Abstract
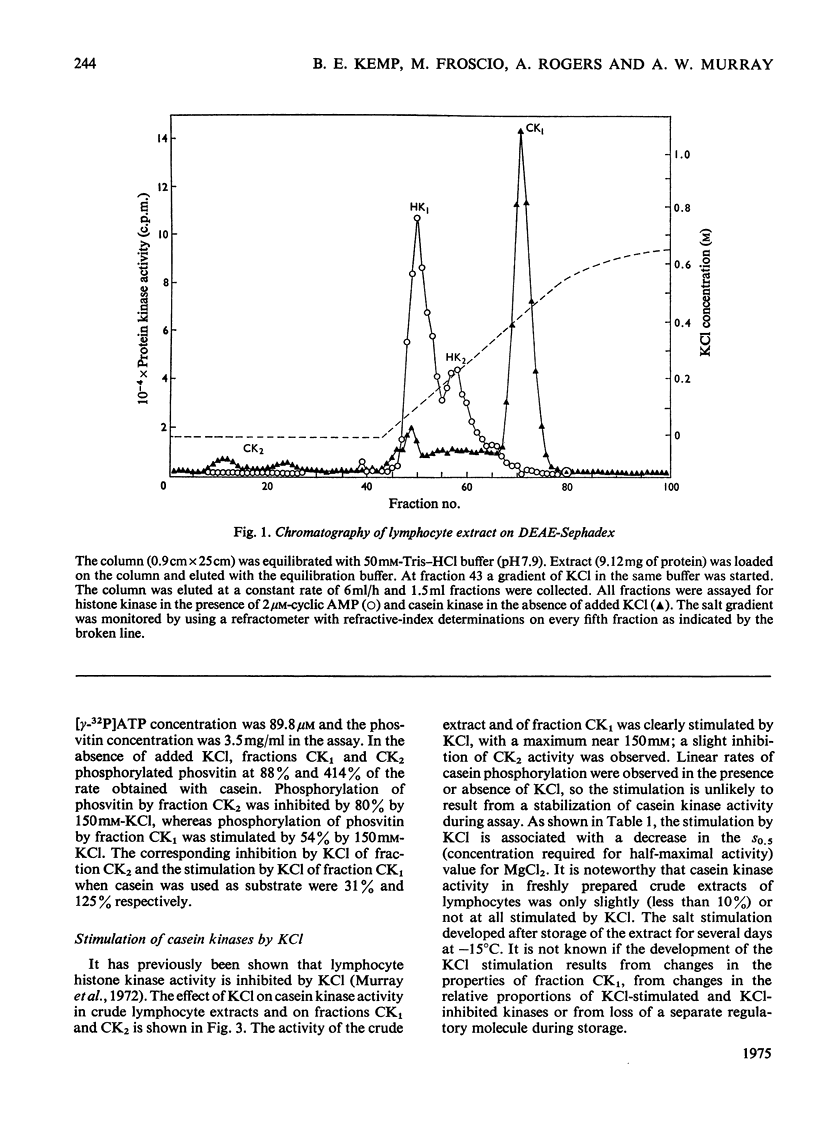
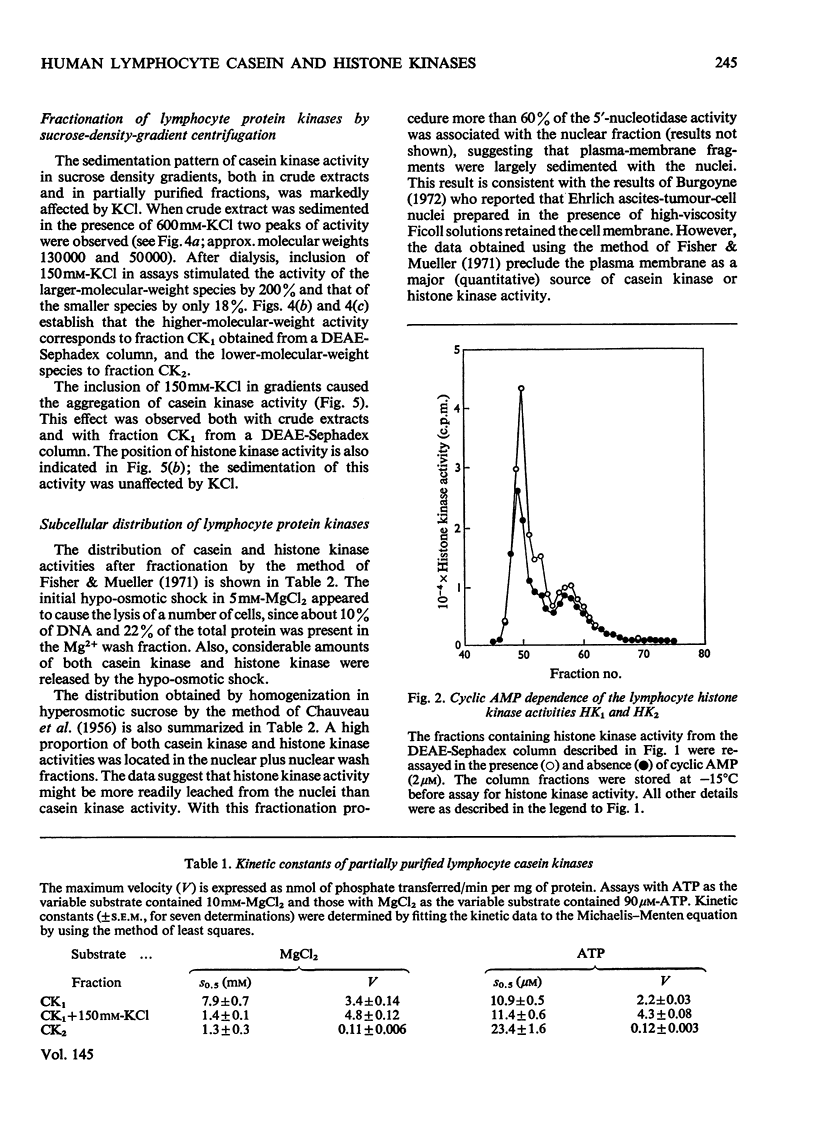
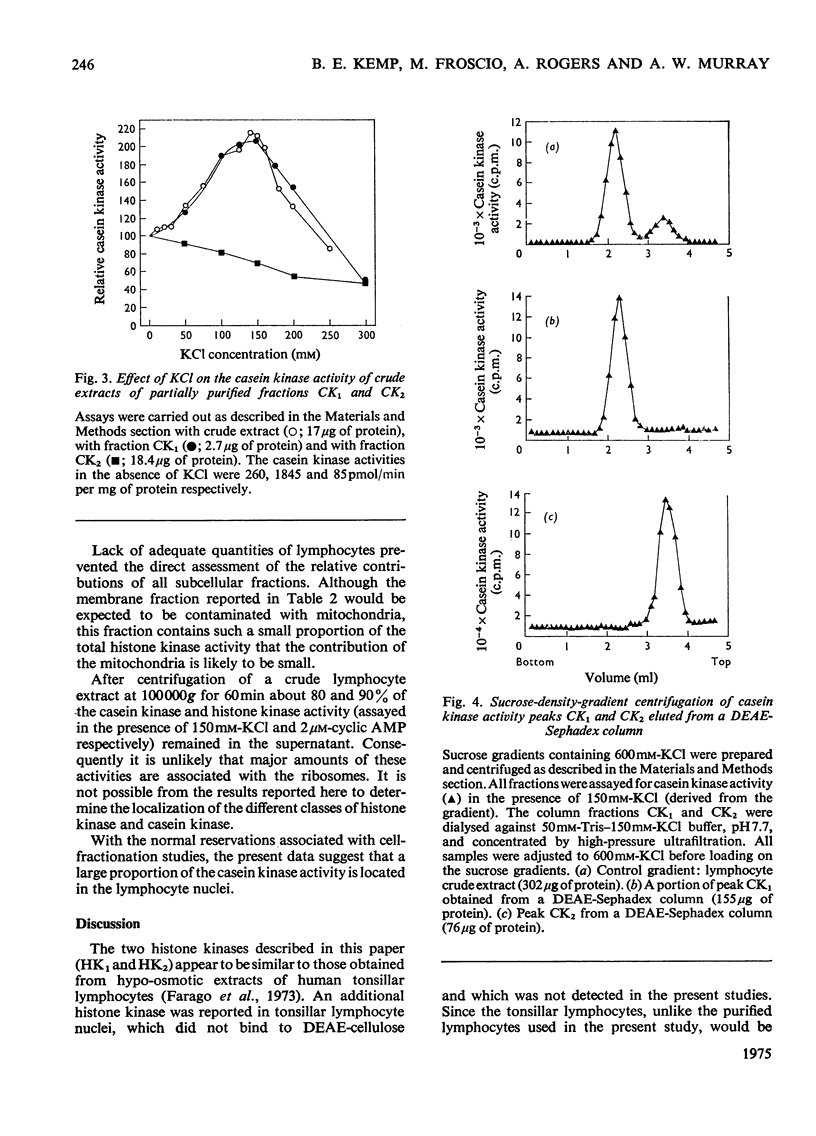
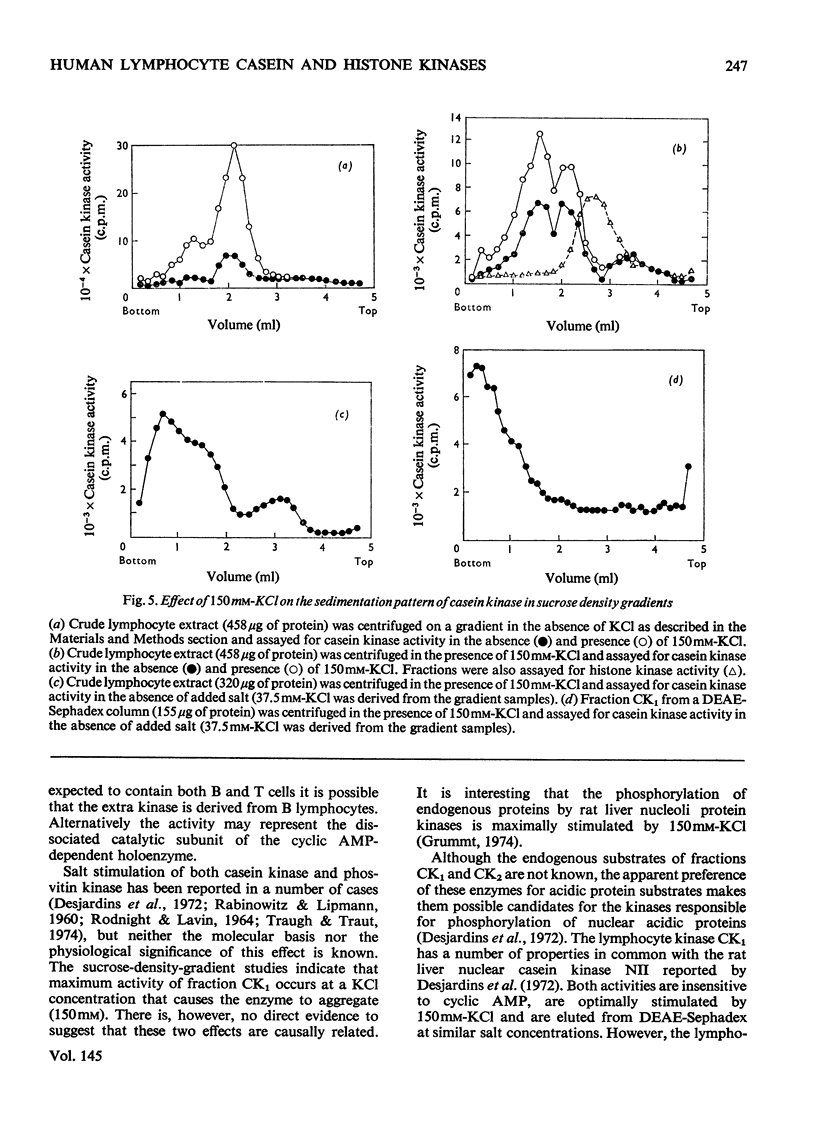
1. Cell-free lysates of human peripheral blood lymphocytes contained two casein kinase activities and two histone kinase activities, which could be separated by chromatography on DEAE-Sephadex. 2. Neither of the casein kinase activities were stimulated by cyclic AMP. The major activity was eluted from DEAE-Sephadex between 0.4 and 0.45M-KCl, had a molecular weight of approx. 130,000 (sucrose density gradients) and was stimulated by KCl (maximum 150mM). It also formed higher-molecular-weight aggregates when centrifuged in sucrose gradients containing 150mM-KCl. The minor activity was not retained by DEAE-Sephadex, had a molecular weight of approx. 50,000 and was not stimulated by KCl. 3. The major histone kinase activity was stimulated by cyclic AMP and was eluted from the DEAE-Sephadex column between 0.05 and 0.2M-KCl. The other activity was not stimulated by cyclic AMP and was insensitive to the rabbit muscle protein kinase inhibitor. 4. Evidence was obtained suggesting that the lymphocyte casein kinases were located primarily in the nuclei.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggio B., Moret V. Multiple forms of phosvitin kinase from rat liver cytosol. Biochim Biophys Acta. 1971 Nov 13;250(2):346–350. doi: 10.1016/0005-2744(71)90190-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A. Deoxyribonucleic acid synthesis in mammalian systems. Permealysed Ehrlich ascites cells in vitro first label Okazaki-type low-molecular-weight deoxyribonucleic acid and then high-molecular-weight deoxyribonucleic acid. Biochem J. 1972 Dec;130(4):959–964. doi: 10.1042/bj1300959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Desjardins P. R., Lue P. F., Liew C. C., Gornall A. G. Purification and properties of rat liver nuclear protein kinases. Can J Biochem. 1972 Dec;50(12):1249–1259. doi: 10.1139/o72-170. [DOI] [PubMed] [Google Scholar]
- Faragó A., Antoni F., Takáts A., Fábián F. Adenosine 3':5'-monophosphate-dependent and independent histone kinases isolated from human tonsillar lymphocytes. Biochim Biophys Acta. 1973 Feb 28;297(2):517–526. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I. Studies on the phosphorylation of nucleolar proteins. Identification of a nucleolus-associated protein kinase activity. FEBS Lett. 1974 Feb 15;39(2):125–128. doi: 10.1016/0014-5793(74)80033-5. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Froscio M., Murray A. W. Protein kinase activity in commercially available crystalline yeast alcohol dehydrogenase. Biochem J. 1973 Feb;131(2):271–274. doi: 10.1042/bj1310271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphorylation of nuclear protein early in the course of gene activation in lymphocytes. Science. 1966 Nov 11;154(3750):780–781. doi: 10.1126/science.154.3750.780. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Froscio M., Kemp B. E. Histone phosphatase and cyclic nucleotide-stimulated protein kinase from human lymphocytes. Biochem J. 1972 Oct;129(5):995–1002. doi: 10.1042/bj1290995a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- Raff M. C. T and B lymphocytes and immune responses. Nature. 1973 Mar 2;242(5392):19–23. doi: 10.1038/242019a0. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rodnight R., Lavin B. E. Phosvitin kinase from brain: activation by ions and subcellular distribution. Biochem J. 1964 Oct;93(1):84–91. doi: 10.1042/bj0930084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddon R. W., Anderson S. L. Presence of multiple protein kinase activities in rat liver nuclei. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1499–1508. doi: 10.1016/0006-291x(72)90777-2. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Takeda M., Yamamura H., Oga Y. Phosphoprotein kinases associated with rat liver chromatin. Biochem Biophys Res Commun. 1971 Jan 8;42(1):103–110. doi: 10.1016/0006-291x(71)90368-8. [DOI] [PubMed] [Google Scholar]
- Traugh J. A., Traut R. R. Characterization of protein kinases from rabbit reticulocytes. J Biol Chem. 1974 Feb 25;249(4):1207–1212. [PubMed] [Google Scholar]
- WILLIAMS J., SANGER F. The grouping of serine phosphate residues in phosvitin and casein. Biochim Biophys Acta. 1959 May;33(1):294–296. doi: 10.1016/0006-3002(59)90545-1. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D. Protein kinases: aspects of their regulation and diversity. Recent Prog Horm Res. 1973;29:329–359. doi: 10.1016/b978-0-12-571129-6.50012-9. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]


