Abstract
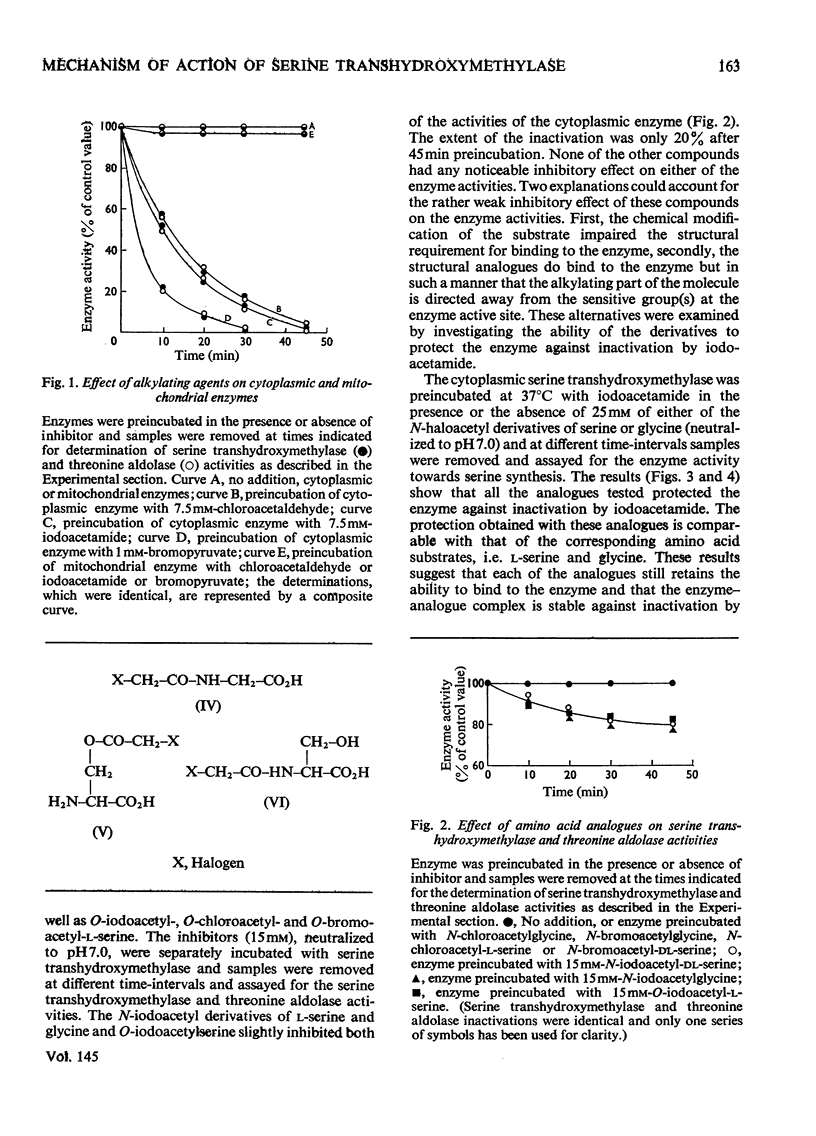
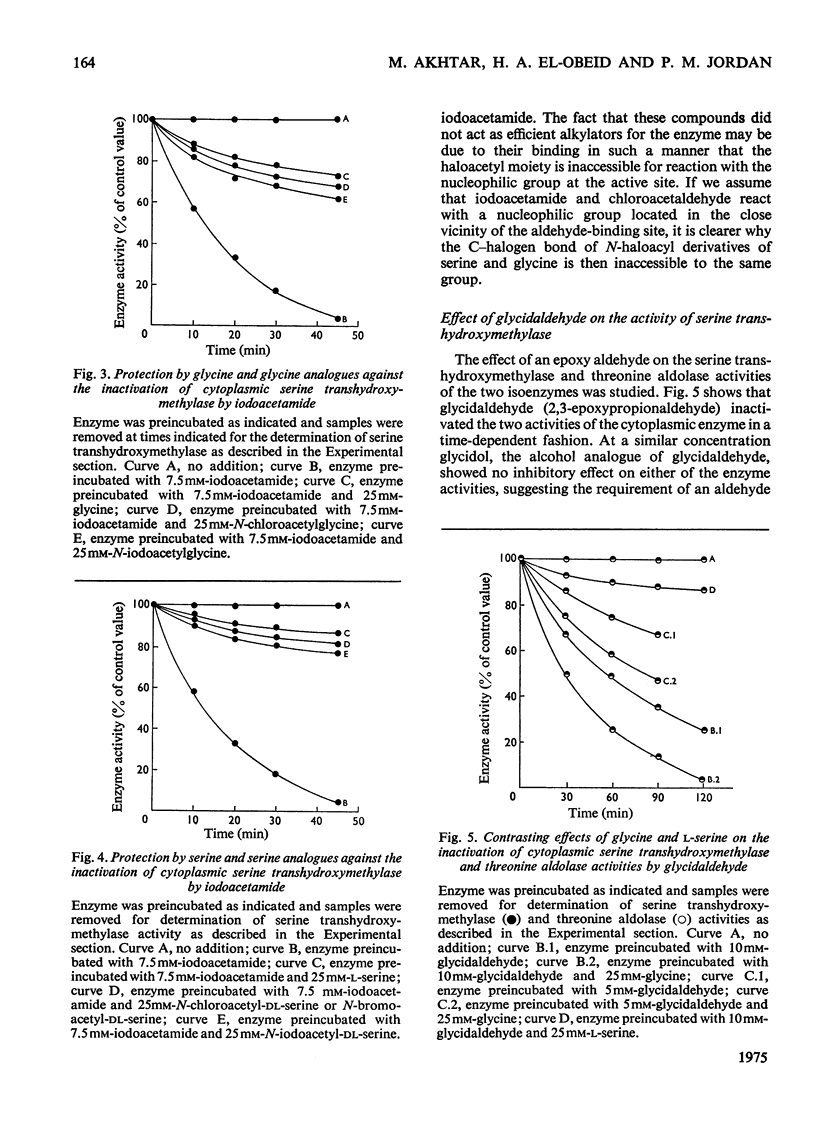
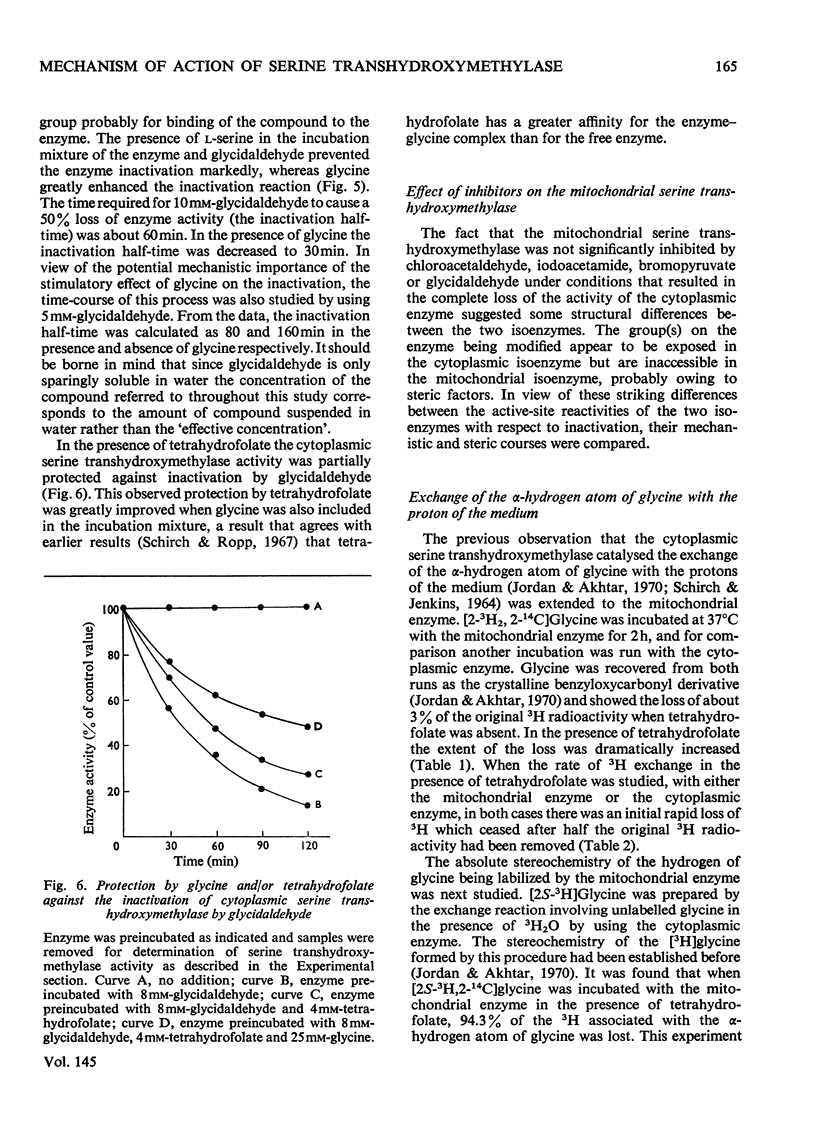
By using cytoplasmic and mitochondrial serine transhydroxymethylase isoenzymes from rabbit liver, it was shown that both enzymes exhibited similar ratios of serine transhydroxymethylase/threonine aldolase activities. Both enzymes catalysed the removal of the pro-S hydrogen atom of glycine, which was greatly enhanced by the presence of tetrahydrofolate. The cytoplasmic as well as the mitochondrial enzyme catalysed the synthesis of serine from glycine and [3H2]formaldehyde in the absence of tetrahydrofolate. The results are consistent with our previous suggestion that a role of tetrahydrofolate in the serine transhydroxymethylase reaction is to transport formaldehyde in and out of the active site (Jordan & Akhtar, 1970). The isoenzymes, however, showed remarkable differences in their inactivation by inhibitors. The serine transhydroxymethylase as well as the threonine aldolase activities of the cytoplasmic enzyme were inactivated in a similar fashion by chloroacetaldehyde, iodoacetamide, bromopyruvate and glycidaldehyde (2,3-epoxypropionaldehyde). These inhibitors had no effect on the two activities of the mitochondrial enzyme. The rate of inactivation of the cytoplasmic enzyme by glycidaldehyde was enhanced by the presence of glycine but decreased by the presence of serine. The implications of these results to the mechanism of catalysis and the nature of the active site of the enzymes are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., el-Obeid H. A. Inactivation of serine transhydroxymethylase and threonine aldolase activities. Biochim Biophys Acta. 1972 Mar 8;258(3):791–799. doi: 10.1016/0005-2744(72)90180-5. [DOI] [PubMed] [Google Scholar]
- Broadbent W., Morley J. S., Stone B. E. Polypeptides. V. The use of t-butyl 2,4,5-trichlorophenyl carbonate in the synthesis of N-t-butoxycarbonyl amino-acids and their 2,4,5-trichlorophenyl esters. J Chem Soc Perkin 1. 1967;24:2632–2636. doi: 10.1039/j39670002632. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Schirch L. V. Serine transhydroxymethylase. A kinetic study of the synthesis of serine in the absence of tetrahydrofolate. J Biol Chem. 1973 May 25;248(10):3631–3635. [PubMed] [Google Scholar]
- Fujioka M. Purification and properties of serine hydroxymethylase from soluble and mitochondrial fractions of rabbit liver. Biochim Biophys Acta. 1969;185(2):338–349. doi: 10.1016/0005-2744(69)90427-6. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
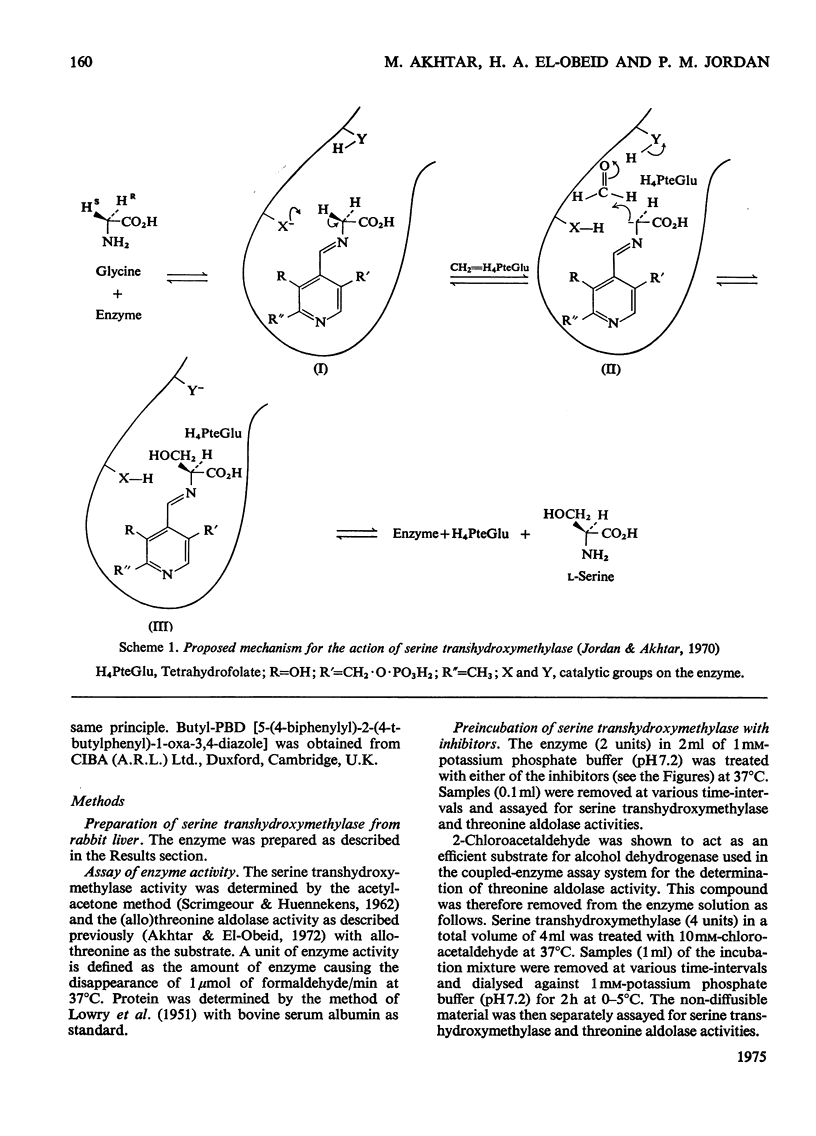
- Jordan P. M., Akhtar M. The mechanism of action of serine transhydroxymethylase. Biochem J. 1970 Jan;116(2):277–286. doi: 10.1042/bj1160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Palekar A. G., Tate S. S., Meister A. Rat liver aminomalonate decarboxylase. Identity with cytoplasmic serine hydroxymethylase and allothreonine aldolase. J Biol Chem. 1973 Feb 25;248(4):1158–1167. [PubMed] [Google Scholar]
- SCHIRCH L., JENKINS W. T. SERINE TRANSHYDROXYMETHYLASE. PROPERTIES OF THE ENZYME-SUBSTRATE COMPLEXES OF D-ALANINE AND GLYCINE. J Biol Chem. 1964 Nov;239:3801–3807. [PubMed] [Google Scholar]
- Schirch L., Gross T. Serine transhydroxymethylase. Identification as the threonine and allothreonine aldolases. J Biol Chem. 1968 Nov 10;243(21):5651–5655. [PubMed] [Google Scholar]
- Schirch L., Ropp M. Serine transhydroxymethylase. Affinity of tetrahydrofolate compounds for the enzyme and enzyme-glycine complex. Biochemistry. 1967 Jan;6(1):253–257. doi: 10.1021/bi00853a039. [DOI] [PubMed] [Google Scholar]


