Abstract
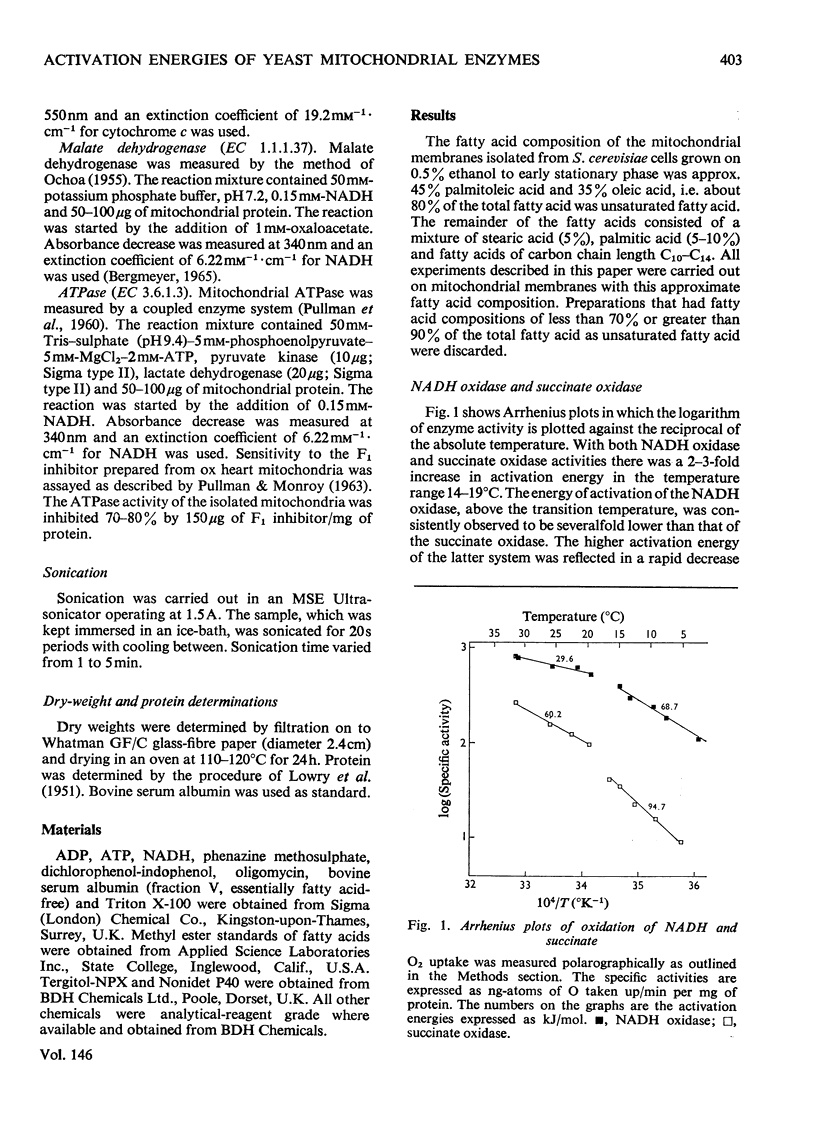
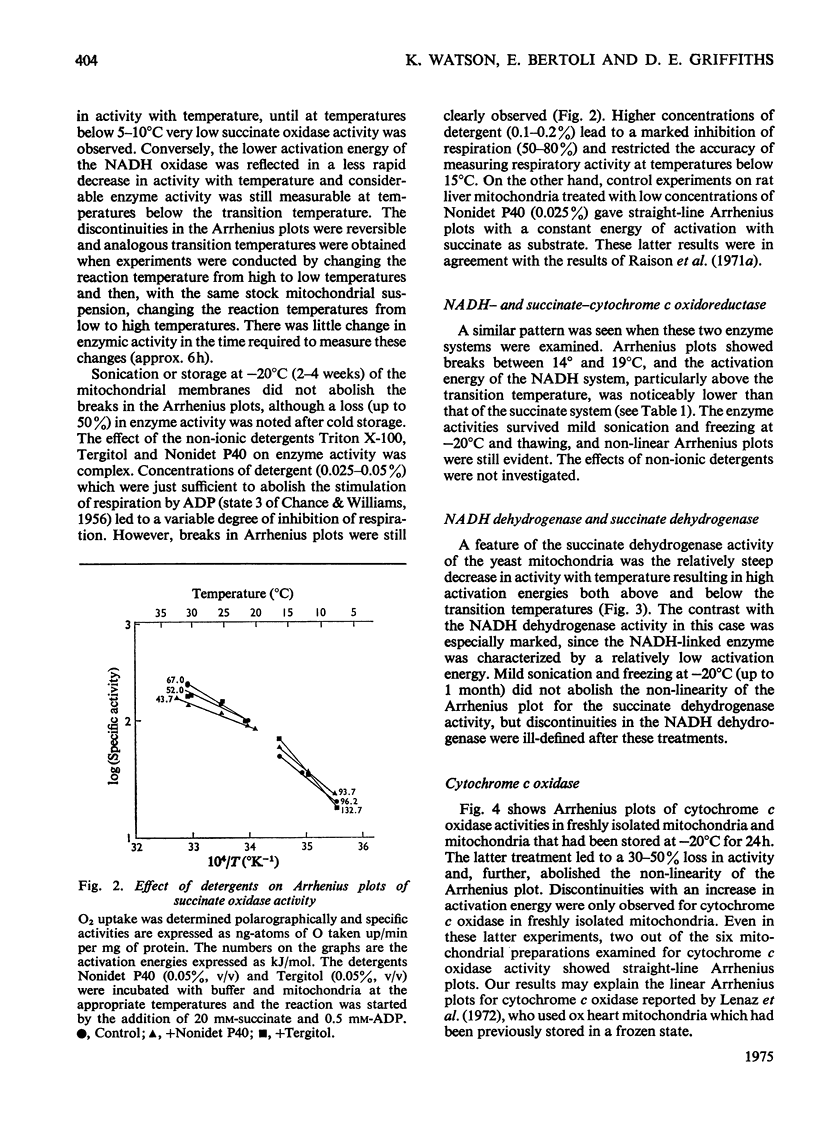
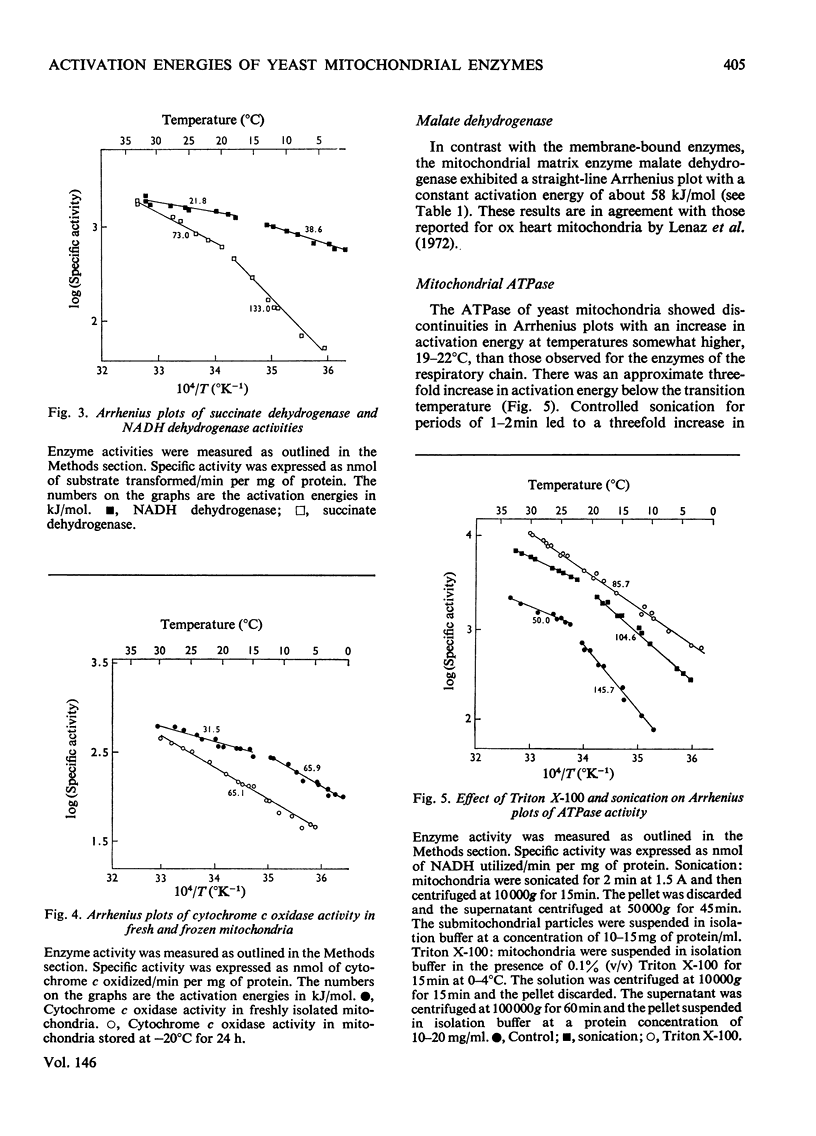
The effect of temperature on the activation energies of mitochondrial enzymes of the yeast Saccharomyces cerevisiae was examined. Non-linear Arrhenius plots with discontinuities in the temperature range 14-19 degrees C and 19-22 degrees C were observed for the respiratory enzymes and mitochondrial ATPase (adenosine triphosphatase) respectively. A straight-line Arrhenius plot was observed for the matrix enzyme, malate dehydrogenase. The activation energies of the enzymes associated with succinate oxidation, namely, succinate oxidase, succinate dehydrogenase and succinate-cytochrome c oxidoreductase, were in the range 60-85kJ/mol above the transition temperature and 90-160kJ/mol below the transition temperature. In contrast, the corresponding enzymes associated with NADH oxidation showed significantly lower activation energies, 20-35kJ/mol above and 40-85kJ/mol below the transition temperature. The discontinuities in the Arrhenius plots were still observed after sonication, treatment with non-ionic detergents or freezing and thawing of the mitochondrial membranes. Discontinuities for cytochrome c oxidase activity were only observed in freshly isolated mitochondria, and no distinct breaks were observed after storage at -20 degrees C. Mitochondrial ATPase activity still showed discontinuities after sonication and freezing and thawing, but a linear plot was observed after treatment with non-ionic detergents. The results indicate that the various enzymes of the respiratory chain are located in a similar lipid macroenvironment within the mitochondrial membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Ainsworth P. J., Tustanoff E. R., Ball A. J. Membrane phase (transitions) as a diagnostic tool for studying mitochondriogenesis. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1299–1305. doi: 10.1016/0006-291x(72)90214-8. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Eletr S., Keith A. D. Spin-label studies of dynamics of lipid alkyl chains in biological membranes: role of unsaturated sites. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1353–1357. doi: 10.1073/pnas.69.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenaz G., Sechi A. M., Parenti-Castelli G., Landi L., Bertoli E. Activation energies of different mitochondrial enzymes: breaks in Arrhenius plots of membrane-bound enzymes occur at different temperatures. Biochem Biophys Res Commun. 1972 Oct 17;49(2):536–542. doi: 10.1016/0006-291x(72)90444-5. [DOI] [PubMed] [Google Scholar]
- Massey V., Curti B., Ganther H. A temperature-dependent conformational change in D-amino acid oxidase and its effect on catalysis. J Biol Chem. 1966 May 25;241(10):2347–2357. [PubMed] [Google Scholar]
- Oldfield E., Chapman D., Derbyshire W. Lipid mobility in Acholeplasma membranes using deuteron magnetic resonance. Chem Phys Lipids. 1972 Jul;9(1):69–81. doi: 10.1016/0009-3084(72)90034-5. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Racker E. The two faces of the inner mitochondrial membrane. Essays Biochem. 1970;6:1–22. [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]
- Zeylemaker W. P., Jansen H., Veeger C., Slater E. C. Studies on succinate dehydrogenase. VII. The effect of temperature on the succinate oxidation. Biochim Biophys Acta. 1971 Jul 21;242(1):14–22. doi: 10.1016/0005-2744(71)90083-0. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Klingenberg M. Pathways of hydrogen in mitochondria of Saccharomyces carlsbergensis. Eur J Biochem. 1970 Feb;12(3):583–592. doi: 10.1111/j.1432-1033.1970.tb00890.x. [DOI] [PubMed] [Google Scholar]


