Abstract
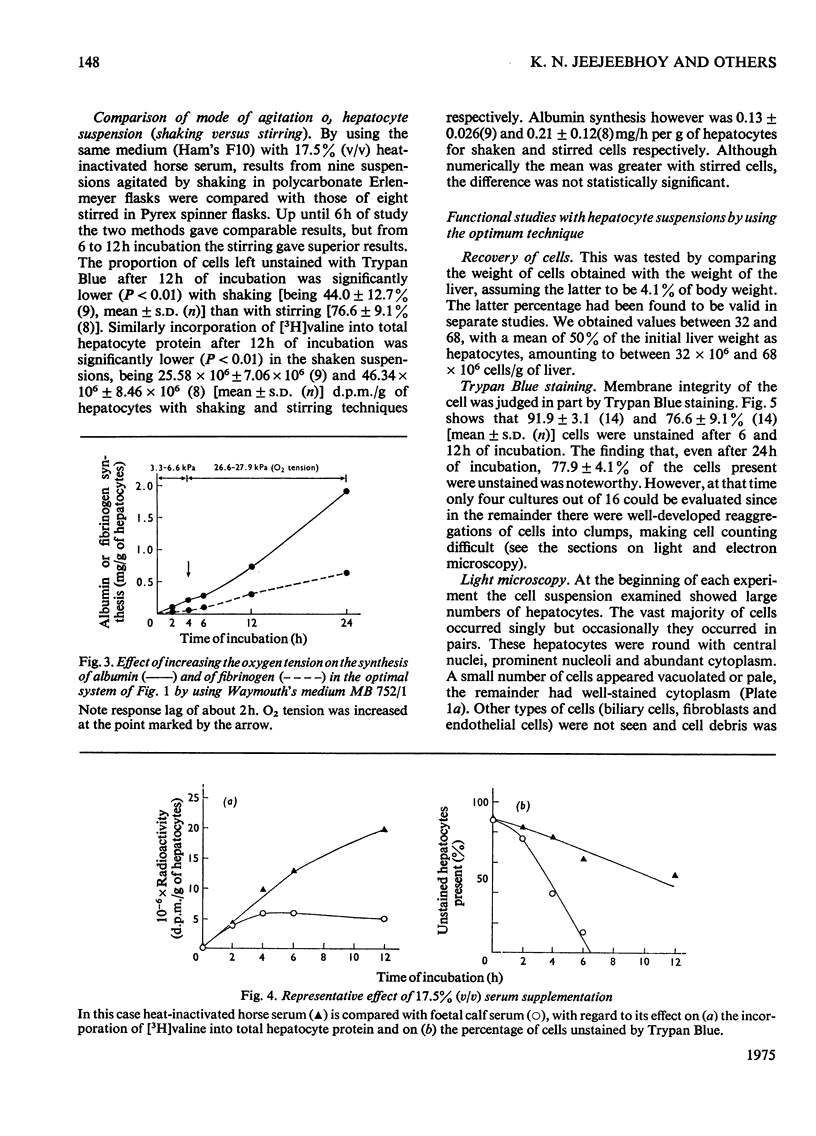
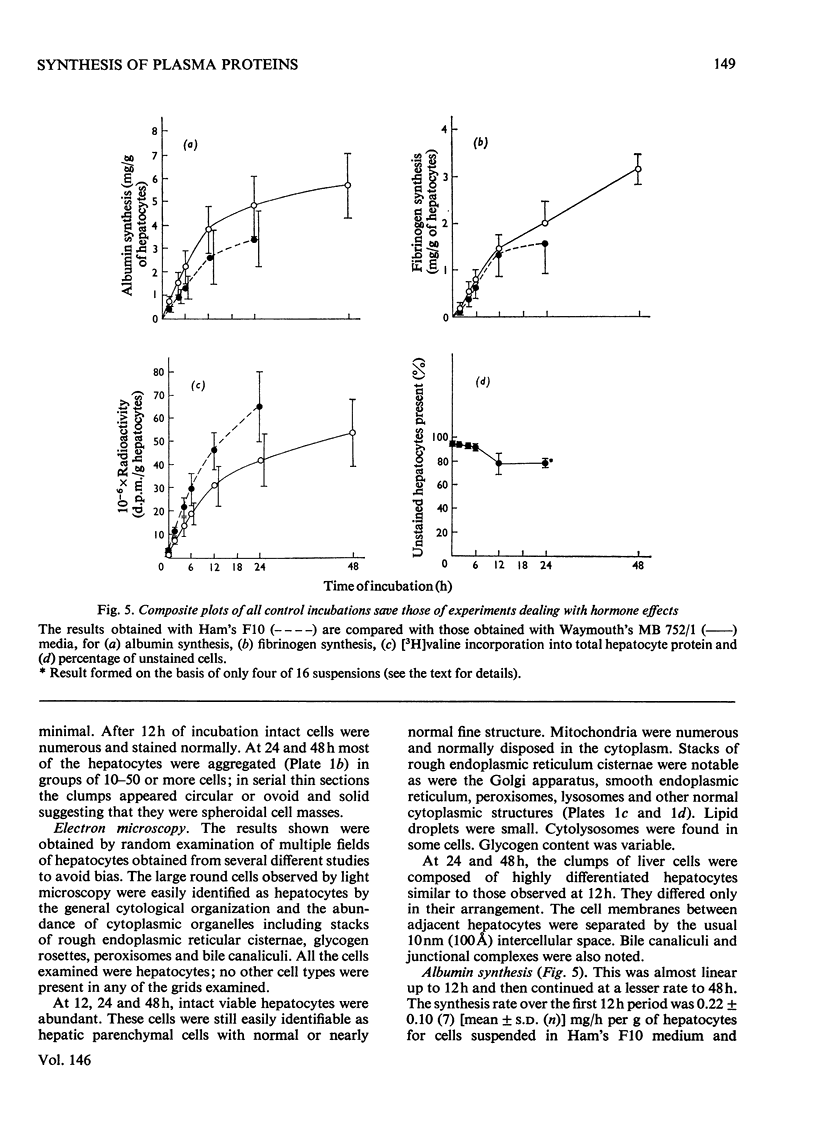
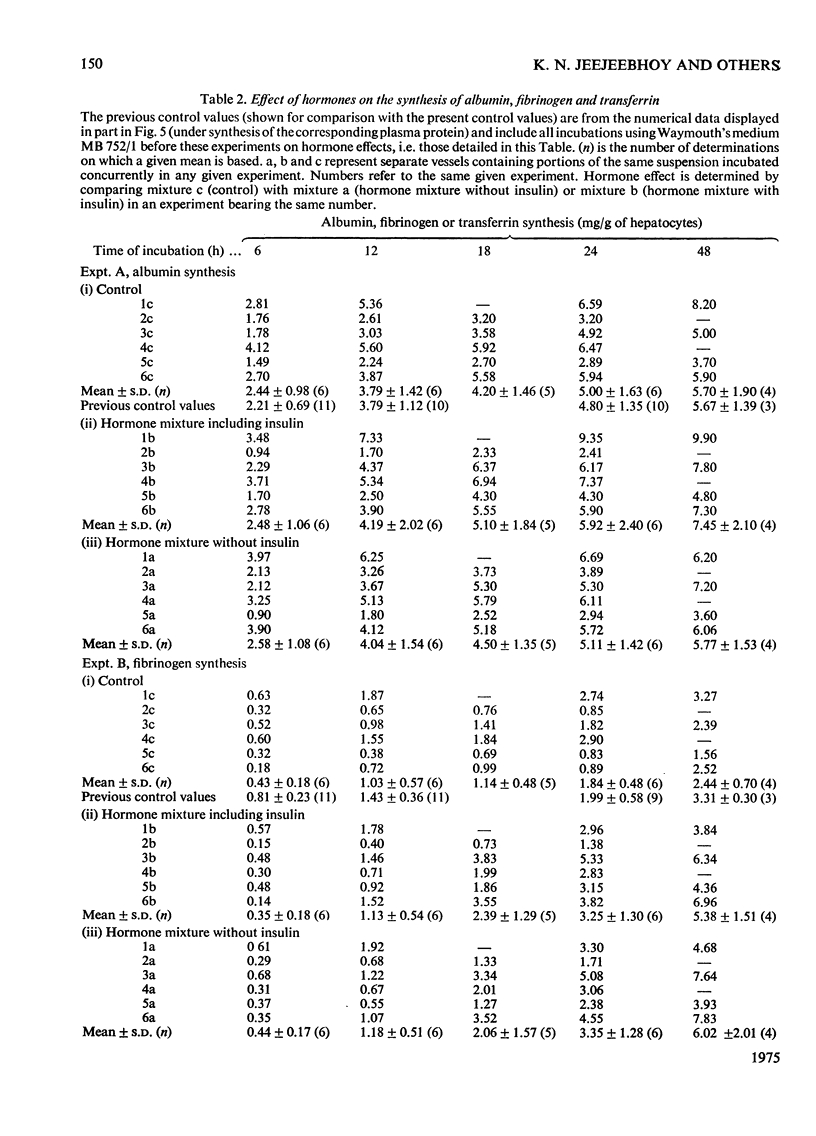
A system using hepatocyte suspensions in vitro was developed for studying the synthesis of albumin, fibrinogen and transferrin. Conditions for optimum survival of the hepatocyte and for synthesis of these plasma proteins were defined for this system. These conditions included the use of horse serum (17.5 percent, v/v, heat-inactivated), an enriched medium (Waymouth's MB 752/1), an O2 tension of between 18.7 times 10(3) and 26.7 times 10(3) Pa and constant stirring. Albumin, fibrinogen and transferrin synthesis rates were obtained of 0.32 p 0.094(10), 0.12 p 0.030(11) and 0.097 p 0.017(10) [mean p S.D. (n)]mg/h per g of hepatocytes respectively. These rates were maintained for the first 12h of study and synthesis continued at a diminished rate up to 48h. The synthesis of albumin was decreased in a medium containing less amino acids and glucose, but that of fibrinogen was substantially unaffected. ATP concentrations up to 12h and RNA/DNA ratios up to 24h were comparable with values in vivo. The ability to study cells up to 48h permitted us to find that the addition of a mixture of hormones consisting of glucagon, cortisol, tri-iodothyronine and growth hormone enhanced fibrinogen synthesis. Addition of insulin to the above mixture resulted in increased synthesis for albumin and transferrin but not for fibrinogen.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. H., Patel D. G., Jeejeebhoy K. N. Design and evaluation by nitrogen balance and blood aminograms of an amino acid mixture for total parenteral nutrition of adults with gastrointestinal disease. J Clin Invest. 1974 Mar;53(3):904–912. doi: 10.1172/JCI107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Leonard E. J. Solid phase radioimmunoassay of human beta 1C globulin. Immunochemistry. 1970 Jan;7(1):29–41. doi: 10.1016/0019-2791(70)90028-5. [DOI] [PubMed] [Google Scholar]
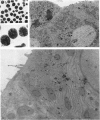
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxam D. L. Nutritional aspects of amino acid metabolism. 1. A rat liver perfusion method for the study of amino acid metabolism. Br J Nutr. 1971 Nov;26(3):393–422. doi: 10.1079/bjn19710046. [DOI] [PubMed] [Google Scholar]
- East A. G., Louis L. N., Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973 Jan;76(1):41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ho J., Jeejeebhoy K. N., Painter R. H. A plasma protein fractionation procedure for use in studies of protein metabolism. Biochem J. 1974 Sep;141(3):655–665. doi: 10.1042/bj1410655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. B., Lee J. C., Pesch L. A. The fine structure, potassium content, and respiratory activity of isolated rat liver parenchymal cells prepared by improved enzymatic techniques. J Cell Biol. 1973 Jun;57(3):642–658. doi: 10.1083/jcb.57.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Bruce-Robertson A., Ho J., Sodtke U. The comparative effects of nutritional and hormonal factors on the synthesis of albumin, fibrinogen and transferrin. Ciba Found Symp. 1972;9:217–247. doi: 10.1002/9780470719923.ch12. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Bruce-Robertson A., Ho J., Sodtke U. The effect of cortisol on the synthesis of rat plasma albumin, fibrinogen and transferrin. Biochem J. 1972 Nov;130(2):533–538. doi: 10.1042/bj1300533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. W., Miller L. L. Regulation of net biosynthesis of serum albumin and acute phase plasma proteins. Induction of enhanced net synthesis of fibrinogen, alpha1-acid glycoprotein, alpha2 (acute phase)-globulin, and haptoglobin by amino acids and hormones during perfusion of the isolated normal rat liver. J Biol Chem. 1969 Nov 25;244(22):6134–6142. [PubMed] [Google Scholar]
- Kaighn M. E., Prince A. M. Production of albumin and other serum proteins by clonal cultures of normal human liver. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2396–2400. doi: 10.1073/pnas.68.10.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGIO R., SIEKEVITZ P., PALADE G. E. STUDIES ON ISOLATED NUCLEI. I. ISOLATION AND CHEMICAL CHARACTERIZATION OF A NUCLEAR FRACTION FROM GUINEA PIG LIVER. J Cell Biol. 1963 Aug;18:267–291. doi: 10.1083/jcb.18.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Majumdar C., Tsukada K., Lieberman I. Liver protein synthesis after partial hepatectomy and acute stress. J Biol Chem. 1967 Feb 25;242(4):700–704. [PubMed] [Google Scholar]
- Morgan E. H., Peters T., Jr Intracellular aspects of transferrin synthesis and secretion in the rat. J Biol Chem. 1971 Jun 10;246(11):3508–3511. [PubMed] [Google Scholar]
- OBRINK K. J. A modified Conway unit for microdiffusion analysis. Biochem J. 1955 Jan;59(1):134–136. doi: 10.1042/bj0590134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS T., Jr The biosynthesis of rat serum albumin. I. Properties of rat albumin and its occurrence in liver cell fractions. J Biol Chem. 1962 Apr;237:1181–1185. [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]
- Quistorff B., Bondesen S., Grunnet N. Preparation and biochemical characterization of parenchymal cells from rat liver. Biochim Biophys Acta. 1973 Sep 14;320(2):503–516. doi: 10.1016/0304-4165(73)90331-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Rayford P. L., Cooper J. A., Ross G. T. Statistical quality control of radioimmunoassays. J Clin Endocrinol Metab. 1968 Oct;28(10):1412–1418. doi: 10.1210/jcem-28-10-1412. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Schreiber M. Protein synthesis in single cell suspensions from rat liver. I. General properties of the system and permeability of the cells for leucine and methionine. J Biol Chem. 1972 Oct 10;247(19):6340–6346. [PubMed] [Google Scholar]
- Schreiber G., Schreiber M. The preparation of single cell suspensions from liver and their use for the study of protein synthesis. Subcell Biochem. 1973;2(4):307–353. [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. Rapid proliferation of sublines of NCTC clone 929 (strain L) mouse cells in a simple chemically defined medium (MB 752/1). J Natl Cancer Inst. 1959 May;22(5):1003–1017. doi: 10.1093/jnci/22.5.1003. [DOI] [PubMed] [Google Scholar]
- Weignand K., Müller M., Urban J., Schreiber G. Intact endoplasmic reticulum and albumin synthesis in rat liver cell suspensions. Exp Cell Res. 1971 Jul;67(1):27–32. doi: 10.1016/0014-4827(71)90617-3. [DOI] [PubMed] [Google Scholar]