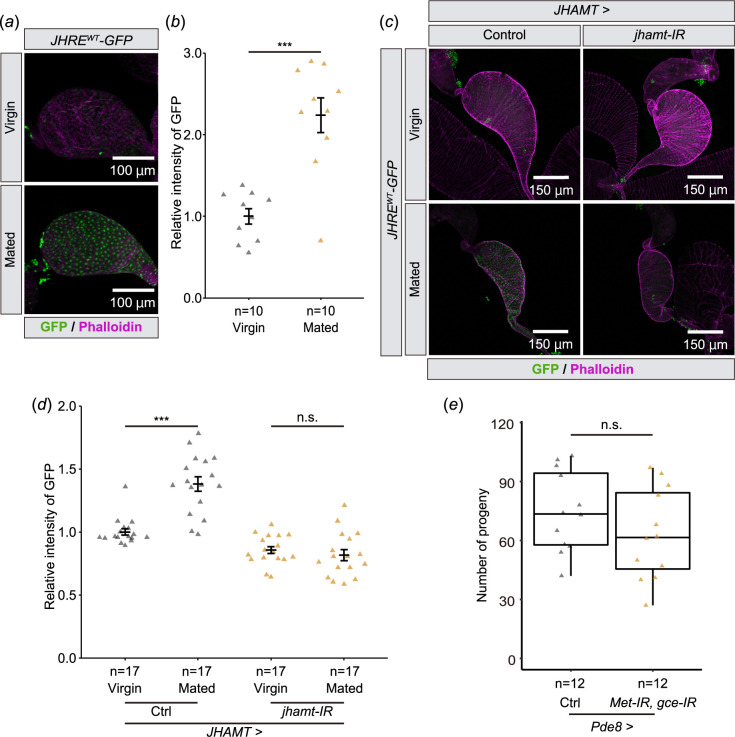
Next, we tested whether JHRE-GFP expression in the seminal vesicle is responsive to natural processes reported to impact JH signalling. In D. melanogaster males, JH signalling may increase in a mating-dependent manner [24,25]. These previous observations motivated us to compare JHRE-GFP signals in the seminal vesicle between virgin and mated males. We found that JHRE-GFP signal in the seminal vesicle epithelial cells increased in mated males as compared with virgin males (figure 3a,b). In addition, the increase of the JHRE-GFP signal upon mating was reduced by jhamt RNAi using the JHAMT-GAL4 driver (figure 3c,d). These results suggest that JH signalling in the seminal vesicle epithelium is responsive to mating. These results raise the possibility that JH signalling in the seminal vesicle influences fertility after mating. However, a double knockdown of Met and gce did not impact on the number of progeny (figure 3e). This result indicates that JH signalling in the seminal vesicle does not play a major role in fertility.
Figure 3.
JHRE-GFP signal in the seminal vesicle is increased after mating. Samples were derived from males 6 days after eclosion. In all photos, GFP and phalloidin (F-actin) signals are shown in green and magenta, respectively. (a,b) JHRE-GFP signals in the seminal vesicle of virgin or mated males. (a) Representative images of the seminal vesicles. (b) Quantification of JHRE-GFP signals in the seminal vesicles. (c,d) JHRE-GFP signals in the seminal vesicles of control and JHAMT-GAL4-driven jhamt RNAi males with or without mating. Control RNAi was achieved with VDRC KK control line noted in the Methods section. (c) Representative images of the seminal vesicles. (d) Quantification of JHRE-GFP signals in the seminal vesicles. (e) Number of progeny from control and Pde8-GAL4-driven Met and gce RNAi males after mating. Control was Pde-GAL4 males crossed to w1118 females. Control flies were obtained by crossing w1118 with Pde8-GAL4 driver. Values in b and d are presented as mean ± s.e.. Statistical analysis: Student’s t‐test for b. Tukey–Kramer test for d. Wilcoxon rank sum exact test for e. *p < 0.05, ***p < 0.001. n.s.: not significant.
2.4. JH induces expression of Ldh in the seminal vesicle
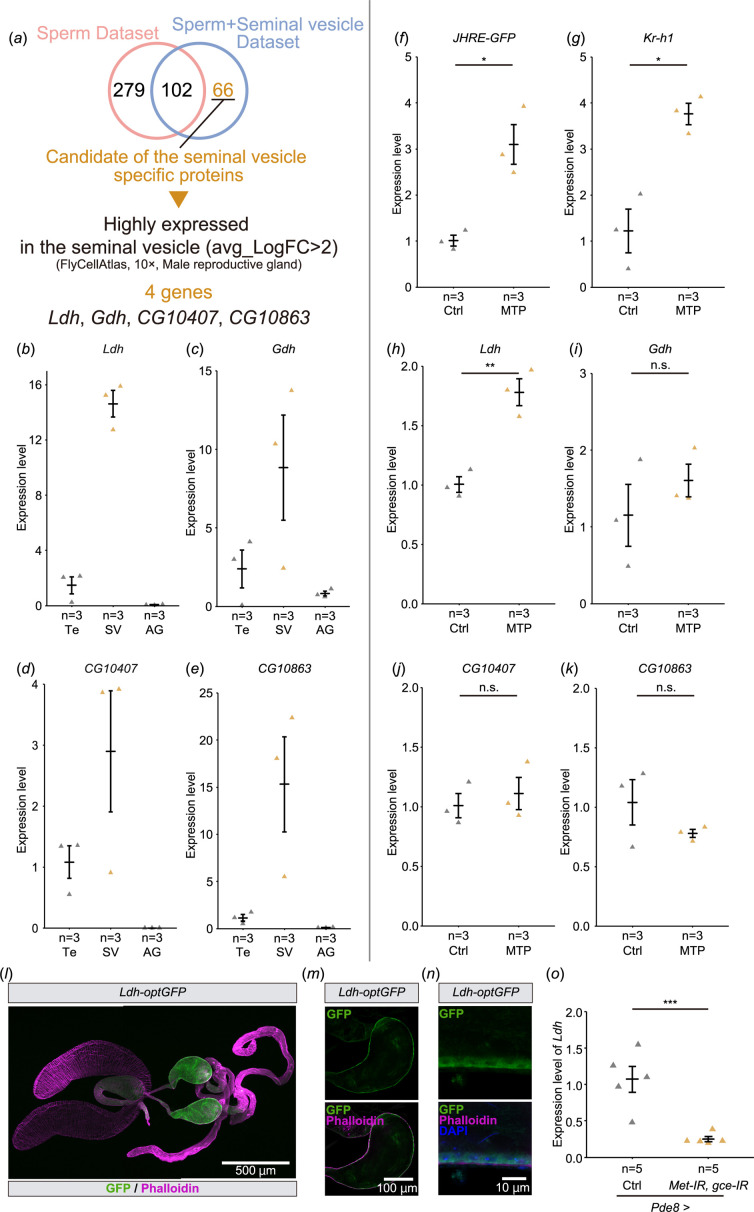
In JH-responsive cells/tissues, JH signalling affects gene expression through Met and Gce [6,13]. Therefore, we searched for genes that are highly expressed in the seminal vesicles and potentially regulated by JH. First, we listed genes that might be highly expressed in the seminal vesicles using the results of proteomic analyses performed in two previous studies [30,31]. Among these proteome studies, one study used mixed samples of the seminal vesicles and sperm [30], while the other study only used sperm samples [31]. Comparing these two data sets, 66 proteins were considered candidates highly enriched in the seminal vesicles but not sperm (figure 4a, table 1). We also searched for the canonical Met/Gce binding E-box (CACGTG) or C-box (CACGCG) sequences in upstream regions of the 66 candidates. We found that many genes have E-boxes or C-boxes (electronic supplementary material, figure S2). Although the presence of these motifs does not directly imply that they are downstream genes of JH signalling, these data may provide clues to clarify the significance of JH signalling in seminal vesicles.
Figure 4.
Screening of genes highly expressed in the seminal vesicle. (a) A flowchart to identify candidate genes that are highly and predominantly expressed in the seminal vesicles. See §§2 and 4§ for details. (b–e) Reverse transcription-quantitative PCR (RT-qPCR of the candidate genes in JHREWT-GFP males. mRNA levels were compared among the testes (Te), seminal vesicles (SV), and male accessory glands (AG). Each dot represents the levels of mRNA derived from 8 virgin males 6 days after eclosion. (b) Ldh. (c) Gdh. (d) CG10407. (e) CG10863. (f–k) RT-qPCR of the candidate genes in male reproductive tracts, including the seminal vesicles, of JHREWT-GFP males with (MTP) or without (Ctrl) oral administration of methoprene. Each dot represents the levels of mRNA derived from 5 virgin males 7 days after eclosion. (f) RT-JHRE-GFP positive control. (g) Kr-h1 positive control. (h) Ldh. (i) Gdh. (j) CG10407. (k) CG10863. (l–n) Immunostaining with anti-GFP antibody (green), phalloidin (magenta) and DAPI (Blue) of Ldh-optGFP virgin males 4 days after eclosion. (l) Image of the male reproductive tract. (m) Cross-section image of the seminal vesicle. (n) Magnified view of the seminal vesicle epithelial cells. (o) RT-qPCR of Ldh in the seminal vesicle of Pde8-GAL4-driven Met and gce RNAi flies. Control flies were obtained by crossing w1118 with Pde8-GAL4 driver. Each dot represents the levels of mRNA derived from 10 seminal vesicles of virgin males 7 days after eclosion. Values in b–k and o are presented as mean ± s.e.. Statistical analysis: Welch’s t‐test for f–k. Student’s t‐test for o. *p < 0.05, **p < 0.01, ***p < 0.001. n.s.: not significant.
Table 1.
Candidate proteins that are specifically and highly expressed in the seminal vesicles.
| gene | avg_logFC (male reproductive gland) | p‐value | Gene | avg_logFC (male reproductive gland) | p‐value |
|---|---|---|---|---|---|
| CG10407 | 5.660312653 | 0 | Prx2540-2 | not found | not found |
| CG10863 | 3.085217476 | 8.75402 × 10−11 | GstE12 | not found | not found |
| Gdh | 2.438033104 | 5.63537 × 10−9 | Gdi | not found | not found |
| Ldh | 2.38763833 | 2.66 × 10−18 | Idgf3 | not found | not found |
| Argk1 | 1.832550764 | 1.34915 × 10−13 | Cam | not found | not found |
| regucalcin | 1.310050249 | 1.75488 × 10−14 | CG1648 | not found | not found |
| GstD1 | 1.146826625 | 7.57 × 10−18 | CG4520 | not found | not found |
| Idgf4 | 0.629053414 | 0.000129652 | CG7264 | not found | not found |
| Vha26 | 0.506168485 | 0.021732058 | CG14282 | not found | not found |
| awd | −0.363285989 | 0.015698655 | CG15125 | not found | not found |
| Est-6 | −0.43701005 | 1.56277 × 10−5 | CG5177 | not found | not found |
| Idh | −0.485521317 | 0.002354908 | CG6287 | not found | not found |
| Rack1 | −0.502643883 | 0.000129842 | Ogdh | not found | not found |
| Obp44a | not found | not found | Rpi | not found | not found |
| Gs1 | not found | not found | CG34107 | not found | not found |
| Dip-B | not found | not found | ND-19 | not found | not found |
| scpr-C | not found | not found | ATPsynO | not found | not found |
| Inos | not found | not found | ND-B22 | not found | not found |
| AdSS | not found | not found | RecQ5 | not found | not found |
| Acsf2 | not found | not found | Fkbp12 | not found | not found |
| Bfc | not found | not found | Swim | not found | not found |
| CG11042 | not found | not found | Mp20 | not found | not found |
| Pglym78 | not found | not found | Tm2 | not found | not found |
| pyd3 | not found | not found | Tm1 | not found | not found |
| Pgk | not found | not found | Prm | not found | not found |
| Got2 | not found | not found | Mf | not found | not found |
| LManII | not found | not found | Mhc | not found | not found |
| Fdh | not found | not found | Tsf1 | not found | not found |
| CG8036 | not found | not found | Zasp66 | not found | not found |
| CG3609 | not found | not found | porin | not found | not found |
| Mfe2 | not found | not found | PPO1 | not found | not found |
| Chd64 | not found | not found | Sod2 | not found | not found |
| Irp-1B | not found | not found | CG11815 | not found | not found |
avg_LogFC: average_Log fold change. ‘not found’ indicates that the presence of mRNA in the seminal vesicle cluster could not be confirmed on the Fly Cell Atlas.
Next, we browsed the D. melanogaster single-cell transcriptome database Fly Cell Atlas (https://flycellatlas.org/) [32] to obtain the gene expression dataset derived from the male reproductive glands. According to the Fly Cell Atlas dataset, the following four genes among the 66 candidate genes are highly enriched in the seminal vesicles as compared with other cells in the male reproductive glands (avg_logFC>2): Ldh, Glutamate dehydrogenase (Gdh), CG10407 and CG10863 (figure 4a, table 1). We then conducted reverse transcription-quantitative PCR (RT-qPCR) to confirm whether these genes were expressed in the seminal vesicles. The mRNA levels of all candidate genes were higher in the seminal vesicles compared with the testes and the male accessory glands (figure 4b–e).
To determine whether expression of these candidate genes is regulated by JH, we did RT-qPCR to measure mRNA levels in male reproductive tracts containing the seminal vesicles dissected from JHREWT-GFP flies with and without methoprene administration. The mRNA levels of JHRE-GFP and the JH-responsive gene Kr-h1, used as positive controls, were upregulated by methoprene treatment (figure 4f,g). Among the candidate genes, Ldh mRNA levels were upregulated by methoprene treatment (figure 4h), while Gdh, CG10407 and CG10863 showed no change in mRNA levels (figure 4i–k). These results suggest that JH signalling in the seminal vesicle induces the expression of Ldh.
To confirm whether Ldh is expressed in the seminal vesicle epithelial cells, we used the transgenic strain, Ldh-optGFP, expressing GFP-tagged Ldh under the control of Ldh regulatory sequences [33,34]. We found that Ldh-optGFP signal was higher in the seminal vesicles, compared with other parts of male reproductive tracts (figure 4l). The magnified images show that Ldh-optGFP is expressed in the seminal vesicle epithelial cells (figure 4m,n), suggesting that Ldh is highly expressed in the seminal vesicle epithelial cells. Importantly, two canonical Met/Gce binding E-box sequences (CACGTG) are found in the Ldh locus, one motif is located in the Ldh-RA promoter region and the other motif is located within the first intron (electronic supplementary material, figure S3a). Then, we examined whether the expression of Ldh was regulated by Met and Gce. We found that Ldh mRNA level was decreased by a double knockdown of Met and gce in the seminal vesicle epithelial cells using Pde8-GAL4 driver (figure 4o). Together, these results indicate that Ldh is a JH-responsive gene in the seminal vesicle epithelial cells. Finally, we conducted a luciferase-based assay in D. melanogaster S2 cultured cells using the promoter/enhancer region of Ldh. In cells expressing luciferase under the control of JHRE, methoprene treatment resulted in increased luciferase activity (electronic supplementary material, figure S3b), as reported previously [10]. However, in cells expressing luciferase driven by the region corresponding to −1160 + 1776 of Ldh, including the two E-boxes (electronic supplementary material, figure S3a), luciferase activity was not increased (electronic supplementary material, figure S3b). Together, these findings suggest that while Ldh expression in seminal vesicles is regulated by Met/Gce in vivo, in vitro JHA does not appear to influence Ldh expression by the two proximal E-boxes.
3. Discussion
The seminal vesicles are known to store, nourish and maintain sperm before they are transferred into the female reproductive tract [26]. In addition, the seminal vesicles act as secretory organs that may assist in producing seminal fluid proteins in some insects [35–40]. How seminal vesicles impart these functions or whether there are additional functions is not understood. In this study, we identified the seminal vesicle as a JH-responsive tissue in adult male D. melanogaster. While neither our current study nor previous studies have been able to clarify the biological significance of the action of JH on the seminal vesicles, our findings here implicate JH-dependent upregulation of the key metabolic enzyme, Ldh. Previous studies on the tasar silkmoth Antheraea mylitta has revealed that topical application of JH III to newly emerged adult males increases the concentration of total seminal vesicle proteins [41], suggesting the JH responsiveness of seminal vesicles might be evolutionarily conserved among insects.
How JH signalling in seminal vesicles supports male fertility or gamete quality is unknown. While JH signalling has been implicated in male fertility, JH is also involved in mating behaviour and memory in male D. melanogaster [24,25,42,43], making tissue-specific requirements for JH signalling challenging. We found that reducing JH signalling in the seminal vesicles did not reduce progeny (figure 3e). However, it should be noted that our male fertility measurements were done under non-competitive conditions. In the mosquito A. aegypti, loss of JH epoxidation in males does not affect the total number of eggs laid by wild-type females but does affect their reproductive fitness under competitive mating conditions [44]. It will be worthwhile to examine whether sperm competition is also affected by JH in D. melanogaster, and also whether it is affected by JH signalling in the seminal vesicles. Molecularly, how JH signalling affects seminal vesicle function remains unclear. JH is known to stimulate secretory activity in the male accessory glands of many insects [45]. In some insects, other than D. melanogaster, seminal vesicle epithelia contain secretory vesicles [37,38,40,46]. Together, these studies leave open the possibility that JH signalling affects the secretory activity of the seminal vesicles in D. melanogaster.
An important finding in this study is that the expression of Ldh in the seminal vesicles is upregulated by activation of JH signalling. While Ldh expression is known to be regulated by ecdysone signalling [47], our study is the first report that Ldh is also influenced by JH signalling. Whether JH induction of Ldh expression by JH signalling is central to seminal vesicle function is not yet clear. Neurobiological studies using D. melanogaster have shown that Ldh has an important role in supplying lactate from glial cells to neurons, known as a lactate shuttle, in response to neural activity in order to supply nutrients to neurons [48–50]. Considering the storage of many sperm in the seminal vesicles and the high expression of Ldh in the seminal vesicle epithelial cells, the lactate shuttle may exist between the sperm stored in the seminal vesicle and the seminal vesicle epithelial cells. It will be intriguing to examine whether JH signalling in the seminal vesicle changes in the quantity and/or quality of sperm.
An interesting previous study has reported that the seminal vesicle expresses multiple clock genes such as period, Clock (Clk) and timeless, all of which are necessary for generating proper circadian rhythm [51]. In the case of the mosquito A. aegypti female, it is reported that JH controls gene expression through a heterodimer of Met and circadian rhythm factor Cycle (CYC) [52]. It was also suggested that Met binds directly to CLK in D. melanogaster [53]. In addition, in the linden bug, Pyrrhocoris apterus, JH alters gene expression through Met, CLK and CYC in the gut [54]. Considering these previous reports and our results, circadian rhythm factors and JH may cooperate to regulate gene expression in the seminal vesicles.
In this study, we used both JHRE-GFP and JHRR-LacZ lines to analyse JH-responsive tissues. Unexpectedly, we found that JHRR-LacZ and JHRE-GFP differentially responded to methoprene administration in the seminal vesicle. JHRE-GFP signal was upregulated in response to methoprene feeding, while JHRR-LacZ signal was not. In addition, the expression pattern of these reporter lines was different in adult males. For example, JHRE-GFP signal was not observed in the male accessory gland, which has been reported as a JH-responsive tissue [24,55–57]. On the other hand, JHRR-LacZ signal was observed in the male accessory gland (electronic supplementary material, figure S1b). This difference may be due to the origin of JHRE and JHRR. JHRE in JHREWT-GFP strain is derived from the early trypsin gene of A. aegypti [22,23], while JHRR is derived from D. melanogaster Kr-h1 [14]. Alternatively, differences in reporter activity may reflect differences in response element number, with JHRE-GFP having eight tandem elements and JHRR-LacZ having one, or in genomic context, as both JHREWT-GFP and JHREMut-GFP transgenes are inserted into the attP2 site of the third chromosome while the JHRR-LacZ is randomly integrated into the third chromosome. Nonetheless, activities of both reporters are restricted to a limited number of cell types of male reproductive tracts. Previous studies reported that Met-T2A-GAL4 and gce-T2A-GAL4 labelled male accessory glands, ejaculatory duct, testes and seminal vesicles. On the other hand, we found that JHRE-GFP only labels cells in seminal vesicles and testes [21]. Considering that Met and Gce are expressed in almost all cell types of male reproductive tract [21], more comprehensive JH reporter strains will be needed in D. melanogaster and other insects in future studies.
Nevertheless, we propose that the JHREWT-GFP and JHREMut-GFP strains [22] are nice tools to approximate JH signalling in vivo in adult male seminal vesicles. For example, in this study, we found that JHRE-GFP in the seminal vesicles is elevated after mating. This observation is consistent with the fact that JH titer is elevated after mating through the action of Ecdysis-triggering hormone [43]. Since JHREWT-GFP strain has the tandem of eight JHREs [22], it may have the advantage of sensitivity for JH signalling. While direct measurements of actual JH titers are crucial [58], indirect approximation of JH titers through JHREWT-GFP and JHREMut-GFP reporter activity in adult males is very easy and convenient. Use of JHRE-GFP signals in the seminal vesicles as a marker of JH signalling will facilitate future studies to increase our understanding of JH-dependent insect male physiology.
4. Materials and methods
4.1. Drosophila melanogaster strains and maintenance
Drosophila melanogaster flies were raised on a standard yeast-cornmeal-glucose fly medium (0.275 g agar, 5.0 g glucose, 4.5 g cornmeal, 2.0 g yeast extract, 150 μl propionic acid and 175 μl 10% butyl p-hydroxybenzoate (in 70% ethanol) in 50 ml water) at 25°C under a 12 : 12 h light/dark cycle. For the methoprene oral administration (figures 2a,b,l,m and 4f–k; electronic supplementary material, figure S1a,e, f), virgin male flies were collected 0 to 8 h after eclosion, aged for 4 days on standard food, and then transferred for 3 days into new tubes in the presence of food supplemented with 60 µM methoprene (Sigma-Aldrich, St Louis, MO, PESTANAL 33375, racemic mixture; 1.5 M stock was prepared in ethanol) or 0.8% ethanol (control). To analyse the effect of mating (figure 3a–d), virgin male flies were collected at eclosion, aged for 4 days on standard food and then transferred for 2 days into new tubes in the presence of w1118 4 days after eclosion virgin females. The ratio of males to females in a vial for mating was 1 : 2. For experiments other than methoprene administration and mating, adult males were aged for 2 to 7 days on standard food.
The following transgenic strains were used: how-GAL4 (Bloomington Drosophila stock center [BDSC] #1767), JHAMT-GAL4 [59] (a gift from Sheng Li, South China Normal University, China), JHREMut-GFP [22], JHREWT-GFP [22,23], JHRR-LacZ (a gift from Sheng Li), KK control (Vienna Drosophila resource center [VDRC] #60100), Ldh-optGFP (BDSC #94704), Pde8-GAL4 (BDSC #65635), UAS-GFP, mCD8::GFP [60] (a gift from Kei Ito, University of Cologne, Germany), UAS-gce-IR (VDRC #101814), UAS-jhamt-IR (VDRC #103958), UAS-Met-IR (VDRC #45852), UAS- mCD8::RFP (BDSC #32219) and UAS-stinger (BDSC #84277).
4.2. Immunohistochemistry
The tissues were dissected in phosphate-buffered Saline (PBS) and fixed in 4% paraformaldehyde in PBS for 30–60 min at 25–27°C. The fixed samples were rinsed thrice in PBS, washed for 15 min with PBS containing 0.3% Triton X-100 (PBT), and treated with a blocking solution (2% bovine serum albumin in PBT; Sigma-Aldrich #A9647) for 1 h at 25–27°C or overnight at 4°C. The samples were incubated with a primary antibody in blocking solution overnight at 4°C. The primary antibodies used were as follows: chicken anti-GFP antibody (Abcam #ab13970, 1 : 2,000), mouse anti-LacZ (β-galactosidase; Developmental Studies Hybridoma Bank #40–1 a; 1 : 50), anti-RFP (Medical & Biological Laboratories PM005, 1 : 2,000). The samples were rinsed thrice with PBS and then washed for 15 min with PBT, followed by incubation with fluorophore (Alexa Fluor 488)-conjugated secondary antibodies (Thermo Fisher Scientific; 1 : 200) and in blocking solution for 2 h at RT or overnight at 4°C. Nuclear stains used in this study were 4',6-diamidino-2-phenylindole (DAPI; final concentration 1 μg ml−1 Sigma-Aldrich, St. Louis, MO, USA). F-Actin was stained with Alexa Fluor 568 phalloidin (1 : 200; Invitrogen, #A12380) or Alexa Fluor 647 phalloidin (1 : 500; Invitrogen, #A30107). For DAPI and phalloidin staining, after the incubation with the secondary antibodies, the samples were washed and then incubated with DAPI and phalloidin for at least 20 min at RT or overnight 4°C. After another round of washing, all the samples were mounted on glass slides using FluorSave reagent (Merck Millipore, #345789). For the quantification of JHRE-GFP signal (figures 2a–f, l,m, and 3a–c), only DAPI and phalloidin was stained after fixation. Confocal images were captured using the LSM 700 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). Quantification of immunostaining signal was conducted using the ImageJ software version 1.53q [61]. Fluorescence intensity of JHRE-GFP was normalized to the area of the seminal vesicle.
4.3. Sperm isolation
Sperm were isolated from 2 day old males carrying the JHRE-GFP reporter using the testes squash protocols from [62,63]. Briefly, testes were dissected in cold testes isolation buffer composed of 10 mM Tris-HCl pH 6.8, 183 mM KCl, 47 mM NaCl and 10 uM taxol (Thermo Fisher Scientific, #AC328420010) then placed on polysine adhesion slides (Thermo Fisher Scientific, #1254578). A coverslip was added on dissected testes, which were then squashed by dropping a pencil with the rubber eraser pointing down from a 0.5-inch height five times. Slides were placed in liquid nitrogen for approximately 7 s and then the coverslip was carefully removed with forceps. Testes and sperm were fixed in 4% paraformaldehyde for seven minutes, washed in 0.1% TritonX-100 in PBS (PBT), then washed twice with PBT containing 0.3% sodium deoxycholate (Thermo Fisher Scientific, #BP349-100) for 15 min each. Samples were washed twice more in PBT and then blocked with 2% BSA in PBT at 4°C overnight. Samples were stained with chicken anti-GFP (Aves Labs, #GFP-1020, 1 : 500) and mouse anti-1B1 (Developmental Studies Hybridoma Bank, 1 : 50) at 4°C for two nights. Samples were washed five times in PBT before incubating with donkey anti-mouse Cy3 (Jackson ImmunoResearch, #715-165-151, 1 : 500) and donkey anti-chicken 488 (Jackson ImmunoResearch, #703-545-155, 1 : 500) at 4°C for 4 h. Samples were washed in PBT, stained for DAPI at 1 : 10 000 (Thermo Fisher Scientific, #D1306, 1 : 10,000), and mounted in Vectashield (VectorLabs, #H-1000-10). Sperm were imaged using a Dragonfly 200 spinning disk confocal and images were processed using Imaris.
4.4. Ex vivo male reproductive tract culture
We collected JHREWT-GFP virgin males 4 days after eclosion. The male reproductive tracts were dissected in Schneider’s Drosophila Medium (SDM; Thermo Fisher Scientific, #21720024), and male accessory glands were removed from the male reproductive tracts using forceps. Approximately 5–6 male reproductive tracts were immediately transferred to a dish containing 3 ml of SDM supplemented with 15% fetal calf serum and 0.6% penicillin-streptomycin with/without the addition of 1 µM methoprene (Sigma-Aldrich, St Louis, MO, PESTANAL 33375, racemic mixture; 1.5 M stock was prepared in ethanol) or 0.7% ethanol (control). The cultures were incubated at 25°C for 16 h, and the samples were immunostained to check the JHRE-GFP signal.
4.5. Screening of GAL4 lines that label the seminal vesicle epithelial cells
To knock down Met and gce in the seminal vesicle, we needed a GAL4 driver active in the seminal vesicle epithelial cells. For this purpose, we first surveyed which genes are highly and predominantly expressed in the seminal vesicles. Candidates of the seminal vesicle-specific genes were extracted from the single-cell transcriptome database, Fly Cell Atlas [32]. In the database, a transcriptomic cluster of the seminal vesicle was annotated in the 10 × Genomics dataset from the whole body and the male reproductive gland samples. We extracted the gene profile of the seminal vesicle cluster derived from the whole-body sample and the male reproductive gland sample. The two profiles of gene expression datasets were filtered by p‐value (p‐value < 0.05) and log fold change (avg_logFC>5). The avg_log FC indicates how specific the expression of a gene is in the certain cluster. Finally, 11 candidate genes were obtained (table 2). Of the published GAL4 strains under the control of each of the 11 candidates, we promptly obtained Pde8-GAL4 and confirmed the expression pattern of Pde8-GAL4 in the seminal vesicle as described in the main text (figure 2g–j).
Table 2.
Candidate seminal vesicle-specific genes for suitable GAL4 identification.
| male reproductive gland | whole body | |||
|---|---|---|---|---|
| gene | avg_logFC(5>) | p‐value (0.05<) | avg_logFC(5>) | p‐value (0.05<) |
| DIP-zeta | 7.488148689 | 3.30132 × 10−8 | 5.467468262 | 6.72 × 10−8 |
| CG14301 | 7.418711185 | 0 | 5.476506233 | 0 |
| CG13460 | 7.363236427 | 5.49847 × 10−6 | 6.622333527 | 3.69572 × 10−6 |
| CG9664 | 7.152559757 | 0 | 5.024883747 | 0 |
| Obp93a | 6.669476509 | 0.041431502 | 5.393202782 | 0.035683934 |
| CG5612 | 6.657152176 | 0 | 6.221313 | 0 |
| CG42828 | 6.422353268 | 0 | 6.050003529 | 0 |
| CG18628 | 5.873726845 | 0 | 7.880100727 | 0 |
| Pde8 | 5.734490395 | 0 | 5.000965118 | 0 |
| NT5E-2 | 5.662868977 | 0 | 5.325617313 | 0 |
| CG10407 | 5.660312653 | 0 | 5.038795471 | 0 |
avg_LogFC: average_Log fold change
4.6. Quantifying progeny from male Drosophila
Virgin male flies were collected at eclosion, aged for 4 days on standard food and then transferred into new tubes in the presence of w1118 4 day old virgin females. The number of females was double that of the males. After 2 days, females were removed, and the males were kept alone for an additional 4 days. The males were then transferred to new tubes in the presence of w1118 4 day old virgin females. Here, males and females were paired one to one. After 1 day, the males were removed, and the females were kept in the same vial for another 6 days and then the number of pupae emerging from each vial was counted.
4.7. Screening of candidate genes that are specifically and highly expressed in the seminal vesicles
Candidate proteins highly enriched in the seminal vesicle were determined by comparing the two independent proteomics datasets. One dataset [30] annotates 168 proteins as being enriched in the seminal vesicle and/or sperm stored in the seminal vesicle. Another dataset [31] annotates 381 proteins as being enriched in the sperm isolated from the seminal vesicle. We found that two datasets share 102 proteins, suggesting that these shared proteins are enriched in the sperm but not the seminal vesicle, with the remaining 66 proteins (168 minus 102) as candidate proteins enriched in the seminal vesicle (table 1). Next, we checked whether each of the genes encoding the 66 proteins is predominantly expressed in the seminal vesicles by the single-cell transcriptome database Fly Cell Atlas [32]. We extracted gene profiles of the seminal vesicle cluster in male reproductive gland sample. The candidate genes were filtered by p‐value (p < 0.05) and log fold change (avg_logFC>5). Finally, we obtained four candidate genes, Ldh, Gdh, CG10407 and CG10863.
4.8. Reverse transcription-quantitative PCR (RT-qPCR)
RNA from tissues was extracted using RNAiso Plus (Takara Bio) and reverse-transcribed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO). Synthesized cDNA samples were used as templates for quantitative PCR using THUNDERBIRD SYBR qPCR Mix (TOYOBO) on a Thermal Cycler Dice Real Time System (Takara Bio). The amount of target RNA was normalized to the endogenous control ribosomal protein 49 gene (rp49) and the relative fold change was calculated. The expression levels of each gene were compared using the ΔΔCt method [64]. The following primers were used for this analysis: rp49 F (5′-CGGATCGATATGCTAAGCTGT-3′), rp49 R (5′-GCGCTTGTTCGATCCGTA-3′), GFP F (5′-GAACCGCATCGAGCTGAA-3′), GFP R (5′-TGCTTGTCGGCCATGATATAG-3′), CG10407 F (5′-ACTGGACAACAGCCAAACCTC-3′), CG10407 R (5′-GTGTCTAGGTCGGGTGCATTG-3′), Ldh F (5′-CGTTTGGTCTGGAGTGAACA-3′), Ldh R (5′-GCAGCTCGTTCCACTTCTCT-3′), Gdh F (5′-GGAGGACTACAAGAACGAGCA-3′), Gdh R (5′-CAGCCACTCGAAGAAGGAGA-3′), CG10863 F (5′-CATCGGACTGGGCACCTATAC-3′), CG10863 R (5′-TTCTCGTAGAAATAGGCGGTGTC-3′), Kr-h1 F (5′-TCACACATCAAGAAGCCAACT-3′) and Kr-h1 R (5′-GCTGGTTGGCGGAATAGTAA-3′).
4.9. Construction of luciferase reporter plasmids
We amplified a −1160 to+1776 bp upstream region of Ldh from w1118 genomic DNA using primers (Fwd: 5′-ACTGAGCTCTACAGATCTCTTGAGGACTCTCTATGG-3′, Rev: 5′-TGACTCGAGTAACTTTAATATTCCGCCAAAGAAAGC-3′) to add Sac1 and Xho1 sites to the 5′ and 3′ ends, respectively. These amplified Ldh upstream regions were digested with Sac1 and Xho1 and ligated into a Sac1-Xho1-digested pGL3-Basic vector luciferase reporter plasmid (Promega #E175A).
4.10. Transfection and luciferase reporter assays
S2 cells were seeded in 500 µl Schneider’s Drosophila Medium (SDM; Thermo Fisher Scientific, #21720024) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, #10270106) and 1% penicillin-streptomycin solution (Fujifilm Wako, #168-23191) in a 24-well plate (TPP, #92424) 24 h before transfection. Transfection of S2 cells was performed using the Effectene Transfection Reagent (Qiagen, #301425). JHREWT-luc and JHREMut-luc plasmids (generous gifts from Dr Marek Jindra) [10], Ldh-luc plasmid (in this study) and an empty pGL3-Basic plasmid were transfected along with the luciferase reporter plasmids. The Copia Renilla control plasmid (Addgene, #38093) was used as the reference. The cells were incubated for 48 hr after transfection. Subsequently, 5 µl of 99.5% EtOH (Nacalai Tesque, #14712-63) or 100 µM methoprene (Fujifilm #136-17621) in 99.5% EtOH was added and incubated for 8 h. Then, they were processed by using the Dual-Luciferase Reporter Assay System (Promega, #E1960) in accordance with the manufacturer’s instructions and were analysed with Fluoroskan ascent FL (Thermo Fisher Scientific).
5. Statistical analysis
All experiments were performed independently at least twice. The sample sizes were chosen based on the number of independent experiments required for statistical significance and technical feasibility. The experiments were not randomized, and the investigators were not blinded. All statistical analyses were performed using the ‘R’ software version 4.0.3. Details of the statistical analyses are described in figure legends.
Acknowledgements
We thank Sheng Li, Kei Ito, Naoki Yamanaka, Addgene, Bloomington Stock Center (NIH P40OD018537), Vienna Drosophila Resource Center for fly strains, Developmental Studies Hybridoma Bank (created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242) for antibodies, Marek Jindra for JHRE-luciferase plasmids, and Jason Tennessen, Daiki Fujinaga, Ryo Hoshino, Eisuke Imura, Yuto Yoshinari and Yoshiki Hayashi for helpful discussions.
Contributor Information
Yoshitomo Kurogi, Email: yoshitomo.kurogi@gmail.com.
Yosuke Mizuno, Email: kuuma0109@gmail.com.
Ryosuke Hayashi, Email: s2420951@u.tsukuba.ac.jp.
Krystal Goyins, Email: krystal.goyins@utsa.edu.
Naoki Okamoto, Email: naoki-okamoto@tara.tsukuba.ac.jp.
Lacy Barton, Email: Lacy.Barton@utsa.edu.
Ryusuke Niwa, Email: ryusuke-niwa@tara.tsukuba.ac.jp.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Electronic supplementary material 2 provides raw numerical data generated in this study. All other source data are provided upon request to R.N.
Supplementary material is available online [65].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
Y.K.: conceptualization, data curation, formal analysis, funding acquisition, investigation, validation, visualization, writing—original draft, writing—review and editing; Y.M.: funding acquisition, investigation, validation, visualization, writing—review and editing; R.H.: investigation, validation, visualization, writing—review and editing; K.G.: investigation, validation, visualization, writing—review and editing; N.O.: methodology, resources, writing—review and editing; L.B.: methodology, resources, writing—review and editing; R.N.: conceptualization, investigation, project administration, resources, supervision, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors have declared no competing interest.
Funding
This work was supported by the Japan Society of the Promotion of Science KAKENHI (21J20365 to Y.K. and 23KJ0252 to Y.M.), the Japan Science and Technology Agency grant SPRING JPMJSP2124 and NIH R00 (R00HD097306 to L.B.) from NICHD. Y.K. and Y.M. received fellowships from the JSPS.
References
- 1. Li K, Jia QQ, Li S. 2019. Juvenile hormone signaling—a mini review. Insect Sci. 26, 600–606. ( 10.1111/1744-7917.12614) [DOI] [PubMed] [Google Scholar]
- 2. Noriega FG. 2014. Juvenile hormone biosynthesis in insects: what is new, what do we know, and what questions remain? Int. Sch. Res. Notices 2014, 967361. ( 10.1155/2014/967361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qu Z, Bendena WG, Tobe SS, Hui JHL. 2018. Juvenile hormone and sesquiterpenoids in arthropods: biosynthesis, signaling, and role of MicroRNA. J. Steroid Biochem. Mol. Biol. 184, 69–76. ( 10.1016/j.jsbmb.2018.01.013) [DOI] [PubMed] [Google Scholar]
- 4. Riddiford LM. 2020. Rhodnius, golden oil, and met: a history of juvenile hormone research. Front. Cell Dev. Biol. 8, 679. ( 10.3389/fcell.2020.00679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodman WG, Cusson M. 2012. The juvenile hormones. In Insect endocrinology (ed. Gilbert LI), pp. 310–365. London, UK: Academic Press. ( 10.1016/B978-0-12-384749-2.10008-1) [DOI] [Google Scholar]
- 6. Rivera-Pérez C, Clifton ME, Noriega FG, Jindra M. 2020. Juvenile hormone regulation and action. In Advances in invertebrate (neuro)endocrinology, pp. 1–76. Palm Bay, FL: Apple Academic Press. ( 10.1201/9781003029861-1) [DOI] [Google Scholar]
- 7. Shinoda T. 2021. Juvenile hormone. In Handbook of hormones: comparative endocrinology for basic and clinical research (eds Ando H, Ukena K, Nagata S), pp. 987–989. London, UK: Academic Press. [Google Scholar]
- 8. Kurogi Y, Mizuno Y, Imura E, Niwa R. 2021. Neuroendocrine regulation of reproductive dormancy in the fruit fly Drosophila melanogaster: a review of juvenile hormone-dependent regulation. Front. Ecol. Evol. 9, 715029. ( 10.3389/fevo.2021.715029) [DOI] [Google Scholar]
- 9. Abdou MA, et al. 2011. Drosophila met and gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 41, 938–945. ( 10.1016/j.ibmb.2011.09.003) [DOI] [PubMed] [Google Scholar]
- 10. Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. 2015. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 11, e1005394. ( 10.1371/journal.pgen.1005394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson TG. 1996. Genetic evidence that mutants of the methoprene-tolerant gene of Drosophila melanogaster are null mutants. Arch. Insect Biochem. Physiol. 32, 641–649. () [DOI] [PubMed] [Google Scholar]
- 12. Tumova S, Dolezel D, Jindra M. 2024. Conserved and unique roles of bHLH-PAS transcription factors in insects—from clock to hormone reception. J. Mol. Biol. 436, 168332. ( 10.1016/j.jmb.2023.168332) [DOI] [PubMed] [Google Scholar]
- 13. Jindra M, Bellés X, Shinoda T. 2015. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11, 39–46. ( 10.1016/j.cois.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 14. He Q, Wen D, Jia Q, Cui C, Wang J, Palli SR, Li S. 2014. Heat shock protein 83 (Hsp83) facilitates methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J. Biol. Chem. 289, 27874–27885. ( 10.1074/jbc.M114.582825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kayukawa T, et al. 2012. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc. Natl Acad. Sci. USA 109, 11729–11734. ( 10.1073/pnas.1204951109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li M, Mead EA, Zhu J. 2011. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl Acad. Sci. USA 108, 638–643. ( 10.1073/pnas.1013914108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li M, Liu P, Wiley JD, Ojani R, Bevan DR, Li J, Zhu J. 2014. A steroid receptor coactivator acts as the DNA-binding partner of the methoprene-tolerant protein in regulating juvenile hormone response genes. Mol. Cell. Endocrinol. 394, 47–58. ( 10.1016/j.mce.2014.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. 2011. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J. Biol. Chem. 286, 8437–8447. ( 10.1074/jbc.M110.191684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Li S, Liu S. 2021. Juvenile hormone studies in Drosophila melanogaster. Front. Physiol. 12, 785320. ( 10.3389/fphys.2021.785320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riddiford LM, Truman JW, Mirth CK, Shen YC. 2010. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137, 1117–1126. ( 10.1242/dev.037218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumann AA, Texada MJ, Chen HM, Etheredge JN, Miller DL, Picard S, Warner R, Truman JW, Riddiford LM. 2017. Genetic tools to study juvenile hormone action in Drosophila. Sci. Rep. 7, 2132. ( 10.1038/s41598-017-02264-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barton LJ, Sanny J, Packard Dawson E, Nouzova M, Noriega FG, Stadtfeld M, Lehmann R. 2024. Juvenile hormones direct primordial germ cell migration to the embryonic gonad. Curr. Biol. 34, 505–518.( 10.1016/j.cub.2023.12.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noriega FG, Shah DK, Wells MA. 1997. Juvenile hormone controls early trypsin gene transcription in the midgut of Aedes aegypti. Insect Mol. Biol. 6, 63–66. ( 10.1046/j.1365-2583.1997.00154.x) [DOI] [PubMed] [Google Scholar]
- 24. Wolfner MF, Partridge L, Lewin S, Kalb JM, Chapman T, Herndon LA. 1997. Mating and hormonal triggers regulate accessory gland gene expression in male Drosophila. J. Insect Physiol. 43, 1117–1123. ( 10.1016/s0022-1910(97)00062-0) [DOI] [PubMed] [Google Scholar]
- 25. Meiselman MR, Ganguly A, Dahanukar A, Adams ME. 2022. Endocrine modulation of primary chemosensory neurons regulates Drosophila courtship behavior. PLoS Genet. 18, e1010357. ( 10.1371/journal.pgen.1010357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman RF. 2012. The insects structure and function, 5th edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27. Fyrberg C, Becker J, Barthmaier P, Mahaffey J, Fyrberg E. 1997. A Drosophila muscle-specific gene related to the mouse quaking locus. Gene 197, 315–323. ( 10.1016/s0378-1119(97)00278-3) [DOI] [PubMed] [Google Scholar]
- 28. Shinoda T, Itoyama K. 2003. Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc. Natl Acad. Sci. USA 100, 11986–11991. ( 10.1073/pnas.2134232100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niwa R, Niimi T, Honda N, Yoshiyama M, Itoyama K, Kataoka H, Shinoda T. 2008. Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect Biochem. Mol. Biol. 38, 714–720. ( 10.1016/j.ibmb.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 30. Takemori N, Yamamoto MT. 2009. Proteome mapping of the Drosophila melanogaster male reproductive system. Proteomics 9, 2484–2493. ( 10.1002/pmic.200800795) [DOI] [PubMed] [Google Scholar]
- 31. Dorus S, Busby SA, Gerike U, Shabanowitz J, Hunt DF, Karr TL. 2006. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat. Genet. 38, 1440–1445. ( 10.1038/ng1915) [DOI] [PubMed] [Google Scholar]
- 32. Li H, et al. 2022. Fly cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432. ( 10.1126/science.abk2432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bawa S, et al. 2020. Drosophila TRIM32 cooperates with glycolytic enzymes to promote cell growth. eLife 9, e52358. ( 10.7554/eLife.52358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H, et al. 2017. Drosophila larvae synthesize the putative oncometabolite L-2-hydroxyglutarate during normal developmental growth . Proc. Natl Acad. Sci. USA 114, 1353–1358. ( 10.1073/pnas.1614102114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riemann JG, Thorson BJ. 1976. Ultrastructure of the vasa deferentia of the mediterranean flour moth. J. Morphol. 149, 483–505. ( 10.1002/jmor.1051490404) [DOI] [PubMed] [Google Scholar]
- 36. Couche GA, Gillott C. 1988. Development of secretory activity in the seminal vesicle of the male migratory grasshopper, Melanoplus sanguinipes (fabr.) (Orthoptera: Acrididae). Int. J. Insect Morphol. Embryol. 17, 51–61. ( 10.1016/0020-7322(88)90030-X) [DOI] [Google Scholar]
- 37. Xie S, Hua B. 2010. Ultrastructure of the seminal vesicle and sperm storage in Panorpidae (Insecta: Mecoptera). Micron 41, 760–768. ( 10.1016/j.micron.2010.05.012) [DOI] [PubMed] [Google Scholar]
- 38. Viscuso R, Brundo MV, Marletta A, Vitale DGM. 2015. Fine structure of male genital tracts of some Acrididae and Tettigoniidae (Insecta: Orthoptera). Acta Zool. 96, 418–427. ( 10.1111/azo.12084) [DOI] [Google Scholar]
- 39. Spiegel CN, Bretas JAC, Peixoto AA, Vigoder FM, Bruno RV, Soares MJ. 2013. Fine structure of the male reproductive system and reproductive behavior of Lutzomyia longipalpis sandflies (Diptera: Psychodidae: Phlebotominae). PLoS One 8, e74898. ( 10.1371/journal.pone.0074898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lyu QH, Zhang BB, Hua BZ. 2018. Ultrastructure and function of the seminal vesicle of Bittacidae (Insecta: Mecoptera). Arthropod Struct. Dev. 47, 173–179. ( 10.1016/j.asd.2018.02.001) [DOI] [PubMed] [Google Scholar]
- 41. Pendam VR, Tembhare DB. 2013. Effect of JH III and β-ecdysone on seminal vesicle protein secretion in the tropical tasar silkmoth, Antheraea mylitta (Drury) (Lepidoptera: Saturniidae). Int. J. Wild Silkmoth Silk 17, 43–48. ( 10.51011/ijwss.17.0_43) [DOI] [Google Scholar]
- 42. Wijesekera TP, Saurabh S, Dauwalder B. 2016. Juvenile hormone is required in adult males for Drosophila courtship. PLoS One 11, e0151912. ( 10.1371/journal.pone.0151912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee SS, Ding Y, Karapetians N, Rivera-Perez C, Noriega FG, Adams ME. 2017. Hormonal signaling cascade during an early-adult critical period required for courtship memory retention in Drosophila. Curr. Biol. 27, 2798–2809.( 10.1016/j.cub.2017.08.017) [DOI] [PubMed] [Google Scholar]
- 44. Nouzova M, et al. 2021. Epoxidation of juvenile hormone was a key innovation improving insect reproductive fitness. Proc. Natl Acad. Sci. USA 118, e2109381118. ( 10.1073/pnas.2109381118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raikhel AS, Brown MR, Belles X. 2005. Hormonal control of reproductive processes. In Comprehensive molecular insect science (ed. Gilbert LI), pp. 433–491. Amsterdam, The Netherlands: Elsevier. ( 10.1016/B0-44-451924-6/00040-5) [DOI] [Google Scholar]
- 46. Fausto AM, Gambellini G, Taddei AR, Maroli M, Mazzini M. 2000. Ultrastructure of the seminal vesicle of Phlebotomus perniciosus Newstead (Diptera, Psychodidae). Tissue Cell 32, 228–237. ( 10.1054/tice.2000.0110) [DOI] [PubMed] [Google Scholar]
- 47. Abu-Shumays RL, Fristrom JW. 1997. IMP-L3, a 20-hydroxyecdysone-responsive gene encodes Drosophila lactate dehydrogenase: structural characterization and developmental studies. Dev. Genet. 20, 11–22. () [DOI] [PubMed] [Google Scholar]
- 48. Volkenhoff A, Weiler A, Letzel M, Stehling M, Klämbt C, Schirmeier S. 2015. Glial glycolysis is essential for neuronal survival in Drosophila. Cell Metab. 22, 437–447. ( 10.1016/j.cmet.2015.07.006) [DOI] [PubMed] [Google Scholar]
- 49. Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, Bellen HJ. 2017. The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26, 719–737.( 10.1016/j.cmet.2017.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brooks GA. 2018. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785. ( 10.1016/j.cmet.2018.03.008) [DOI] [PubMed] [Google Scholar]
- 51. Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. 2002. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 99, 2134–2139. ( 10.1073/pnas.032426699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shin SW, Zou Z, Saha TT, Raikhel AS. 2012. BHLH-PAS heterodimer of methoprene-tolerant and cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc. Natl Acad. Sci. USA 109, 16576–16581. ( 10.1073/pnas.1214209109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He L, Wu B, Shi J, Du J, Zhao Z. 2023. Regulation of feeding and energy homeostasis by clock-mediated gart in Drosophila. Cell Rep. 42, 112912. ( 10.1016/j.celrep.2023.112912) [DOI] [PubMed] [Google Scholar]
- 54. Bajgar A, Jindra M, Dolezel D. 2013. Autonomous regulation of the insect gut by circadian genes acting downstream of juvenile hormone signaling. Proc. Natl Acad. Sci. USA 110, 4416–4421. ( 10.1073/pnas.1217060110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shemshedini L, Lanoue M, Wilson TG. 1990. Evidence for a juvenile hormone receptor involved in protein synthesis in Drosophila melanogaster. J. Biol. Chem. 265, 1913–1918. ( 10.1016/S0021-9258(19)39917-X) [DOI] [PubMed] [Google Scholar]
- 56. Wilson TG, DeMoor S, Lei J. 2003. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant27 mutant phenotype. Insect Biochem. Mol. Biol. 33, 1167–1175. ( 10.1016/j.ibmb.2003.06.007) [DOI] [PubMed] [Google Scholar]
- 57. Yamamoto K, Chadarevian A, Pellegrini M. 1988. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science 239, 916–919. ( 10.1126/science.3124270) [DOI] [PubMed] [Google Scholar]
- 58. Rivera-Perez C, Nouzova M, Noriega FG. 2012. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLoS One 7, e43784. ( 10.1371/journal.pone.0043784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wen D, et al. 2015. Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet. 11, e1005038. ( 10.1371/journal.pgen.1005038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. 1998. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster meigen. Learn. Mem. 5, 52–77. [PMC free article] [PubMed] [Google Scholar]
- 61. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Casal J, Gonzalez C, Wandosell F, Avila J, Ripoll P. 1990. Abnormal meiotic spindles cause a cascade of defects during spermatogenesis in asp males of Drosophila. Development 108, 251–260. ( 10.1242/dev.108.2.251) [DOI] [PubMed] [Google Scholar]
- 63. Shao L, et al. 2023. Eukaryotic translation initiation factor eIF4E-5 is required for spermiogenesis in Drosophila melanogaster. Development 150, dev200477. ( 10.1242/dev.200477) [DOI] [PubMed] [Google Scholar]
- 64. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) method. Methods 25, 402–408. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 65. Kurogi Y, Mizuno Y, Hayashi R, Goyins K, Okamoto N, Barton Let al. 2024. Supplementary material from The seminal vesicle is a juvenile hormone-responsive tissue in adult male Drosophila melanogaster. Figshare ( 10.6084/m9.figshare.c.7576624) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Electronic supplementary material 2 provides raw numerical data generated in this study. All other source data are provided upon request to R.N.
Supplementary material is available online [65].