Abstract
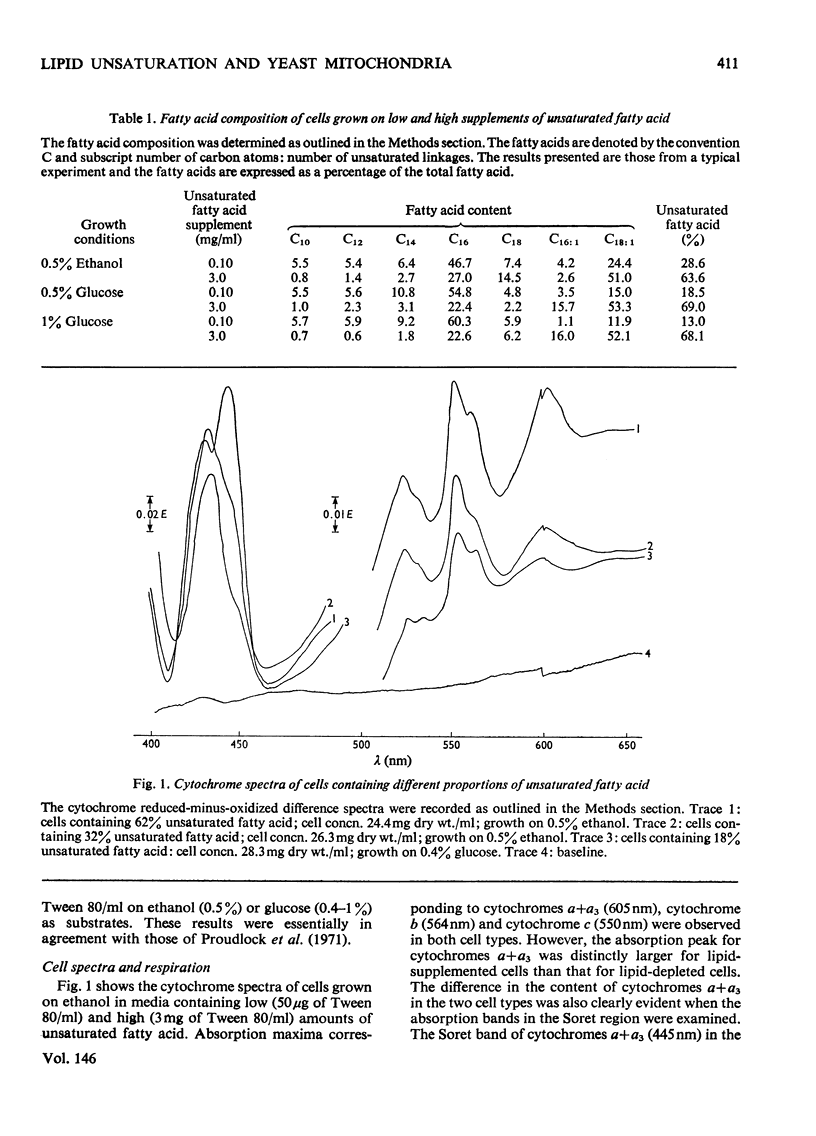
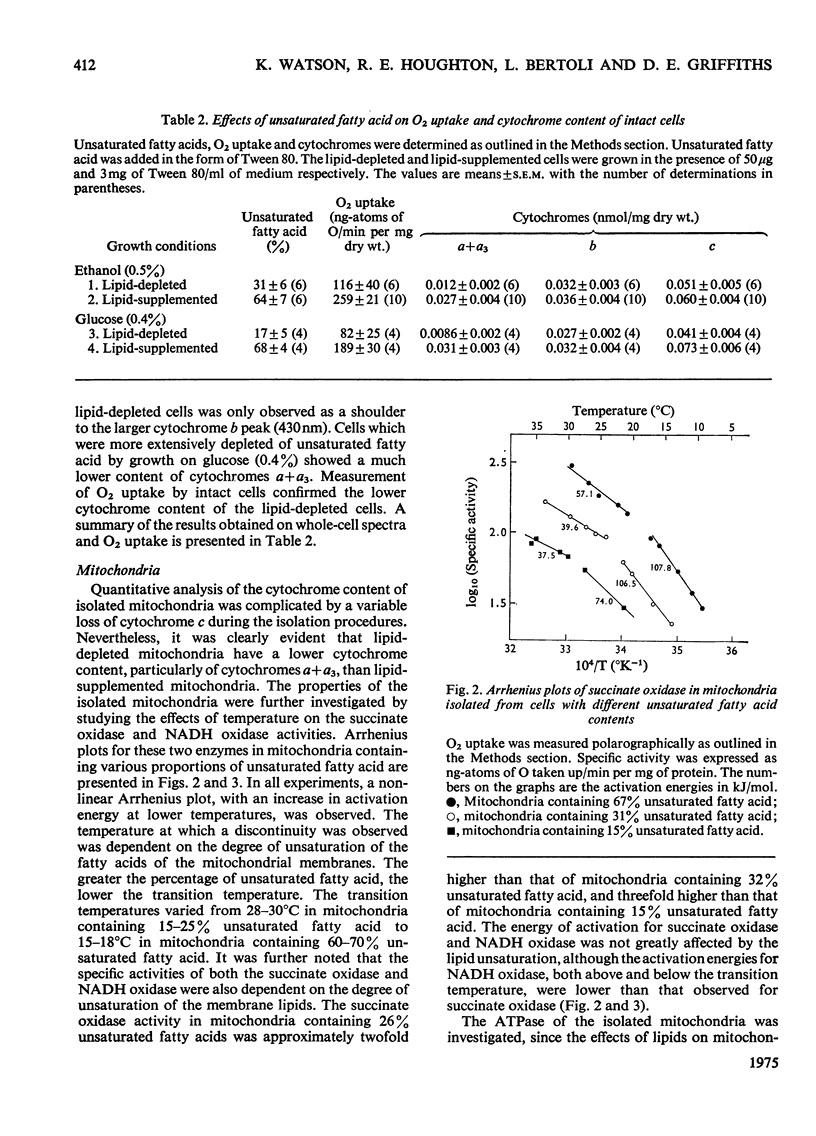
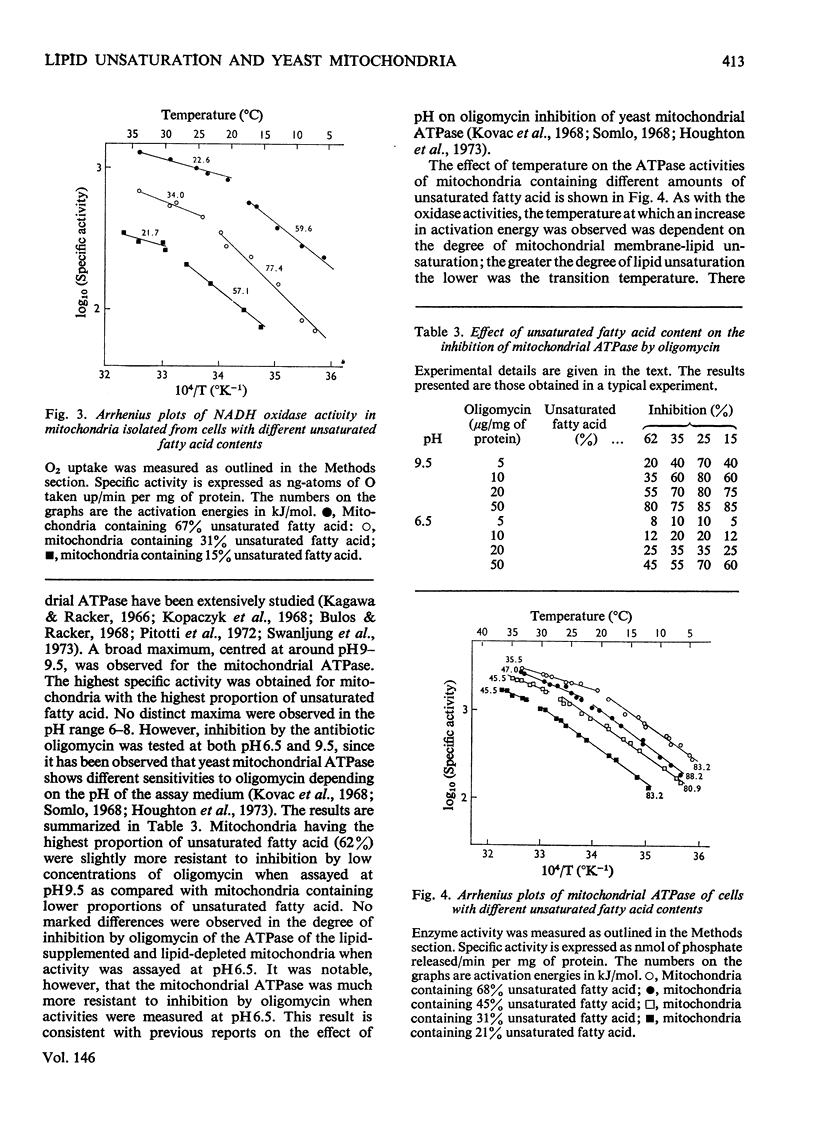
The lipid composition of yeast cells was manipulated by the use of an unsaturated fatty acid auxotroph of Saccharomyces cerevisiae. There was a 2-3-fold decrease in the concentration of cytochromes a+a3 when the unsaturated fatty acid content of the cells was decreased from 60-70% of the total fatty acid to 20-30%. The amounts of cytochromes b and c were also decreased under these conditions, but to a lesser extent. Further lipid depletion, to proportions of less than 20% unsaturated fatty acid, led to a dramatic decrease in the content of all cytochromes, particularly cytochromes a+a3. The ATPase (adenosine triphosphatase), succinate oxidase and NADH oxidase activities of the isolated mitochondria also varied with the degree of unsaturation of the membrane lipids. The lower the percentage of unsaturated fatty acid, the lower was the enzymic activity. Inhibition of mitochondrial ATPase by oligomycin, on the other hand, was not markedly influenced by the membrane-lipid unsaturation. Npn-linear Arrenius plots of mitochondrial membrane-bound enzymes showed transition temperatures that were dependent on the degree of membrane-lipid unsaturation. The greater the degree of lipid unsaturation, the lower was the transition temperature. It was concluded that the degree of unsaturation of the membrane lipids plays an important role in determining the properties of mitochondrial membrane-bound enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Ainsworth P. J., Tustanoff E. R., Ball A. J. Membrane phase (transitions) as a diagnostic tool for studying mitochondriogenesis. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1299–1305. doi: 10.1016/0006-291x(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XVII. Further resolution of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1968 Jul 25;243(14):3891–3900. [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- Eletr S., Zakim D., Vessey D. A. A spin-label study of the role of phospholipids in the regulation of membrane-bound microsomal enzymes. J Mol Biol. 1973 Aug 5;78(2):351–362. doi: 10.1016/0022-2836(73)90121-6. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHER S., BRIERLEY G., KLOUWEN H., SLAUTTERBACK D. B. Studies of the electron transfer system. 47. The role of phospholipids in electron transfer. J Biol Chem. 1962 Oct;237:3264–3272. [PubMed] [Google Scholar]
- Forrester I. T., Watson K., Linnane A. W. Mitochondrial membrane organisation, a determinant of mitochondrial ribosomal RNA synthesis. Biochem Biophys Res Commun. 1971 Apr 16;43(2):409–415. doi: 10.1016/0006-291x(71)90768-6. [DOI] [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R. Effect of lipid status on cytoplasmic and mitochondrial protein synthesis in anaerobic cultures of Saccharomyces cerevisiae. J Gen Microbiol. 1972 Sep;72(2):231–242. doi: 10.1099/00221287-72-2-231. [DOI] [PubMed] [Google Scholar]
- Green D. E., Tzagoloff A. Role of lipids in the structure and function of biological membranes. J Lipid Res. 1966 Sep;7(5):587–602. [PubMed] [Google Scholar]
- Guarnieri M., Johnson R. M., Du J. T. The unsaturated fatty acid content of mitochondria in relation to oxidation of exogenous reduced nicotinamide adenine dinucleotide. Biochim Biophys Acta. 1971 Apr 6;234(1):28–33. doi: 10.1016/0005-2728(71)90125-3. [DOI] [PubMed] [Google Scholar]
- Harder M. E., Beacham I. R., Cronan J. E., Jr, Beacham K., Honegger J. L., Silbert D. F. Temperature-sensitive mutants of Escherichia coli requiring saturated and unsaturated fatty acids for growth: isolation and properties. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3105–3109. doi: 10.1073/pnas.69.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam J. M., Proudlock J. W., Linnane A. W. Biogenesis of mitochondria. 20. The effects of altered membrane lipid composition on mitochondrial oxidative phosphorylation in Saccharomyces cerevisiae. J Bioenerg. 1971 Dec;2(5):351–370. doi: 10.1007/BF01963830. [DOI] [PubMed] [Google Scholar]
- Haslam J. M., Spithill T. W., Linnane A. W., Chappell J. B. Biogenesis of mitochondria. The effects of altered membrane lipid composition on cation transport by mitochondria of Saccharomyces cerevisiae. Biochem J. 1973 Aug;134(4):949–957. doi: 10.1042/bj1340949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURTSHUK P., Jr, SEKUZU I., GREEN D. E. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVI. ON THE FORMATION OF AN ACTIVE COMPLEX BETWEEN THE APO-D(--)-BETA-HYDROXYBUTYRIC DEHYDROGENASE AND MICELLAR LECITHIN. J Biol Chem. 1963 Nov;238:3595–3605. [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN H. P. Synthesis of lipids in resting cells of Saccharomyces cerevisiae. J Bacteriol. 1955 Jun;69(6):620–627. doi: 10.1128/jb.69.6.620-627.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Kandrach A., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J Biol Chem. 1973 Jan 25;248(2):676–684. [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. IX. Reconstruction of oligomycin-sensitive adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2467–2474. [PubMed] [Google Scholar]
- Keith A. D., Resnick M. R., Haley A. B. Fatty acid desaturase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 May;98(2):415–420. doi: 10.1128/jb.98.2.415-420.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Phospholipid requirements for (Na + + K + )-ATPase activity: head-group specificity and fatty acid fluidity. Biochim Biophys Acta. 1972 Sep 1;282(1):277–292. doi: 10.1016/0005-2736(72)90334-3. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopaczyk K., Asai J., Allmann D. W., Oda T., Green D. E. Resolution of the repeating unit of the inner mitochondrial membrane. Arch Biochem Biophys. 1968 Mar 11;123(3):602–621. doi: 10.1016/0003-9861(68)90181-1. [DOI] [PubMed] [Google Scholar]
- Lyons J. M., Wheaton T. A., Pratt H. K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964 Mar;39(2):262–268. doi: 10.1104/pp.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Mycoplasma membrane lipids: variations in fatty acid composition. Science. 1969 Apr 25;164(3878):433–434. doi: 10.1126/science.164.3878.433. [DOI] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van der Neut-Kok E. C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973 Mar 16;298(2):500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Williams M. A., Criddle R. S. Freeze-fracture studies on mitochondria from wild-type and respiratory-deficient yeasts. Biochim Biophys Acta. 1973 Jan 18;292(1):92–104. doi: 10.1016/0005-2728(73)90254-5. [DOI] [PubMed] [Google Scholar]
- Palatini P., Bruni A. Reversal by phospholipids of the oligomycin induced inhibition of membrane associated adenosintriphosphatases. Biochem Biophys Res Commun. 1970 Jul 13;40(1):186–191. doi: 10.1016/0006-291x(70)91064-8. [DOI] [PubMed] [Google Scholar]
- Paltauf F., Schatz G. Promitochondria of anaerobicallly grown yeast. II. Lipid composition. Biochemistry. 1969 Jan;8(1):335–339. doi: 10.1021/bi00829a046. [DOI] [PubMed] [Google Scholar]
- Pitotti A., Contessa A. R., Dabbeni-Sala F., Bruni A. Activation by phospholipids of particulate mitochondrial ATPase from rat liver. Biochim Biophys Acta. 1972 Aug 9;274(2):528–535. doi: 10.1016/0005-2736(72)90198-8. [DOI] [PubMed] [Google Scholar]
- Plattner H., Schatz G. Promitochondria of anaerobically grown yeast. 3. Morphology. Biochemistry. 1969 Jan;8(1):339–343. doi: 10.1021/bi00829a047. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 19. The effects of unsaturated fatty acid depletion on the lipid composition and energy metabolism of a fatty acid desaturase mutant of Saccharomyces cerevisiae. J Bioenerg. 1971 Dec;2(5):327–349. doi: 10.1007/BF01963829. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Mortimer R. K. Unsaturated fatty acid mutants of Saccharomyces cerevisiae. J Bacteriol. 1966 Sep;92(3):597–600. doi: 10.1128/jb.92.3.597-600.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlo M. Induction and repression of mitochondrial ATPase in yeast. Eur J Biochem. 1968 Jul;5(2):276–284. doi: 10.1111/j.1432-1033.1968.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Stancliff R. C., Williams M. A., Utsumi K., Packer L. Essential fatty acid deficiency and mitochondrial function. Arch Biochem Biophys. 1969 May;131(2):629–642. doi: 10.1016/0003-9861(69)90438-x. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanljung P., Frigeri L., Ohlson K., Ernster L. Studies on the activation of purified mitochondrial ATPase by phospholipids. Biochim Biophys Acta. 1973 Jun 28;305(3):519–533. doi: 10.1016/0005-2728(73)90073-x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. I. Characterization of some enzymes of the inner membrane of yeast mitochondria. J Biol Chem. 1969 Sep 25;244(18):5020–5026. [PubMed] [Google Scholar]
- Watson K., Bertoli E., Griffiths D. E. Phase transitions in yeast mitochondrial membranes. The effect of temperature on the energies of activation of the respiratory enzymes of Saccharomyces cerevisiae. Biochem J. 1975 Feb;146(2):401–407. doi: 10.1042/bj1460401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 13. The isolation of mitochondrial structures from anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1970 Jul;46(1):88–96. doi: 10.1083/jcb.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. The organization of ribosomal granules within mitochondrial structures of aerobic and anaerobic cells of Saccharomyces cerevisae. J Cell Biol. 1972 Dec;55(3):721–726. doi: 10.1083/jcb.55.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Stancliff R. C., Packer L., Keith A. D. Relation of unsaturated fatty acid composition of rat liver mitochondria to oscillation period, spin label motion, permeability and oxidative phosphorylation. Biochim Biophys Acta. 1972 Jun 23;267(3):444–456. doi: 10.1016/0005-2728(72)90172-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Epel D. The cytochrome system of sea urchin sperm. Arch Biochem Biophys. 1968 Jul;126(1):83–90. doi: 10.1016/0003-9861(68)90562-6. [DOI] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Fleischer S. Kinetic studies of the lipid requirement of mitochondrial cytochrome c oxidase. J Bioenerg. 1971 Aug;2(3):209–215. doi: 10.1007/BF01648915. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., de Greef W. J., van Eyk R. V., Demel R. A., van Deenen L. L. The effect of different fatty acid and sterol composition on the erythritol flux through the cell membrane of Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Mar 16;298(2):479–499. doi: 10.1016/0005-2736(73)90375-1. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]


