Abstract
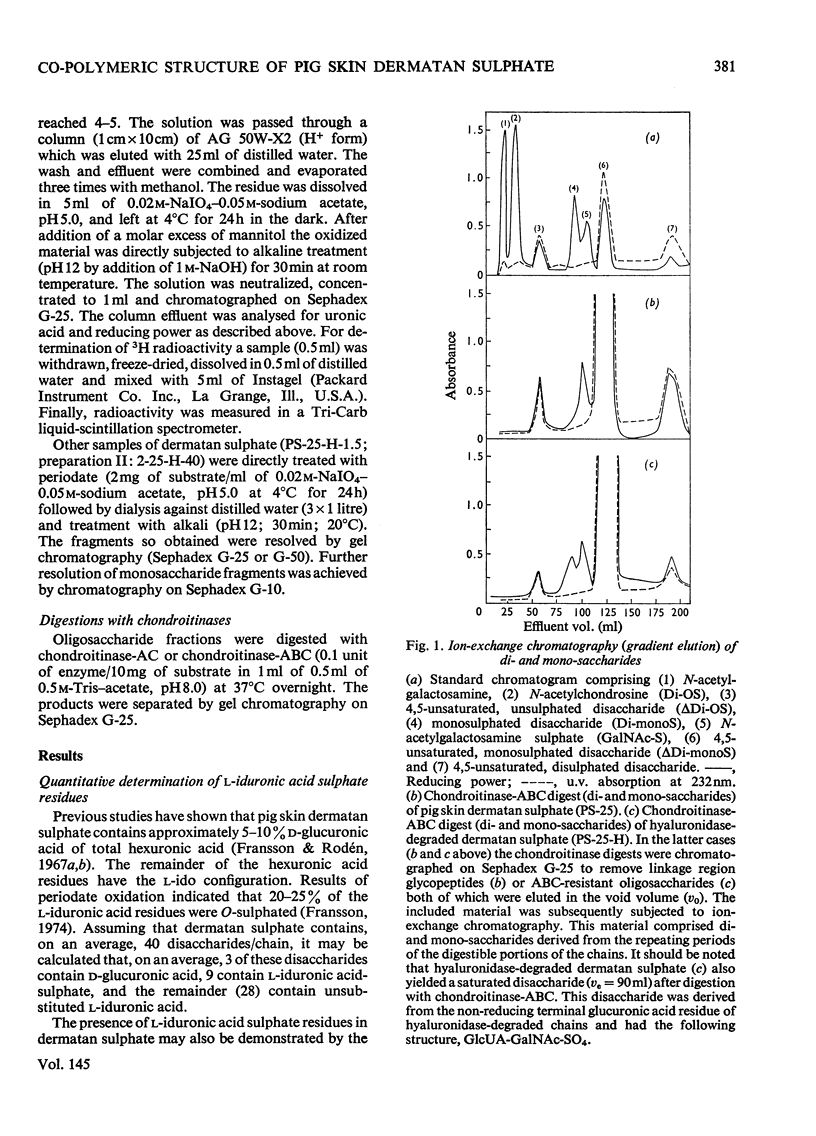
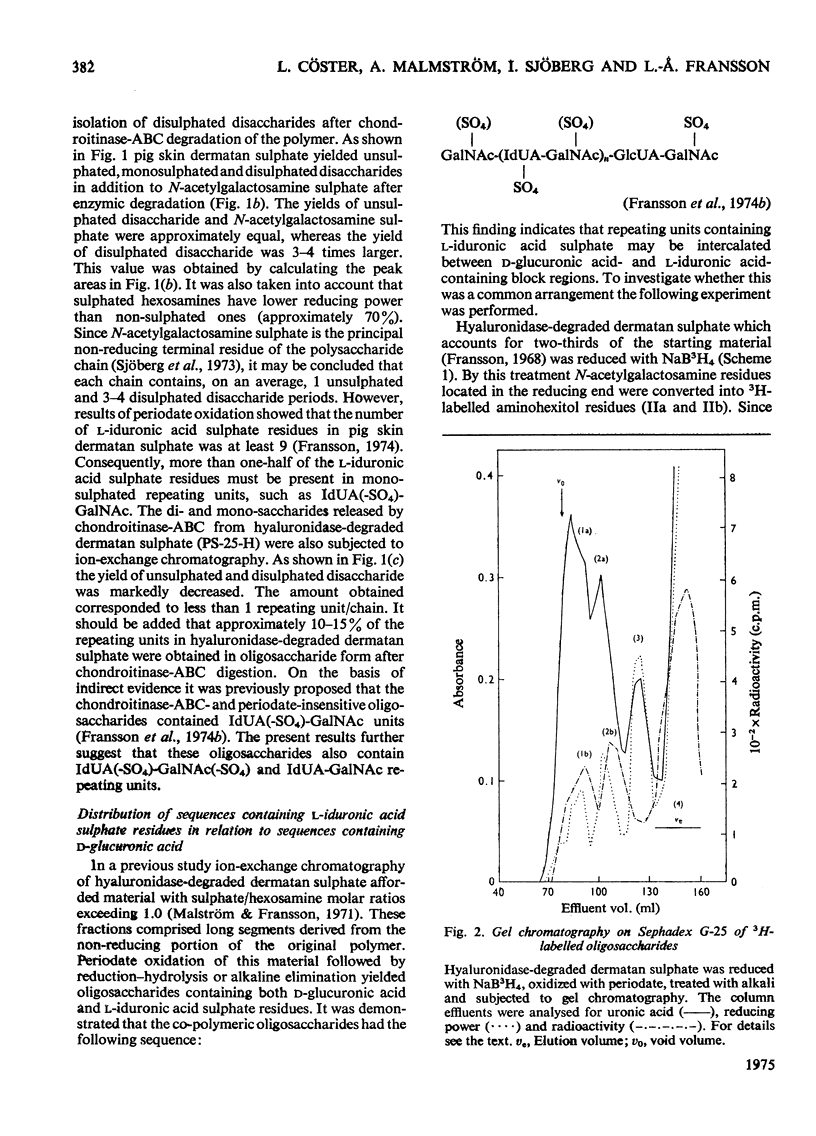
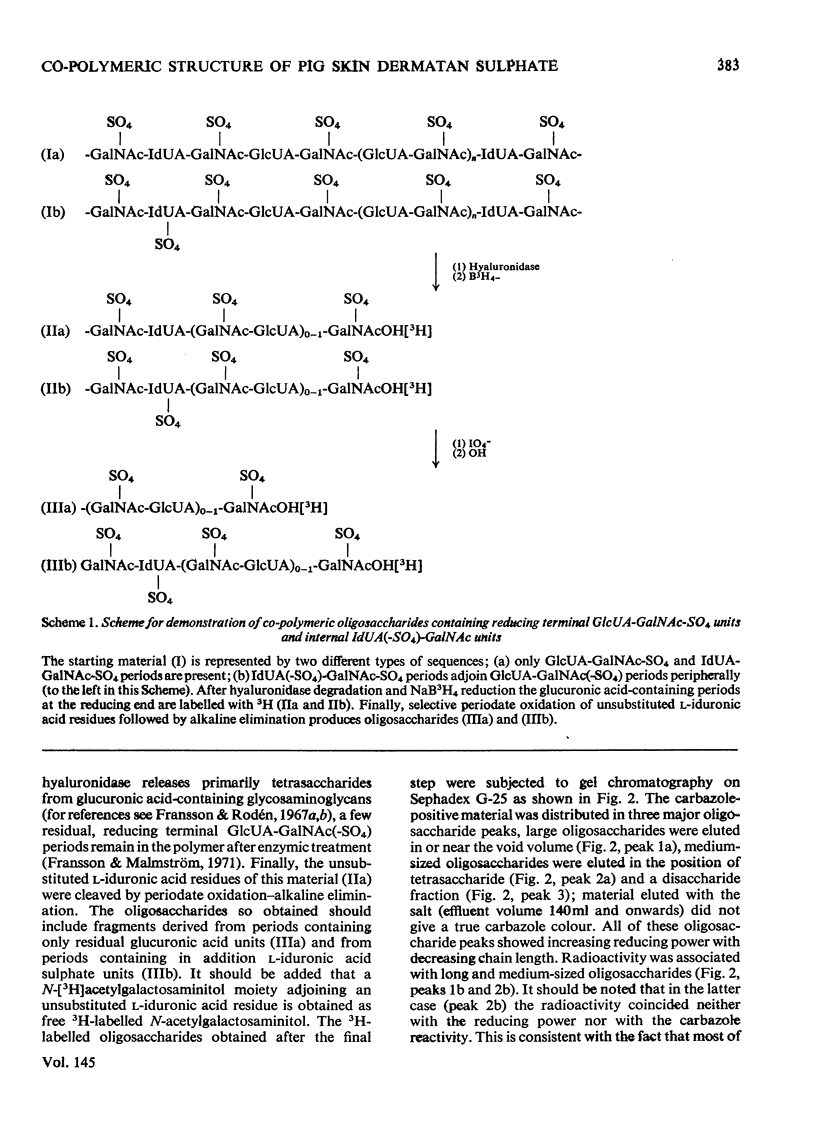
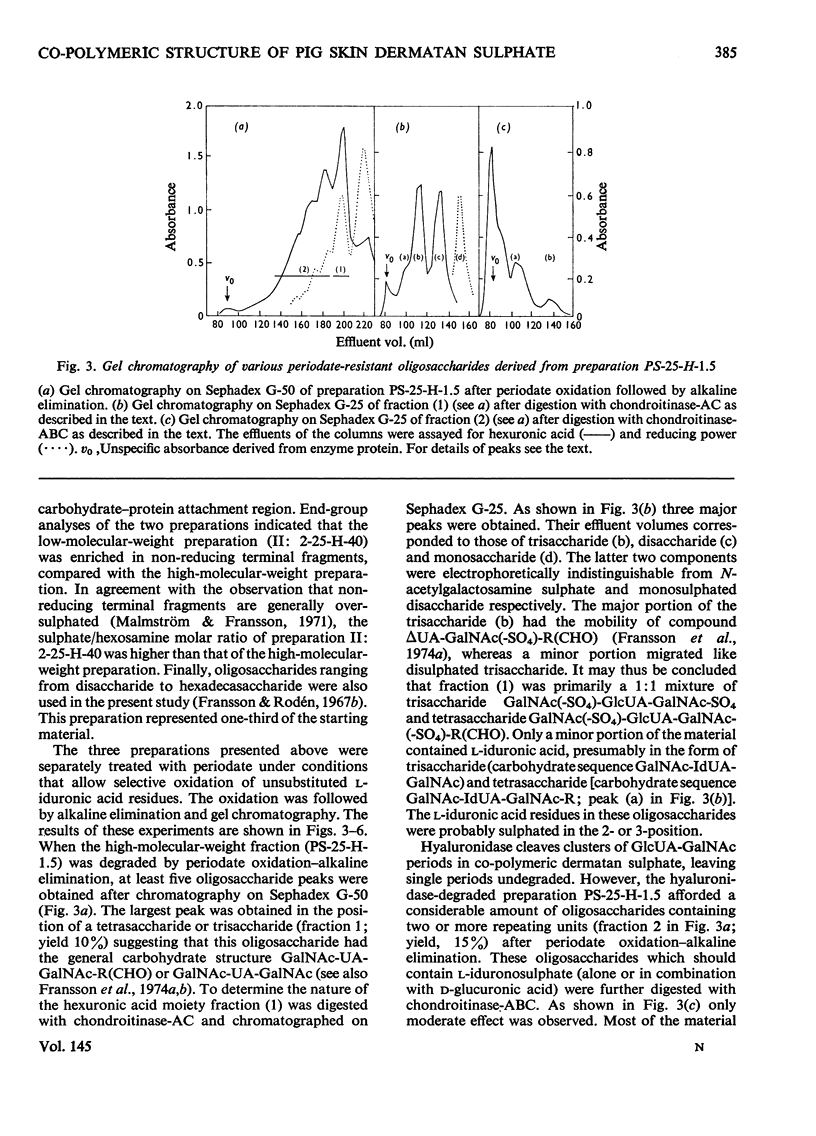
1. Pig skin dermatan sulphate was degraded by periodate oxidation followed by alkaline elimination or by chondroitinase-ABC to quantify irregular repeating units, i.e. those containing D-GlcUA (D-glucuronic acid) and L-IdUA-SO4 (sulphated iduronic acid). 2. Previous results of periodate oxidation (Fransson, 1974) indicated repeating sequences in pig skin dermatan sulphate containing, on average, 3D-GlcUA, 9 L-IdUA-SO4 or 28 L-IdUA units in addition to N-acetylgalactosamine sulphate. However, complete digestion with chondroitinase-ABC yielded, at the most, 3-4 disulphated disaccharides/chain. Consequently, more than one-half of the L-IdUA-SO4 residues were present in monosulphated periods, i.e. IdUA-(SO4)-GalNAc. 3. To determine the location of L-IdUA-SO4 residues along the copolymeric chain dermatan sulphate was digested with testicular hyaluronidase. (This enzyme cleaves GalNAc-GlcUA bonds within block regions containing D-GlcUA.) By NaB3H4 reduction GalNAc residues located in the reducing end of the fragments were converted into [3H]GalNAcOH (N-acetylgalactosaminitol). Finally, the radioactive product was fragmented by periodate oxidation followed by alkaline elimination. The bulk of the radioactivity was associated with periodate-resistant oligosaccharides indicating that clusters of GlcUA-GalNAc-SO4 periods are often adjacent to a varying number of (n = 1-4) of L-IdUA-SO4-containing periods. 4. To study the distribution of L-IdUA-SO4-containing periods in relation to blocks of IdUA-GalNAc-SO4 periods different fractions of hyaluronidase-degraded dermatan sulphate were degraded separately. In all types of fragments (mol. wts. 1,500-10,000) L-IdUA-SO4-containing periods were demonstrated. In short fragments reducing terminal GalNAc-6-SO4 (6-sulphated N-acetylgalactosamine) was found confirming that these sequences were joined to relatively long D-GlcUA-containing block sequences via GalNAc-6-SO4. Moreover, low-molecular-weight oligosaccharides composed of alternating sequences were encountered. An octasaccharide derived from the carbohydrate sequence -GalNAc---GlcUA-GalNAc-IdUA-GalNAc-GlcUA-GalNAc-IdUA-GalNAc---GlcUA-GalNAc (--- indicates the position of cleavage by hyaluronidase) was identified.
Full text
PDF


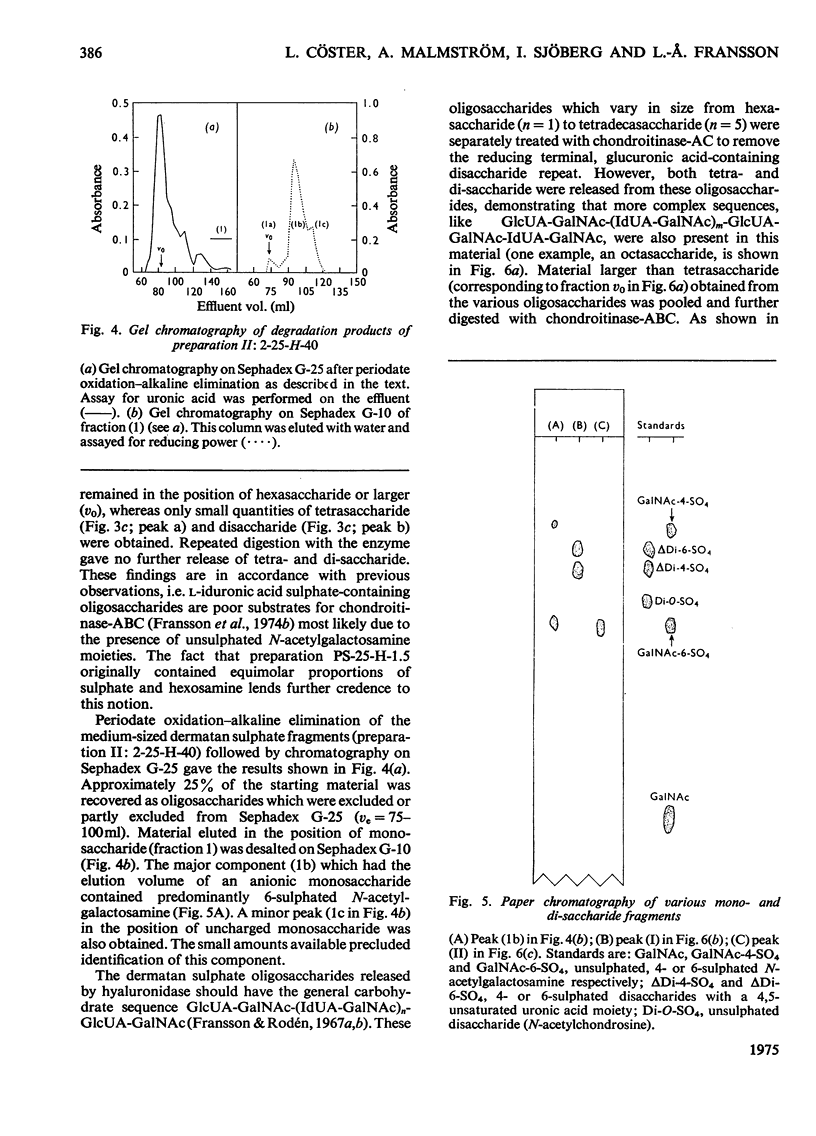
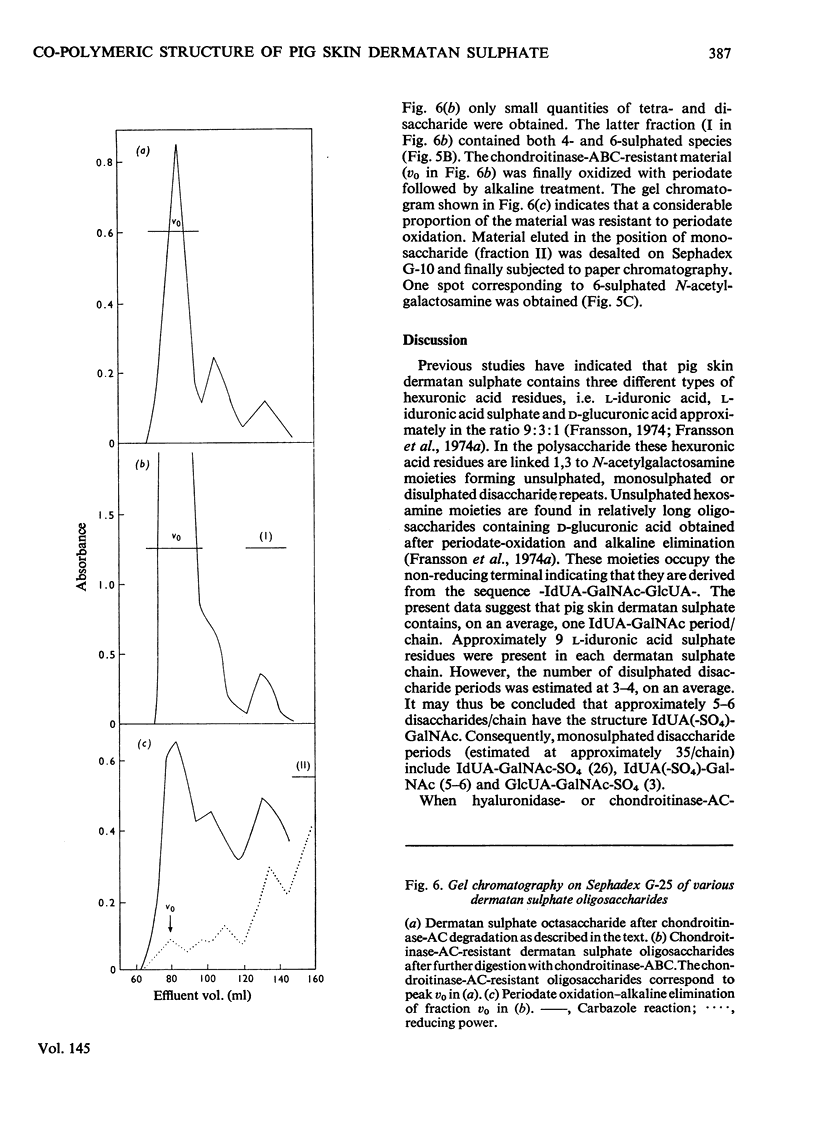


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIDSON E., HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissue. Biochim Biophys Acta. 1956 Sep;21(3):506–518. doi: 10.1016/0006-3002(56)90188-3. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Carlstedt I. Alkaline and smith degradation of oxidized dermatan sulphate-chondroitin sulphate copolymers. Carbohydr Res. 1974 Sep;36(2):349–358. doi: 10.1016/s0008-6215(00)83056-6. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Cöster L., Havasmark B., Malmström A., Sjöberg I. The copolymeric structure of pig skin dermatan sulphate. Isolation and characterization of L-idurono-sulphate-containing oligosaccharides from copolymeric chains. Biochem J. 1974 Nov;143(2):379–389. doi: 10.1042/bj1430379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Cöster L., Malmstrom A., Sjöberg I. The copolymeric structure of pig skin dermatan suplhate. Characterization of D-glucuronic acid-containing oligosaccharides isolated after controlled degradation of oxydermatan sulphate. Biochem J. 1974 Nov;143(2):369–378. doi: 10.1042/bj1430369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Malmström A. Structure of pig skin dermatan sulfate. 1. Distribution of D-glucuronic acid residues. Eur J Biochem. 1971 Feb 1;18(3):422–430. doi: 10.1111/j.1432-1033.1971.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Fransson L. A. Periodate oxidation of L-iduronic acid residues in dermatan sulphate. Carbohydr Res. 1974 Sep;36(2):339–348. doi: 10.1016/s0008-6215(00)83055-4. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Rodén L., Spach M. L. Automated ion-exchange chromatography of uronic acids and uronic acid containing oligosaccharides. Anal Biochem. 1968 May;23(2):317–330. doi: 10.1016/0003-2697(68)90362-x. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Rodén L. Structure of dermatan sulfate. I. Degradation by testicular hyaluronidase. J Biol Chem. 1967 Sep 25;242(18):4161–4169. [PubMed] [Google Scholar]
- Fransson L. A., Rodén L. Structure of dermatan sulfate. II. Characterization of products obtained by hyaluronidase digestion of dermatan sulfate. J Biol Chem. 1967 Sep 25;242(18):4170–4175. [PubMed] [Google Scholar]
- Fransson L. A. Structure of dermatan sulfate. IV. Glycopeptides from the carbohydrate-protein linkage region of pig skin dermatan sulfate. Biochim Biophys Acta. 1968 Mar 11;156(2):311–316. [PubMed] [Google Scholar]
- HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissues. II. Further experiments on chondroitin sulfate B. Arch Biochem Biophys. 1957 Jul;69:435–440. doi: 10.1016/0003-9861(57)90508-8. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Malmström A., Fransson L. A. Biosynthesis of L-iduronic acid in heparin: epimerization of D-glucuronic acid on the polymer level. Biochem Biophys Res Commun. 1972 Jan 31;46(2):985–991. doi: 10.1016/s0006-291x(72)80238-9. [DOI] [PubMed] [Google Scholar]
- Malmström A., Fransson L. A. Structure of pig skin dermatan sulfate. 2. Demonstration of sulfated iduronic acid residues. Eur J Biochem. 1971 Feb 1;18(3):431–435. doi: 10.1111/j.1432-1033.1971.tb01260.x. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Sjöberg I., Fransson L. A., Matalon R., Dorfman A. Hunter's syndrome: a deficiency of L-idurono-sulfate sulfatase. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1125–1132. doi: 10.1016/0006-291x(73)90809-7. [DOI] [PubMed] [Google Scholar]


