Abstract
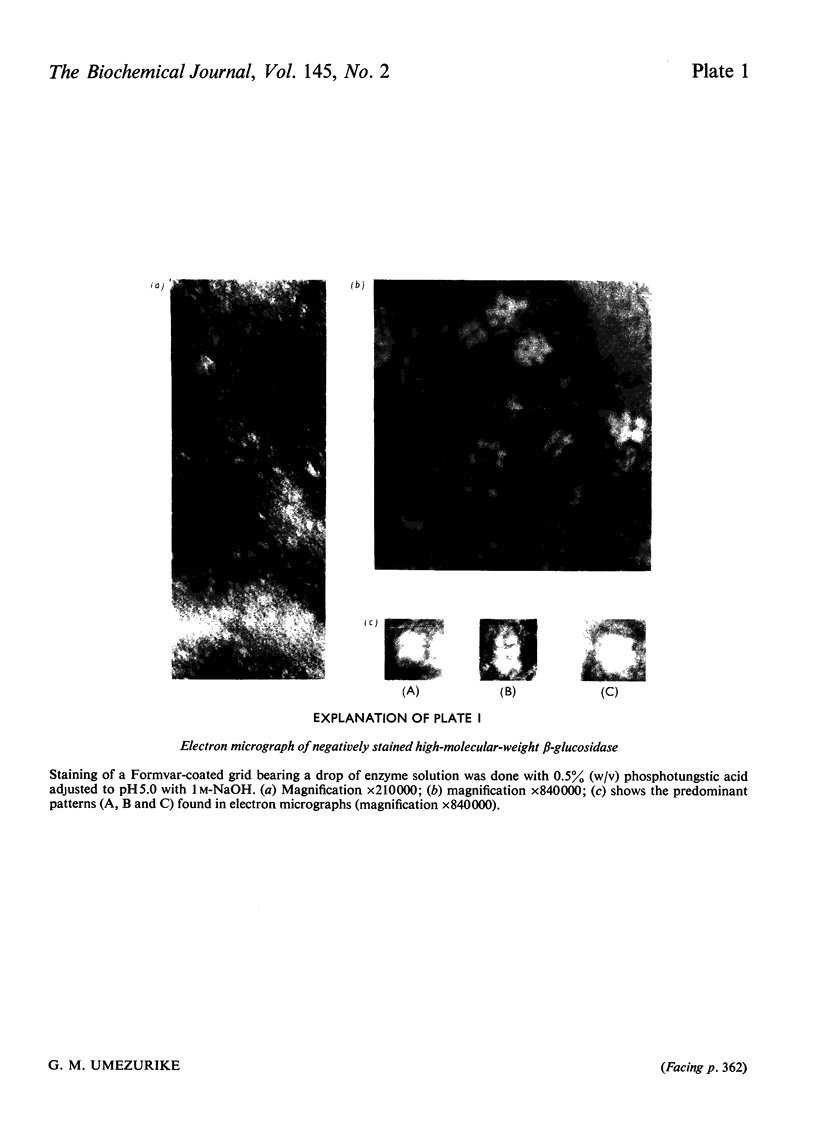
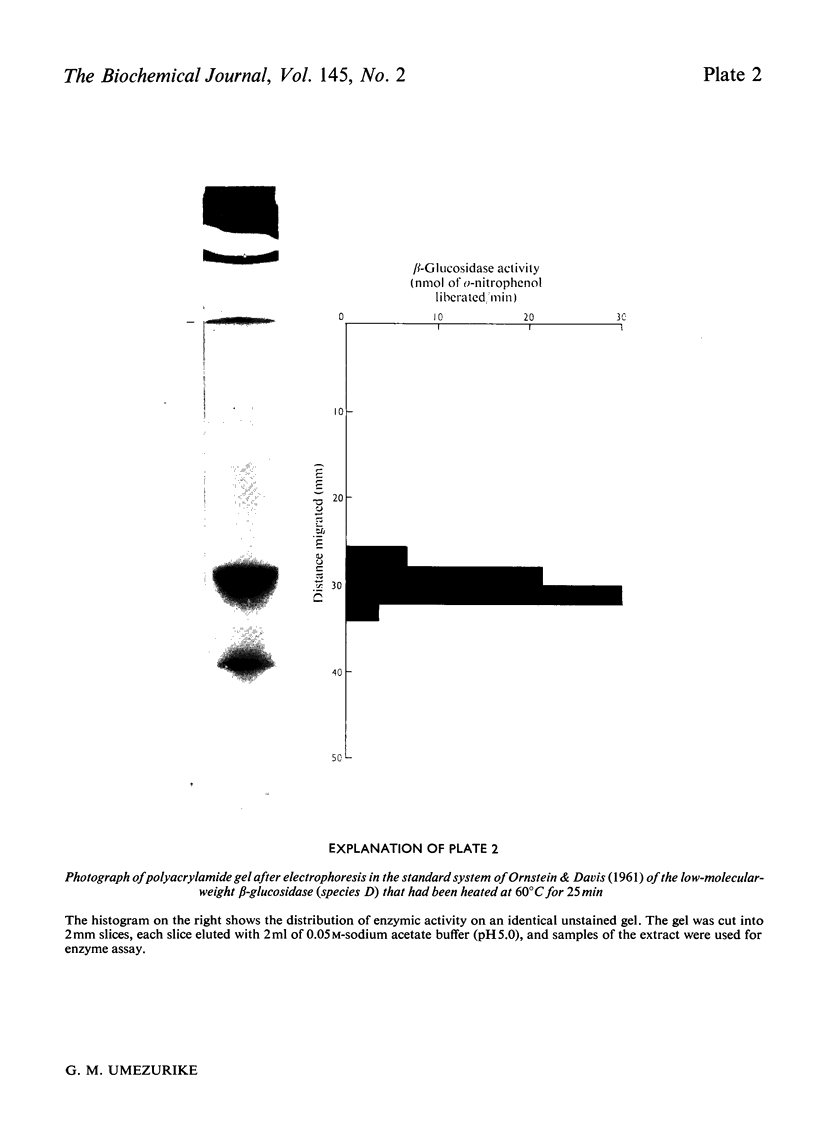
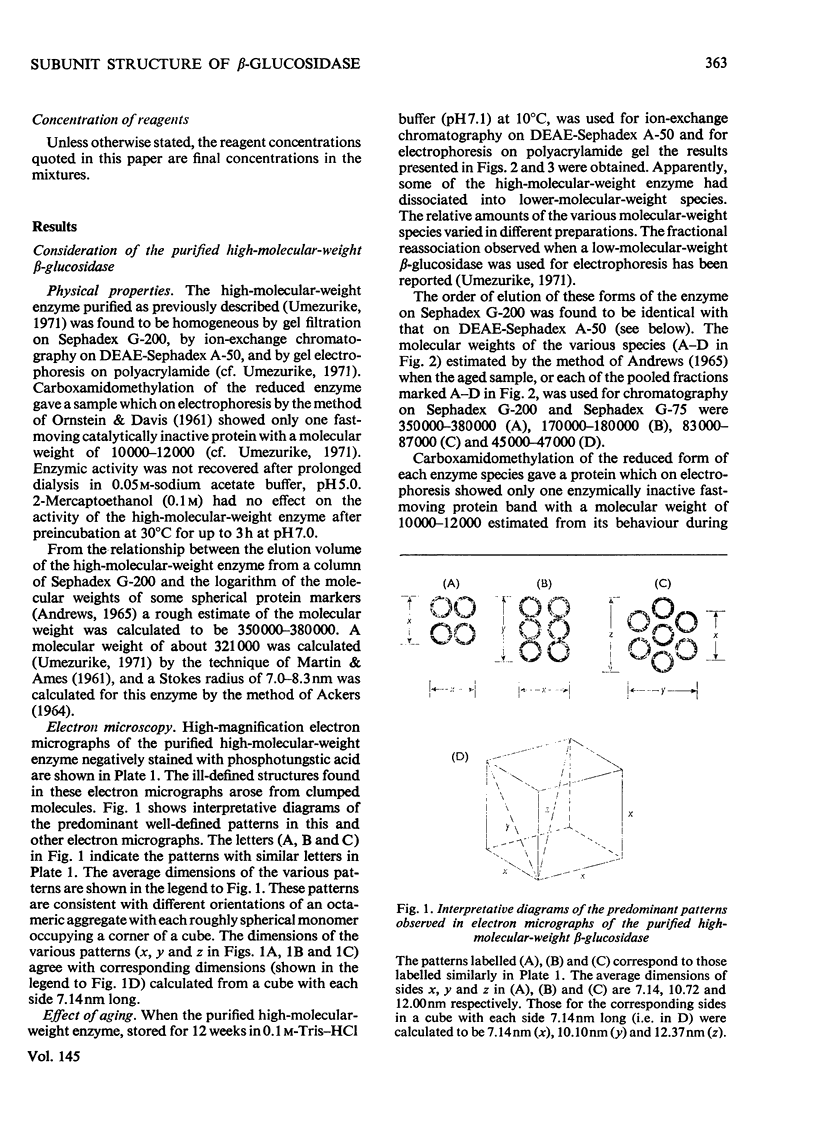
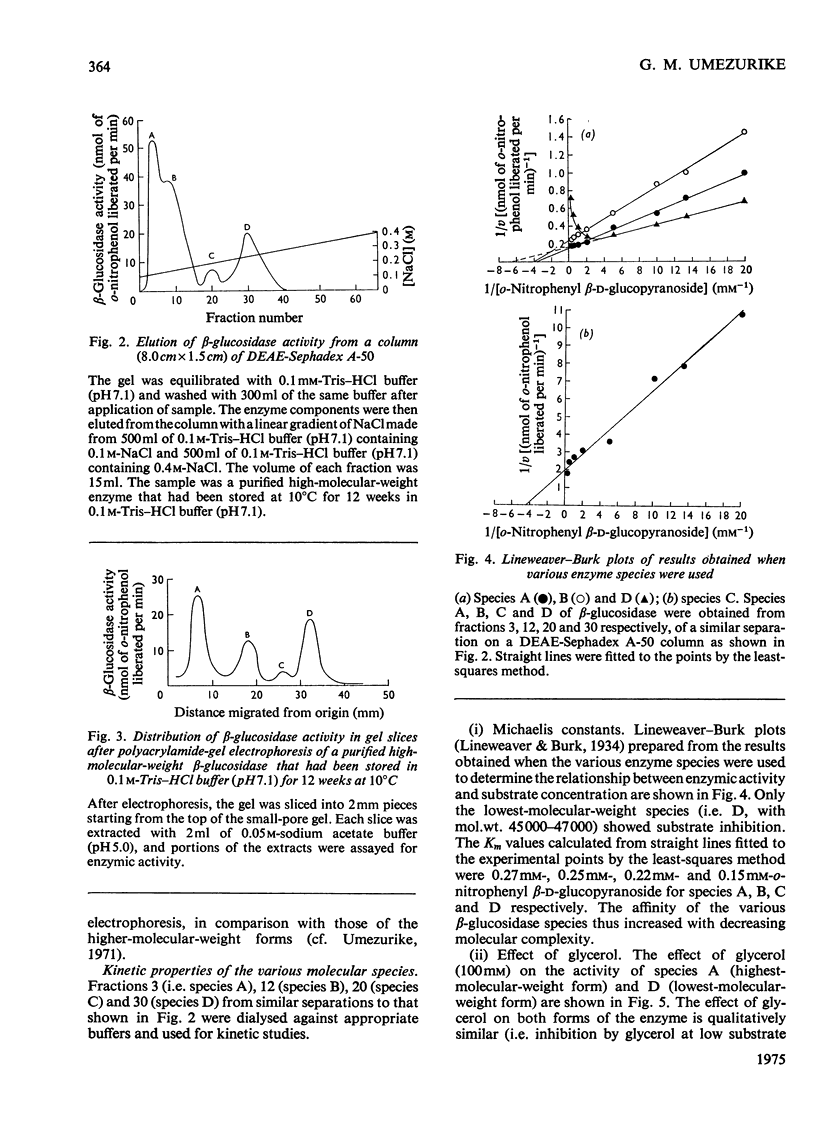
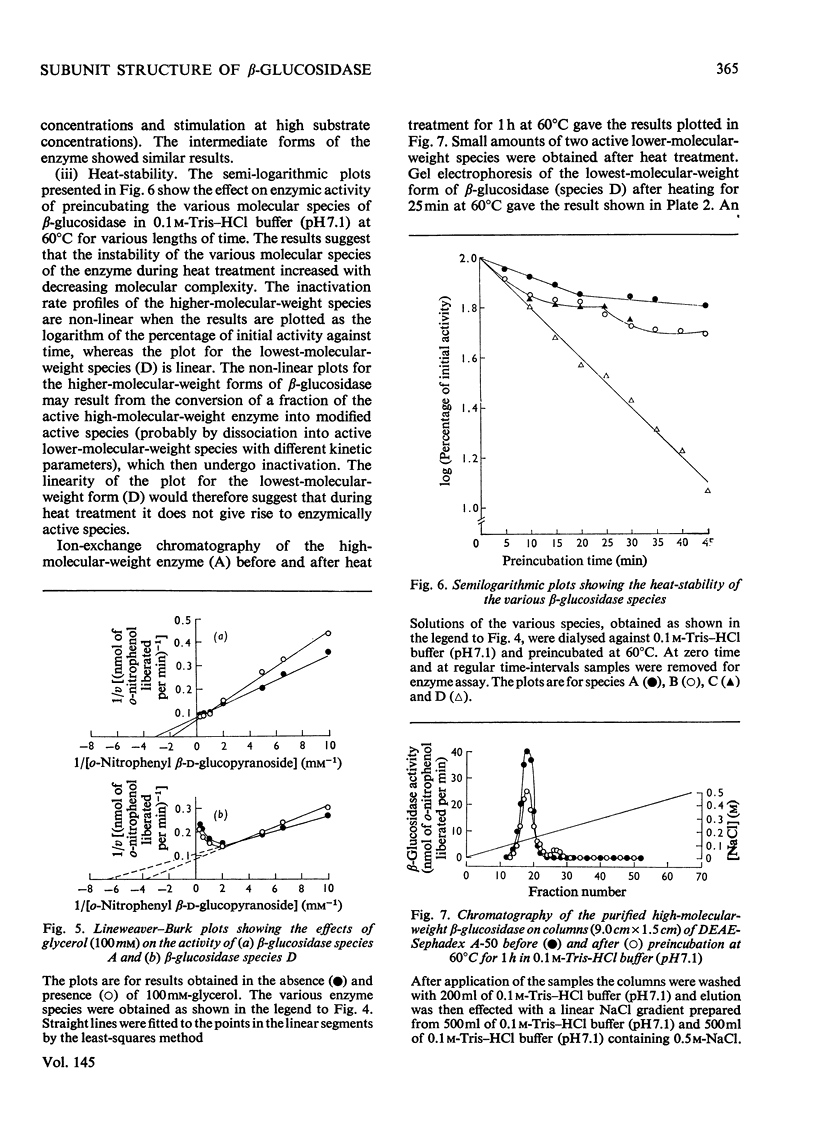
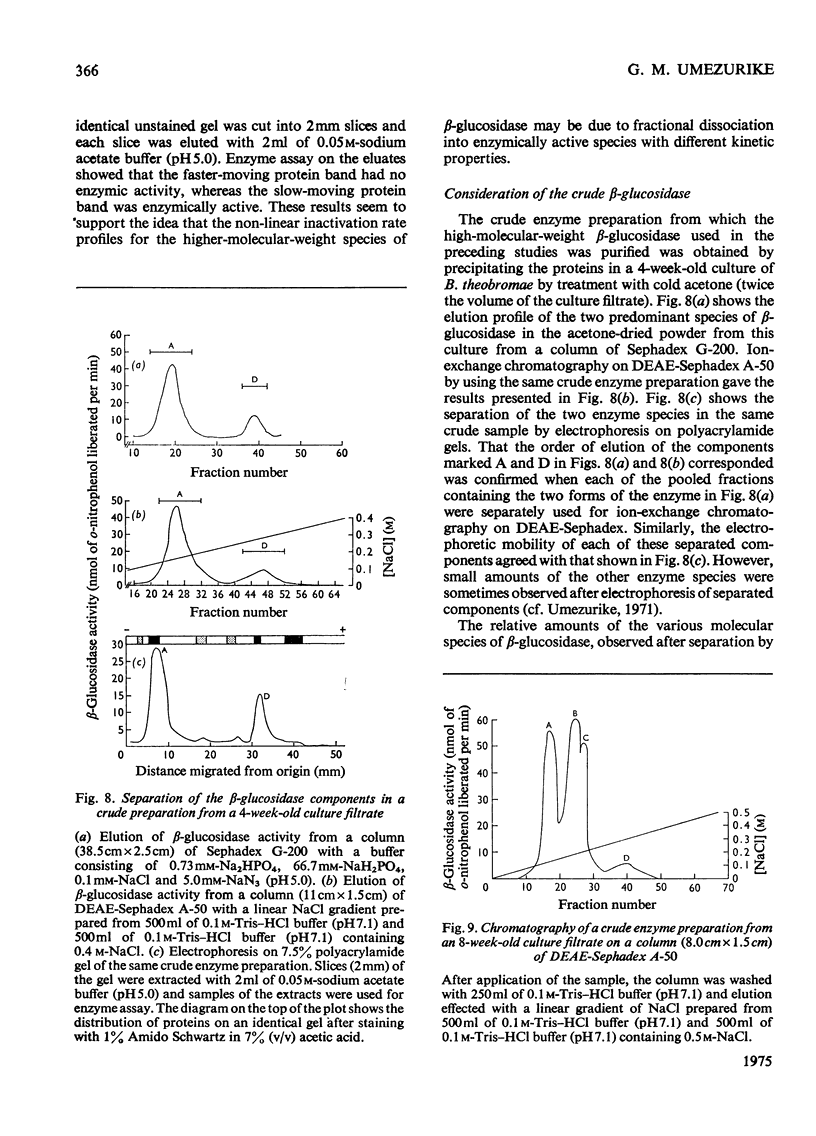
1. A homologous series of beta-glcosidase (beta-D-glcoside glcohydrolase, EC 3.2.1.21), which varied in relative amounts in different preparations from cultures of similar and different age, was observed in cultures od Botryodiplodia theobromae Pat grown for 4-8 week on cotton flock (cellulose) as carbon source. 2. Aging of the purified high-molecular-weight species led to some amount of siddociation into a homolous series of lower-molecular-weight speices. 3. Rough molecular-weight estimates, by gel filtration, of the various species derived from the purifeid high-molecular-weight enzyme were 350000-3800000, 170000, 180000, 83000-87000 and 45000-47000. 4. Electron micrographs of the negatively stained 350000-380000-molecular-weight enzyme showed that the molecule is an octamer in which each roughly spherical monomer occupies a corner of a cube with each side about 7.14nm long. 5. Carboxamidomethylation of the reduced form of each molecular-weight species of the enzyme led to irreversible dissociation of the molecules into electrophoretically identical polypeptides with a moleclar weight of 10000-12000. 6. These results suggest a slow association-dissociation of the type (8n)in equilibrium 2 (4n) in equilibrium 4(2n) in equilibrium 8(n), where n is defined as the monomer. The monomer is in turn made up of four polypeptide a subunits whi-ch are non-catalytic. 7. The Michaelis constants (Km) and heat stability of the four wnzymically active molecular species derived from the purified enzyme increased with molecular complexity, whereas all four species were inhibited by glycerol (100nM) at low concentrations of substrate (o-nitrophenyl beta-D-glucopyranoside) but activated at high substrat concentrations. 8. Only the lowest-molecular-weight species (45species (45,000-47000 mol. wt.) showed substrate inhibition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Abrahams H. E., Robinson D. Beta-D-glucosidases and related enzymic activities in pig kidney. Biochem J. 1969 Mar;111(5):749–755. doi: 10.1042/bj1110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUERKSEN J. D., HALVORSON H. Purification and properties of an inducible beta-glucosidase of yeast. J Biol Chem. 1958 Nov;233(5):1113–1120. [PubMed] [Google Scholar]
- Fleming L. W., Duerksen J. D. Evidence for multiple molecular forms of yeast beta-glucosidase in a hybrid yeast. J Bacteriol. 1967 Jan;93(1):142–150. doi: 10.1128/jb.93.1.142-150.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L. W., Duerksen J. D. Purification and characterization of yeast beta-glucosidases. J Bacteriol. 1967 Jan;93(1):135–141. doi: 10.1128/jb.93.1.135-141.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU A. S., EPSTEIN R., HALVORSON H. O., BOCK R. M. Yeast beta-glucosidase: comparison of the physical-chemical properties of purified constitutive and inducible enzyme. Arch Biochem Biophys. 1960 Dec;91:210–218. doi: 10.1016/0003-9861(60)90492-6. [DOI] [PubMed] [Google Scholar]
- Han Y. W., Srinivasan V. R. Purification and characterization of beta-glucosidase of Alcaligenes faecalis. J Bacteriol. 1969 Dec;100(3):1355–1363. doi: 10.1128/jb.100.3.1355-1363.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Studies on the spore coats of aspergillus oryzae. II. Conidia coat-bound beta-glucosidase. Biochim Biophys Acta. 1965 Nov 1;101(3):352–357. doi: 10.1016/0926-6534(65)90014-x. [DOI] [PubMed] [Google Scholar]
- Legler G. Labelling of the active centre of a beta-glucosidase. Biochim Biophys Acta. 1968 Mar 25;151(3):728–729. doi: 10.1016/0005-2744(68)90033-8. [DOI] [PubMed] [Google Scholar]
- Li L. H., Flora R. M., King K. W. Individual roles of cellulase components derived from Trichoderma viride. Arch Biochem Biophys. 1965 Aug;111(2):439–447. doi: 10.1016/0003-9861(65)90207-9. [DOI] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. ARYL BETA-GLUCOSIDASE OF SOME NEUROSPORA STRAINS. Biochim Biophys Acta. 1964 Jul 15;90:214–215. doi: 10.1016/0304-4165(64)90147-3. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marchin G. L., Duerksen J. D. Purification of beta-glucosidase from Saccharomyces lactis strain Y-123. J Bacteriol. 1968 Oct;96(4):1181–1186. doi: 10.1128/jb.96.4.1181-1186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchin G. L., Duerksen J. D. Purification of beta-glucosidase from Saccharomyces lactis strains Y-14 and Y-1057A. J Bacteriol. 1968 Oct;96(4):1187–1190. doi: 10.1128/jb.96.4.1187-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZUR J. H., KLEPPE K., BALL E. M. THE GLYCOPROTEIN NATURE OF SOME FUNGAL CARBOHYDRASES. Arch Biochem Biophys. 1963 Dec;103:515–516. doi: 10.1016/0003-9861(63)90445-4. [DOI] [PubMed] [Google Scholar]
- Racusen D. Double-disc electrophoresis of proteins. Nature. 1967 Mar 4;213(5079):922–922. doi: 10.1038/213922a0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Price R. G., Dance N. Rat-urine glycosidases and kidney damage. Biochem J. 1967 Feb;102(2):533–538. doi: 10.1042/bj1020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Price R. G., Dance N. Separation and properties of beta-galactosidase, beta-glucosidase, beta-glucuronidase and N-acetyl-beta-glucosaminidase from rat kidney. Biochem J. 1967 Feb;102(2):525–532. doi: 10.1042/bj1020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- Umezurike G. M. The purification and properties of extracellular beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Feb 10;227(2):419–428. doi: 10.1016/0005-2744(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Van den Broek W. J., Van Breemen J. F., Van Bruggen E. F., Veeger C. Pyridine-nucleotide transhydrogenase. 2. Electron-microscopic studies on the transhydrogenase from Azotobacter vinelandii. Eur J Biochem. 1971 Dec 22;24(1):46–54. doi: 10.1111/j.1432-1033.1971.tb19653.x. [DOI] [PubMed] [Google Scholar]
- Wood T. M. The cellulase of Fusarium solani. Purification and specificity of the -(1-4)-glucanase and the -D-glucosidase components. Biochem J. 1971 Feb;121(3):353–362. doi: 10.1042/bj1210353. [DOI] [PMC free article] [PubMed] [Google Scholar]