Abstract
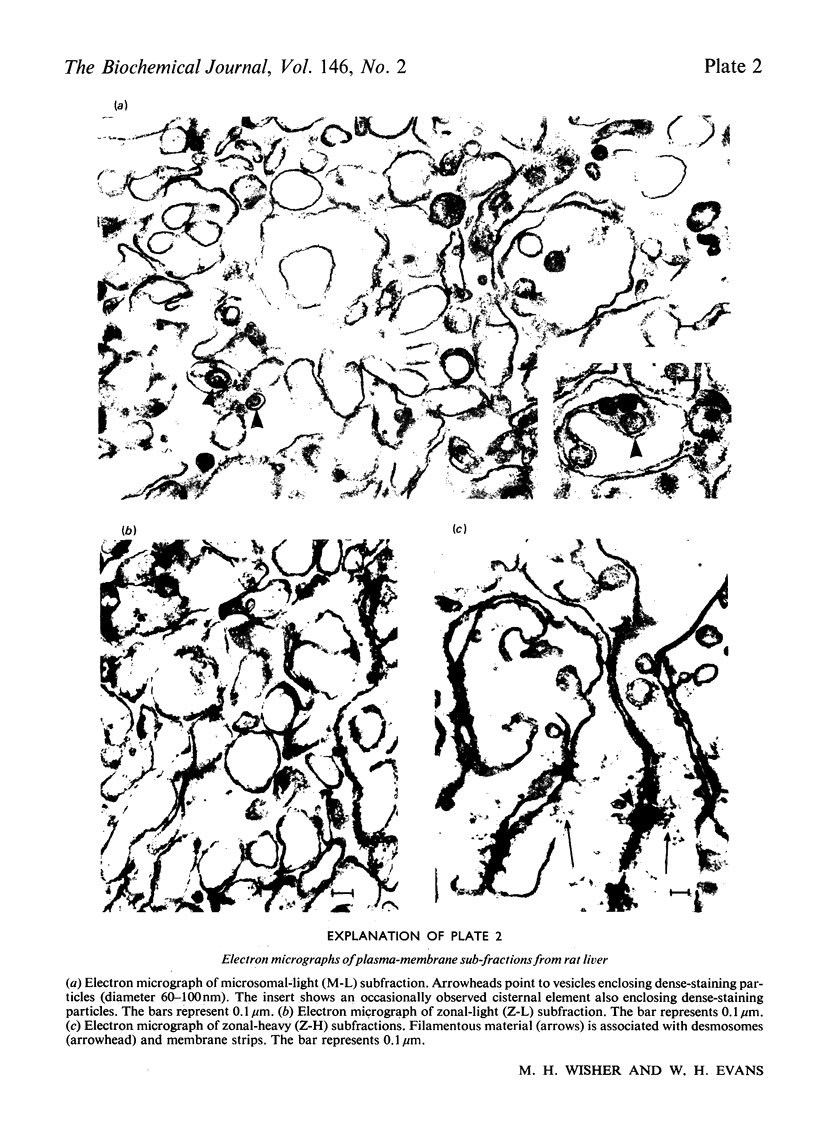
1. Six rat liver plasma-membrane subfractions of different density and morphological, enzymic and chemical properties were prepared from homogenates by a combination of differential, rate-zonal and density-gradient centrifugation. They consisted of three vesicular 'light' subfractions of density 1.12-1.13 and three 'heavy' subfractions of density 1.16-1.18 containing membrane strips and intercellular junctions. 2. All six subfractions contained a basal adenylate cyclase activity. One of the 'light' subfractions that showed the highest glucagon-stimulated adenylate cyclase activity was identified as deriving form the blood-sinusoidal face of the hepatocyte. This subfraction, unlike the others, was contaminated by Golgi components, as indicated by its morphological properties and the presence of galactosyl- and sialyl-transferase activities. 3. All the six subfractions showed high activities of the following plasma-membrane marker enzymes: 5'-nucleotidase, alkaline phosphodiesterase (nucleotide pyrophosphatase), alkaline phosphatase, leucine naphthylamidase and Mg2+-activated adenosine triphosphatase. A 'light' subfraction that showed the highest specific activities of all the above marker enzymes, but lacked a glucagon-stimulated adenylate cyclase activity, was identified as deriving from the bile-canalicular face of the hepatocyte. 4. The 'heavy' subfractions, which showed generally the lowest activities of the above plasma-membrane enzyme markers, and were characterized by the presence of desmosomes and gap junctions, were taken to originate from the contiguous faces of the hepatocyte. 5. The protein composition of the six subfractions was generally similar, as shown by polyacrylamide-gel electrophoresis. Differences in the amounts of various protein and glycoprotein bands among the subfractions correlated with their morphology, enzymic composition and sialic acid content. 6. Hormonal and histochemical evidence supporting the identification of a bile-canalicular subfraction, a blood-sinusoidal subfraction and contiguous-face subfractions is discussed.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Tan L. Y., Peters B. P. Galactosyl transferase-the liver plasma membrane binding-site for asialo-glycoproteins. Biochem Biophys Res Commun. 1973 Jul 2;53(1):112–118. doi: 10.1016/0006-291x(73)91408-3. [DOI] [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970 Dec 10;245(23):6357–6365. [PubMed] [Google Scholar]
- Bennett G., Leblond C. P., Haddad A. Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labelled fucose injection into rats. J Cell Biol. 1974 Jan;60(1):258–284. doi: 10.1083/jcb.60.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. D., Vance J. E., Morgan A., Williams R. H. Effect of pancreozymin on insulin and glucagon levels in blood and bile. Am J Physiol. 1968 Dec;215(6):1293–1298. doi: 10.1152/ajplegacy.1968.215.6.1293. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., Jourdian G. W., Roseman S. The sialic acids. XIV. Synthesis of sialyl-lactose by a sialyltransferase from rat mammary gland. J Biol Chem. 1973 Aug 25;248(16):5742–5750. [PubMed] [Google Scholar]
- Dewald B., Touster O. A new alpha-D-mannosidase occurring in Golgi membranes. J Biol Chem. 1973 Oct 25;248(20):7223–7233. [PubMed] [Google Scholar]
- Duguid J. R., Raftery M. A. Fractionation and partial characterization of membrane particles from Torpedo californica electroplax. Biochemistry. 1973 Sep 11;12(19):3593–3597. doi: 10.1021/bi00743a003. [DOI] [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESSNER E., NOVIKOFF A. B., MASEK B. Adenosinetriphosphatase and 5-nucleotidase activities in the plasma membrane of liver cells as revealed by electron microscopy. J Biophys Biochem Cytol. 1958 Nov 25;4(6):711–716. doi: 10.1083/jcb.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Bergeron J. J., Geschwind I. I. Distribution of insulin receptor sites among liver plasma membrane subfractions. FEBS Lett. 1973 Aug 15;34(2):259–262. doi: 10.1016/0014-5793(73)80807-5. [DOI] [PubMed] [Google Scholar]
- Evans W. H. Fractionation of liver plasma membranes prepared by zonal centrifugation. Biochem J. 1970 Mar;116(5):833–842. doi: 10.1042/bj1160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Biosynthesis of liver membranes. Incorporation of ( 3 H)leucine into proteins and of ( 14 C)glucosamine into proteins and lipids of liver microsomal and plasma-membrane fractions. Biochem J. 1971 Nov;125(2):615–624. doi: 10.1042/bj1250615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Preparation and properties of nexuses and lipid-enriched vesicles from mouse liver plasma membranes. Biochem J. 1972 Jul;128(3):691–700. doi: 10.1042/bj1280691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Nucleotide pyrophosphatase, a sialoglycoprotein located on the hepatocyte surface. Nature. 1974 Aug 2;250(465):391–394. doi: 10.1038/250391a0. [DOI] [PubMed] [Google Scholar]
- Evans W. H. Subfractionation of rat liver plasma membranes. FEBS Lett. 1969 Jun;3(4):237–241. doi: 10.1016/0014-5793(69)80146-8. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S., Ozawa H. Isolation and characterization of Golgi membranes from bovine liver. J Cell Biol. 1969 Oct;43(1):59–79. doi: 10.1083/jcb.43.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S. Preparation and characterization of golgi membranes from rat liver. Biochim Biophys Acta. 1970 Dec 1;219(2):301–319. doi: 10.1016/0005-2736(70)90209-9. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Deumling B., Baerbelermen, Jarasch E. D., Kleinig H. Nuclear membranes from mammalian liver. I. Isolation procedure and general characterization. J Cell Biol. 1970 Aug;46(2):379–395. doi: 10.1083/jcb.46.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Kawai K., Asano S., Nakao M. Protein components of two different regions of an intestinal epithelial cell membrane. Regional singularities. Biochim Biophys Acta. 1973 Apr 25;307(1):141–151. doi: 10.1016/0005-2736(73)90032-1. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio N. A., Johnson C. B., Blecher M. Hormone receptors. 3. Properties of glucagon-binding proteins isolated from liver plasma membranes. J Biol Chem. 1974 Jan 25;249(2):428–437. [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. R. Insulin in body fluids other than blood. Physiol Rev. 1974 Jan;54(1):1–22. doi: 10.1152/physrev.1974.54.1.1. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House P. D., Poulis P., Weidemann M. J. Isolation of a plasma-membrane subfraction from rat liver containing an insulin-sensitive cyclic-AMP phosphodiesterase. Eur J Biochem. 1972 Jan 21;24(3):429–437. doi: 10.1111/j.1432-1033.1972.tb19703.x. [DOI] [PubMed] [Google Scholar]
- Ipata P. L. A coupled optical enzyme assay for 5'-nucleotidase. Anal Biochem. 1967 Jul;20(1):30–36. doi: 10.1016/0003-2697(67)90261-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Iype P. T., Bhargava P. M., Tasker A. D. Some aspects of the chemical and cellular composition of adult rat liver. Exp Cell Res. 1965 Nov;40(2):233–251. doi: 10.1016/0014-4827(65)90257-0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., MONIS B., ROSENBATT D., SELIGMAN A. M. Improvement in the histochemical localization of leucine aminopeptidase with a new substrate, L-leucyl-4-methoxy-2-naphthylamide. J Biophys Biochem Cytol. 1960 Apr;7:261–264. doi: 10.1083/jcb.7.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. Radioautographic comparison of the uptake of galactose-H and glucose-H3 in the golgi region of various cells secreting glycoproteins or mucopolysaccharides. J Cell Biol. 1966 Jul;30(1):137–150. doi: 10.1083/jcb.30.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Pekarthy J. M., Short J., Lansing A. I., Lieberman I. Function and control of liver alkaline phosphatase. J Biol Chem. 1972 Mar 25;247(6):1767–1774. [PubMed] [Google Scholar]
- Perdue J. A., Kletzien R., Miller K., Pridmore G., Wray V. L. The isolation and characterization of plasma membranes from cultured cells. II. The chemical composition of membrane isolated from uninfected and oncogenic RNA virus-converted parenchyma-like cells. Biochim Biophys Acta. 1971 Dec 3;249(2):435–453. doi: 10.1016/0005-2736(71)90121-0. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., La Mont J. T., Isselbacher K. J. Galactosyltransferase and concanavalin A agglutination of cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):904–908. doi: 10.1073/pnas.71.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P. D., Bradford H. F., McIlwain H. Stimulation and solubilization of the sodium ion-activated adenosine triphosphatase of cerebral microsomes by surface-active agents, especially polyoxyethylene ethers: actions of phospholipases and a neuraminidase. Biochem J. 1964 Aug;92(2):235–247. doi: 10.1042/bj0920235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweat F. W., Hupka A. Adenyl cyclase in hepatic parenchymal and reticuloendothelial cells. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1436–1442. doi: 10.1016/s0006-291x(71)80246-2. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Hill R. L. The purification and properties of the A protein of lactose synthetase. J Biol Chem. 1971 Nov;246(21):6666–6675. [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]




