Abstract
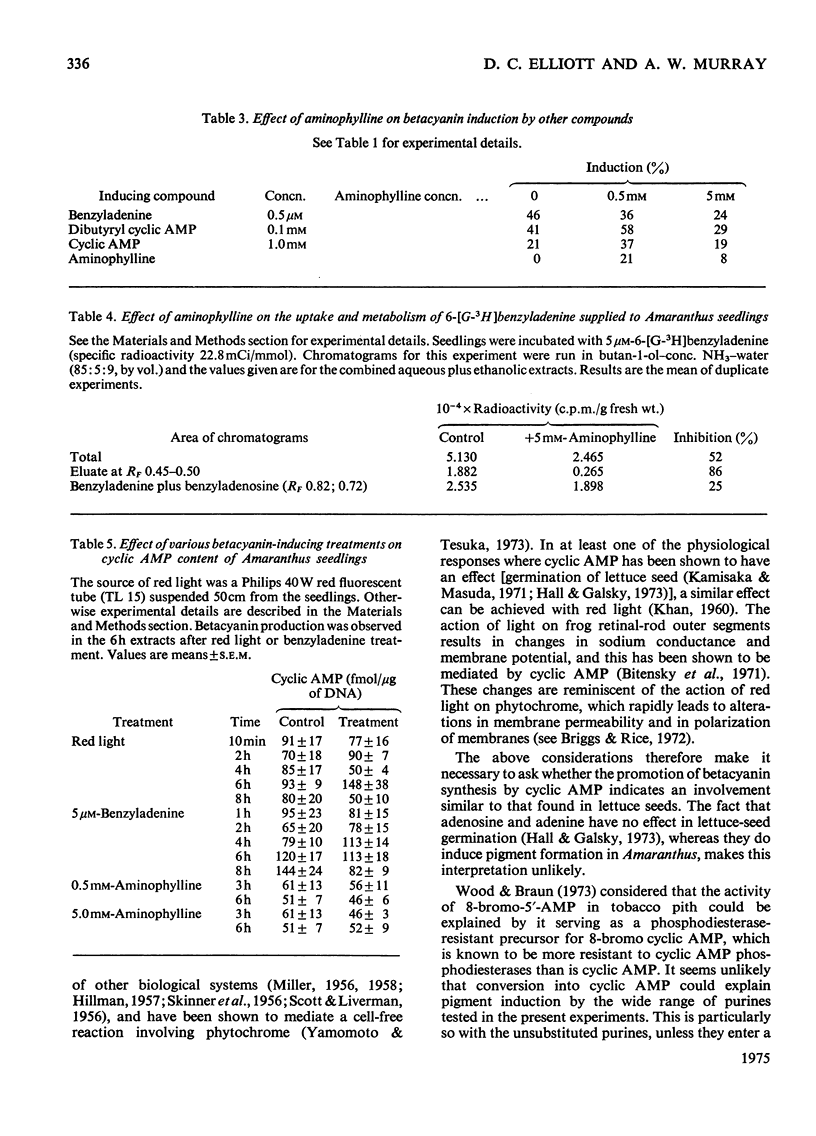
1. A wide range of purine bases, nucleosides and cyclic nucleotides were shown to induce betacyanin synthesis in Amaranthus seedlings. 2. The induction of pigment by benzyladenine, dibutyryl cyclic AMP or cyclic AMP was not potentiated by aminophylline. Aminophylline was shown to inhibit Amaranthus cyclic AMP phosphodiesterase activity. 4. Incubation of seedlings with aminophylline inhibited the conversion of 6-[G--3H]benzyladenine into presumed 9- and 7-glucosylbenzyladenine. 5. Induction of betacyanin synthesis by 6-benzyladenine or by exposure to red light was not accompanied by changes in the total cyclic AMP content in seedlings. 6. It is concluded that the inducers tested act as cytokinin analogues; no evidence was obtained to support cyclic AMP as an intermediate in the induction process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E., Mayer A. M. Effect of Kinetin on Formation of Red Pigment in Seedlings of Amaranthus retroflexus. Science. 1960 Apr 15;131(3407):1094–1095. doi: 10.1126/science.131.3407.1094. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Ralph R. K., Letham D. S. The binding of kinetin to plant ribosomes. Biochem J. 1970 Aug;119(1):75–84. doi: 10.1042/bj1190075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Miller W. H. Adenyl cyclase as a link between photon capture and changes in membrane permeability of frog photoreceptors. Proc Natl Acad Sci U S A. 1971 Mar;68(3):561–562. doi: 10.1073/pnas.68.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYBROOK J. R., SHIVE W., SKINNER C. G., TALBERT F. D. Stimulation of seed germination by 6-(substituted) thiopurines. Arch Biochem Biophys. 1956 Dec;65(2):567–569. doi: 10.1016/0003-9861(56)90214-4. [DOI] [PubMed] [Google Scholar]
- Cowley D. E., MacLeod J. K. The glucosylation of cytokinins. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1370–1376. doi: 10.1016/s0006-291x(73)80045-2. [DOI] [PubMed] [Google Scholar]
- Deleuze G. G., McChesney J. D., Fox J. E. Identification of a stable cytokinin metabolite. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1426–1432. doi: 10.1016/0006-291x(72)90872-8. [DOI] [PubMed] [Google Scholar]
- Dyson W. H., Fox J. E., McChesney J. D. Short term metabolism of urea and purine cytokinins. Plant Physiol. 1972 Apr;49(4):506–513. doi: 10.1104/pp.49.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. C., Murray A. W. A quantitative limit for cytokinin incorporation into transfer ribonucleic acid by soya-bean callus tissue. Biochem J. 1972 Dec;130(4):1157–1160. doi: 10.1042/bj1301157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. R. Discussion on the Relation of the Internal Secretions to the Female Characteristics and Functions in Health and Disease. Proc R Soc Med. 1914;7(OBSTET):83–85. [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. F. Statutory requirements on bioavailability determination of peroral-dosage forms. Boll Chim Farm. 1973 Sep;112(9):565–571. [PubMed] [Google Scholar]
- Hillman W. S. Nonphotosynthetic Light Requirement in Lemna minor and Its Partial Satisfaction by Kinetin. Science. 1957 Jul 26;126(3265):165–166. doi: 10.1126/science.126.3265.165. [DOI] [PubMed] [Google Scholar]
- Kahn A. Promotion of Lettuce Seed Germination by Gibberellin. Plant Physiol. 1960 May;35(3):333–339. doi: 10.1104/pp.35.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller C. O. Similarity of Some Kinetin and Red Light Effects. Plant Physiol. 1956 Jul;31(4):318–319. doi: 10.1104/pp.31.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. The Relationship of the Kinetin and Red-Light Promotions of Lettuce Seed Germination. Plant Physiol. 1958 Mar;33(2):115–117. doi: 10.1104/pp.33.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Liverman J. L. Promotion of Leaf Expansion by Kinetin and Benzylaminopurine. Plant Physiol. 1956 Jul;31(4):321–322. doi: 10.1104/pp.31.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swislocki N. I. Decomposition of dibutyryl cyclic AMP in aqueous buffers. Anal Biochem. 1970 Nov;38(1):260–269. doi: 10.1016/0003-2697(70)90175-2. [DOI] [PubMed] [Google Scholar]
- Wood H. N., Braun A. C. 8-bromoadenosine 3':5'-cyclic monophosphate as a promoter of cell division in excised tobacco pith parenchyma tissue. Proc Natl Acad Sci U S A. 1973 Feb;70(2):447–450. doi: 10.1073/pnas.70.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. N., Lin M. C., Braun A. C. The inhibition of plant and animal adenosine 3':5'-cyclic monophosphate phosphodiesterases by a cell-division-promoting substance from tissues of higher plant species. Proc Natl Acad Sci U S A. 1972 Feb;69(2):403–406. doi: 10.1073/pnas.69.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]


