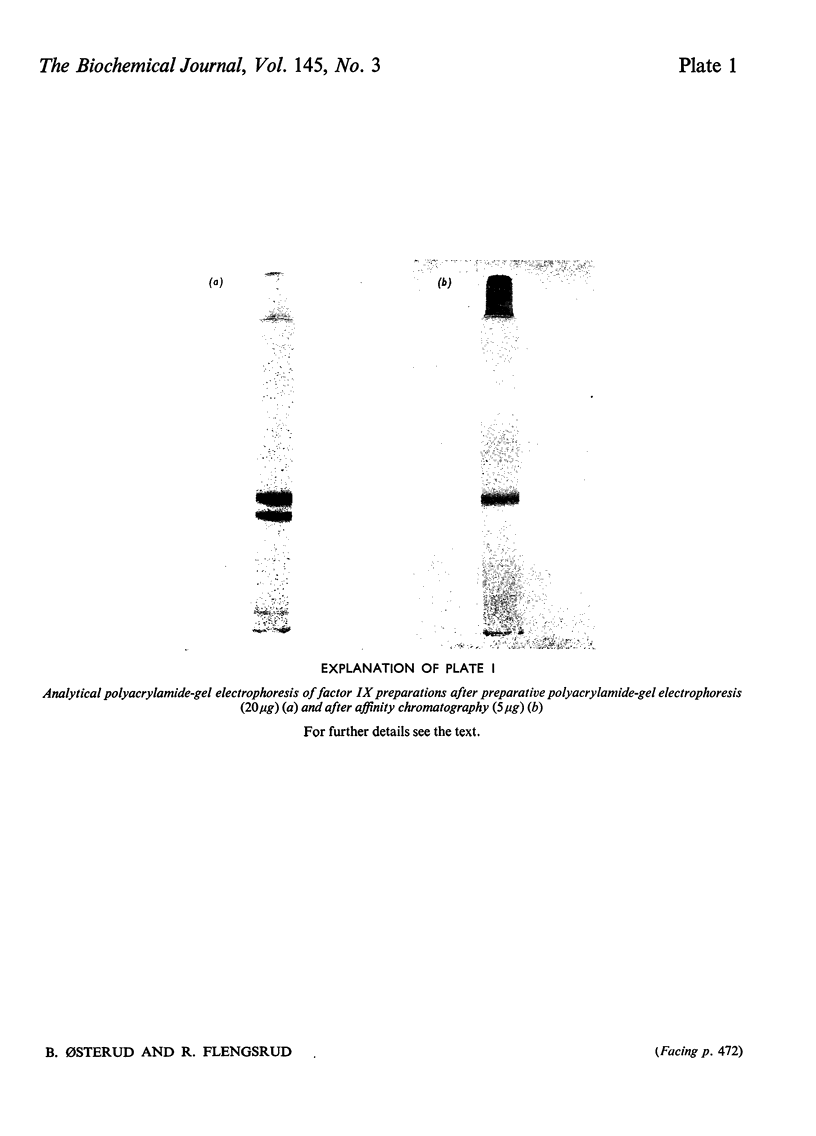
Abstract
Non-activated coagulation factor IX was purified approx. 10,000-fold from human plasma. The final product was electrophoretically homogeneous and comprised a tingle polypeptide chain with a molecular weight of about 70,000 and a pI of 4.3-4.45. The N-terminal amino acid was glycine. The amino acid and the carbohydrate contents were analysed and a monospecific antiserum to the factor was raised in rabbits.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton P. G. Sequence theories of blood coagulation re-evaluated with reference to lipid-protein interactions. Nature. 1967 Sep 30;215(5109):1508–1509. doi: 10.1038/2151508a0. [DOI] [PubMed] [Google Scholar]
- Bjorklid E., Storm E., Prydz H. The protein component of human brain thromboplastin. Biochem Biophys Res Commun. 1973 Dec 10;55(3):969–976. doi: 10.1016/0006-291x(73)91237-0. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Catsimpoolas N. Micro isoelectric focusing in polyacrylamide gel columns. Anal Biochem. 1968 Dec;26(3):480–482. doi: 10.1016/0003-2697(68)90219-4. [DOI] [PubMed] [Google Scholar]
- Chandra S., Pechet L. The separation of clotting factors. II. Purification of human factor IX by isoelectric focusing. Biochim Biophys Acta. 1973 Dec 6;328(2):456–463. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Deyl Z., Rosmus J. Thin layer chromatography of Dansyl amino acid derivatives. J Chromatogr. 1965 Dec;20(3):514–520. doi: 10.1016/s0021-9673(01)97453-9. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Enfield D. L., Titani K., Ericsson L. H., Davie E. W. A comparison of bovine prothrombin, factor IX (Christmas factor), and factor X (Stuart factor). Proc Natl Acad Sci U S A. 1974 Feb;71(2):427–430. doi: 10.1073/pnas.71.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- Howell R. M., Dupe R. J. Observations on factor X activity after adsorption to barium sulphate. Thromb Diath Haemorrh. 1972 Oct 31;28(2):306–316. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I., Schiffman S., Chong M. M. Formation of intrinsic factor-X activator activity, with special reference to the role of thrombin. Br J Haematol. 1971 Dec;21(6):643–660. doi: 10.1111/j.1365-2141.1971.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Synthesis of intrinsic factor X activator. Inhibition of the function of formed activator by antibodies to factor VIII and to factor IX. Biochemistry. 1970 Apr 14;9(8):1854–1861. doi: 10.1021/bi00810a028. [DOI] [PubMed] [Google Scholar]
- Osterud B., Schiffman S. Gel filtration properties of factors II, VII, IX, IX a , and X. Thromb Diath Haemorrh. 1972 Oct 31;28(2):317–324. [PubMed] [Google Scholar]
- Prydz H., Gladhaug A. A specific system for he estimation of prothrombin activity. Thromb Diath Haemorrh. 1970 Dec 31;24(3):601–602. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Skotland T., Holm T., Osterud B., Flengsrud R., Prydz H. The localization of a vitamin K-induced modification in an N-terminal fragment of human prothrombin. Biochem J. 1974 Oct;143(1):29–37. doi: 10.1042/bj1430029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]