Abstract
Background
Patients who achieve return of spontaneous circulation (ROSC) after in‐hospital cardiac arrest (IHCA) may re‐arrest. This phenomenon has not been sufficiently investigated. The aim of this study was to examine the immediate (1‐min) and short‐term (20‐min) risks of re‐arrest in IHCA.
Methods
We retrospectively analyzed four datasets of IHCA episodes, comprising defibrillator recordings collected between 2002 and 2022. Re‐arrest was defined as the resumption of chest compressions following a period of ROSC after cardiac arrest of any duration. Parametric models were applied to calculate the immediate risk of re‐arrest. In addition, we estimated the short‐term risk of re‐arrest within 20 min.
Results
In 763 episodes of IHCA, we observed 316 re‐arrests: 68% to pulseless electrical activity (PEA), 25% to ventricular fibrillation/ventricular tachycardia (VF/VT), and 7% to asystole. Most re‐arrests occurred with the same rhythm as in the initial arrest. When ROSC was achieved from a non‐shockable rhythm, the risk of re‐arrest to a non‐shockable rhythm was initially 2% per minute and decreased to 1% per minute after 9 min. The corresponding risk of re‐arrest to VF/VT was constant at 2% per minute. If ROSC was obtained from a shockable rhythm, the risk of re‐arrest to a shockable rhythm was initially 5% per minute, decreasing to 4% per minute after 9 min. The corresponding risk to a non‐shockable rhythm was constant at 1% per minute. The risk of re‐arrest within 20 min was 27%, and the overall risk of at least one re‐arrest per episode was 33%.
Conclusions
The immediate risk of re‐arrest was approximately 2% per minute, with the highest risk occurring as a reversion to VF/VT if ROSC was obtained from VF/VT. The risk of re‐arrest within 20 min of the initial arrest was 27%, and the overall risk of at least one re‐arrest per episode was 33%.
Keywords: cardiac arrest, IHCA, parametric models, re‐arrest, ROSC
1. INTRODUCTION
Return of spontaneous circulation (ROSC) is achieved in 40%–70% of patients with in‐hospital cardiac arrest (IHCA), many of whom are critically ill and require intensive care. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 These patients are at risk of re‐arrest, defined as cardiac arrest (CA) after ROSC is obtained, including any non‐perfusing state and rhythm. 9 , 10 , 11 International guidelines for advanced life support (ALS) provide a good framework for clinical decision‐making. 12 , 13 Nevertheless, we lack knowledge about the risk of re‐arrest in the minutes immediately after ROSC. Previous studies investigating the occurrence of re‐arrest mainly focus on out‐of‐hospital cardiac arrest (OHCA), where it is reported that 18%–39% of patients obtaining ROSC will re‐arrest. 10 , 14 , 15 , 16 , 17 , 18 Most studies on re‐arrest after IHCA have used national registry data to investigate the occurrence of more than one CA event during a hospital stay, over a span of days and hours. 19 , 20 Bhardwaj et al. investigated the occurrence of re‐arrest in a dataset combining episodes of OHCA and IHCA and reported that it was a common complication, occurring in 24% of CA patients after a median time of 5.4 h. 21
Previous studies suggest that most patients who obtain ROSC after IHCA do so within the first 20 min. 3 , 4 , 22 The aim of this study was to examine the immediate (1‐min) as well as the short‐term (20‐min) risks of re‐arrest in IHCA, considering both the duration and the last observed state before ROSC.
2. METHODS
2.1. Study setting and population
We performed a post hoc analysis of four cohorts of adult patients (>18 years) suffering IHCA. The episodes were collected by emergency personnel at four different hospitals: St. Olav's University Hospital, Norway (two periods from 2009 to 2012 and 2018 to 2022), the University of Chicago Hospitals, USA (2002–2005), the Hospital of the University of Pennsylvania, USA (2008–2010), and the Penn Presbyterian Medical Center, USA (2008–2010). The episodes from Chicago and the first period at St. Olav's were analyzed as part of previous studies and later made available to us. All episodes from St. Olav's were collected for research purposes, while the episodes from the three US hospitals were collected as part of a quality assurance initiative.
The University of Chicago Hospitals (IL, USA) is an academic tertiary care facility. Two previously published studies investigated the quality of cardiopulmonary resuscitation (CPR) using a defibrillator with CPR‐sensing capabilities. 23 , 24 The current study includes episodes of IHCA from these studies. In Chicago, episodes were excluded if the arrest occurred in the emergency department (ED) or the operating room (OR), as these areas were not covered by the resuscitation teams. 23 St. Olav's University Hospital (Trondheim, Norway) is an academic tertiary care facility. All adult IHCA occurring between 2008 and 2013 were collected as part of an earlier study investigating clinical state transitions. 3 We collected additional episodes between 2018 and 2022. The Hospital of the University of Pennsylvania and the Penn Presbyterian Medical Center are both academic medical centers. All IHCA at these two hospitals were collected between 2008 and 2010, with the only inclusion criterion being age >18 years. The researchers responsible for data collection at all sites contribute as co‐authors.
2.2. Data collection and handling
The data consist of defibrillator recordings, including a one‐lead electrocardiogram (ECG) from the defibrillator pads, chest compressions, and ventilations during ongoing CPR. This information was combined with relevant details from patient records. The defibrillator recordings were manually assessed to determine the succession of clinical states during resuscitation. Details regarding the process of retrospective annotation of signal and event data from defibrillator files have been published earlier. 3 , 4 , 25 A recent methodological paper further elaborated on the potential utility of this approach. 26
The start of an episode was defined as the time of the first registered chest compression, detected either as fluctuations in the transthoracic impedance signal or the compression depth signal. 27 , 28 We assessed the ECG during every compression pause to determine the clinical state of the patient.
ROSC was defined as a period of regular QRS complexes that lasted at least 1 min in the absence of chest compressions. It was divided into two groups based on whether the rhythm preceding ROSC was shockable or not. As we do not possess measurements of invasive blood pressure, this 1‐min cut‐off is a pragmatic approach to retrospective ROSC detection, as the resuscitation team would hardly allow a hands‐off time of that duration without the presence of a palpable pulse.
We defined re‐arrest as the resumption of chest compressions subsequent to a period of ROSC. Sustained ROSC is defined in the Utstein guidelines as ROSC lasting more than 20 min. 3 , 4 , 29 Therefore, a new episode of IHCA was declared if the patient experienced a second CA after at least 20 min of ROSC.
PEA was defined as an ECG with regular QRS complexes with a frequency of ≥12 QRS/minute between compressions. This corresponds to at least one QRS every 5 s. Asystole was defined as an isoelectric (“flat”) ECG line or as a rhythm slower than 12 QRS complexes per minute. This cut‐off time was chosen to make it possible to distinguish PEA from asystole during a normal rhythm check. We categorized ventricular tachycardia (VT) and ventricular fibrillation (VF) based on their unique morphologies. 30 We knew from the patient record whether the patient obtained ROSC or was eventually declared dead at the end of an episode. Depending on this information, both ROSC and death were defined from the last compression recorded. The episodes from Chicago and the first period at St. Olav's (2008–2013) were annotated with the same exact criteria as part of a previous study. 3
2.3. Statistical methods and modeling
We investigated any potential time variability for the immediate risk of re‐arrest by estimating the hazard of a spontaneous re‐arrest (from ROSC) to either a shockable or a non‐shockable rhythm that occurred within 20 min of the initial arrest. The hazard rate, or simply hazard, is defined as the conditional probability of a re‐arrest in the next instant, given that the patient is still in ROSC. In the supplementary file, we show how the hazard approximately corresponds to the one‐minute (i.e., immediate) probability for hazard values lower than 0.1. For example, a hazard of 0.02 corresponds to a one‐minute probability of 2%. The shape of the hazard function for re‐arrest over time could develop in several ways, for instance: rising, falling, constant, or unimodal (i.e., rising and then falling). A constant hazard can be modeled with the exponential distribution, while a monotonically rising or falling hazard can be modeled with the Weibull distribution. 31 The Greenwich and lognormal distributions are both suitable for modeling a unimodal course. 31 , 32 We compared these models for each type of re‐arrest to find the most useful model while avoiding overfitting. This approach for model selection is described in detail in the supplemental material of a previous publication from our group. 25
We used a Kaplan–Meier estimator to illustrate the proportion of patients remaining in ROSC up to 20 min. 33 A mixed‐effects Poisson regression model with episode as a random factor was applied to estimate the total risk of re‐arrest per episode, suitable for investigating events clustered within episodes. 34
We applied the software Stata version 17 and R version 4.2.2 for all visualizations and statistical analysis. 35 , 36
2.4. Ethical aspects
This study is based purely on a retrospective analysis of observational data, and inclusion would neither benefit nor disadvantage the patient. All episodes were de‐identified, and the patients remained anonymous during the subsequent data analysis.
The Regional Ethics Committee (REC) granted approval for the collection and analysis of data from both periods at St. Olav's University Hospital. The data collected at the Penn Presbyterian Medical Center and the Hospital of the University of Pennsylvania were provided to us in anonymized form, and the collection of these data was approved by the local institutional review board (IRB).
The present study also represents a novel analysis of anonymous data collected in Chicago between 2002 and 2005 and analyzed by Laerdal Medical in collaboration with the University of Stavanger, originally published in earlier studies. 3 , 24 The original Principal Investigators actively contributed as authors and obtained IRB approval for both primary and secondary analyses at their institutions. Additional ethical approval in Norway was not deemed necessary due to the purely observational and de‐identified nature of the data, and the significant time that has elapsed since data collection.
3. RESULTS
Defibrillator recordings from 763 episodes of IHCA in 703 different patients were included: 160 episodes from the first period at St. Olav's University Hospital (2009–2013), 203 from the second period at St. Olav's University Hospital (2018–2022), 159 from the University of Chicago Medicine, 187 from the Hospital of the University of Pennsylvania, and 54 from the Penn Presbyterian Medical Center. In the 763 episodes, 506 contained at least one period of ROSC.
An overview of the episode and patient characteristics is given in Table 1. The lower part of the table shows the absolute number of re‐arrests stratified by the preceding state and the rhythm that patients presented with during the re‐arrest. In the 316 re‐arrests, 88% recurred to the last observed rhythm before ROSC if ROSC was obtained from a non‐shockable rhythm, and 73% recurred to the last observed rhythm before ROSC if ROSC was obtained from a shockable rhythm.
TABLE 1.
Episode and patient characteristics.
| Patient characteristics (n = 703) | |||
| Median age (years) | 67 (IQR 56–77) | ||
| Sex | 401 male (57%) | ||
| Episode characteristics (n = 763) | |||
| Episodes containing at least one period of ROSC | 506 (66%) | ||
| Episodes containing at least one re‐arrest | 176 (23%) | ||
| Monitored episodes | 485 (64%) | ||
| Obtained sustained ROSC | 384 (50%) | ||
| Presumed cardiac etiology | 330 (43%) | ||
| Survived to discharge | 124 (16%) | ||
| Rhythm of re‐arrest | |||
| PEA | 216 (68%) | ||
| VF/VT | 78 (25%) | ||
| Asystole | 22 (7%) | ||
| Re‐arrests stratified by preceding state | To non‐shockable | To shockable | Sum |
| ROSC from non‐shockable | 220 | 30 | 250 |
| ROSC from shockable | 18 | 48 | 66 |
| Sum | 238 | 78 | 316 |
Since both ROSC and re‐arrests could occur multiple times during an episode, Figure 1 illustrates the distribution of episodes with ROSC (left) and re‐arrests (right). We observed that 75% (n = 380) of the episodes with ROSC contained a single period, while 63% (n = 110) of the episodes with re‐arrests included one such event.
FIGURE 1.
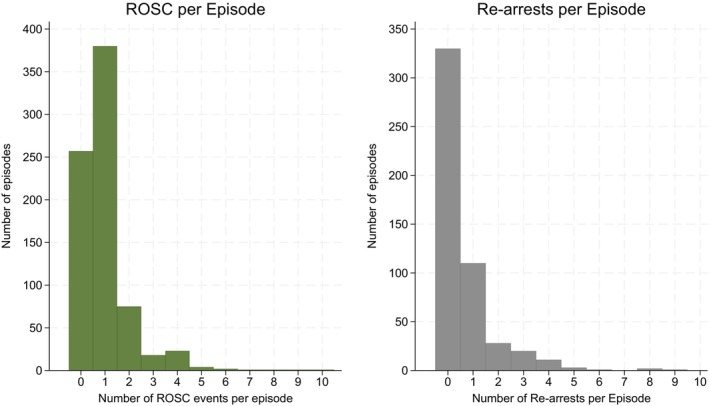
Distribution of the number of periods with return of spontaneous circulation (ROSC) and re‐arrests per episode. Left: Histogram of the number of ROSC events across episodes. The majority of episodes had only one event. Right: Histogram of the number of re‐arrests across episodes. Most episodes contained no or a single re‐arrest.
The immediate, minute‐by‐minute, hazard of re‐arrest may vary with time and possibly differ depending on the nature of re‐arrest and the rhythm that preceded ROSC. Figure 2 shows the hazard functions for re‐arrest to PEA or asystole depending on whether ROSC was preceded by a non‐shockable (left graph) or shockable rhythm (right graph) during the first 20 min after the initial arrest. The hazard of re‐arrest to a non‐shockable rhythm, if ROSC had been preceded by a non‐shockable rhythm, decreased slightly from 0.02 for the first 6 min to 0.01 from 9 to 18 min after the initial event of CA. If ROSC was preceded by VF/VT, the hazard of re‐arrest to a non‐shockable rhythm remained constant at 0.02.
FIGURE 2.
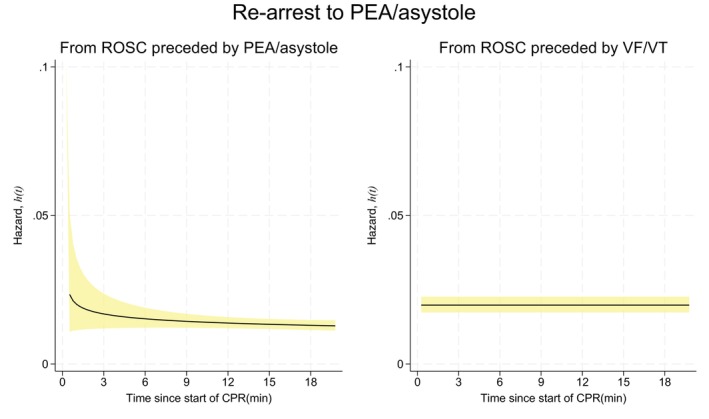
Hazard functions with 95% confidence intervals (CI) for re‐arrest to a non‐shockable rhythm. Left: Hazard if return of spontaneous circulation (ROSC) was preceded by a non‐shockable rhythm. Right: Hazard if ROSC was preceded by a shockable rhythm.
Figure 3 shows the hazard of re‐arrest to VF/VT depending on whether ROSC was preceded by a non‐shockable (left graph) or a shockable rhythm (right graph). ROSC preceded by VF/VT had a hazard of 0.05 for re‐arrest to VF/VT at 3 min, decreasing to 0.04 at 6 min and 0.03 at 12 min. The hazard of re‐arrest to VF/VT, if ROSC was preceded by a non‐shockable rhythm, decreased from about 0.02 during the first 6 min before stabilizing at 0.01 per minute.
FIGURE 3.
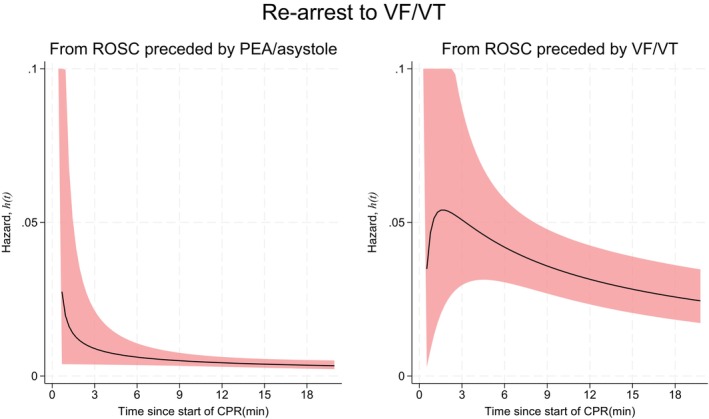
Hazard functions with 95% confidence intervals (CI) for re‐arrest to a shockable rhythm. Left: Hazard if return of spontaneous circulation (ROSC) was preceded by a non‐shockable rhythm. Right: Hazard if ROSC was preceded by a shockable rhythm.
At any one of the time points displayed in Figures 2 and 3, the hazard can be interpreted directly as the approximate probability of a re‐arrest in the next minute. A patient who obtained ROSC from VF/VT and was still in ROSC 3 min after the initial arrest had an approximate 5% probability of a re‐arrest back to VF/VT in the next minute (Figure 3). The same patient had a 2% probability of a re‐arrest to a non‐shockable rhythm (Figure 2). A patient who obtained ROSC from PEA or asystole and was still in ROSC 3 min after the initial arrest had a 1% probability of re‐arrest to VF/VT (Figure 3) and a 2% probability of re‐arrest to a non‐shockable rhythm (Figure 2).
The total risk of re‐arrest in the 506 episodes with ROSC can be reported in three distinct ways. Firstly, by the total number of observed re‐arrests, which was 316 out of 506 episodes (62%; 95% confidence interval (CI), 58%–67%). Secondly, by the proportion of episodes that contained at least one re‐arrest, observed in 176 of 506 episodes (35%; 95% CI, 31%–39%). Thirdly, by the mixed‐effects Poisson model, which yielded an estimated expected proportion of 33% (95% CI, 26%–41%) with significant variability between episodes (p < 0.001).
Figure 4 provides an overall view of the process and illustrates the proportion of patients who were still in ROSC within 20 min of the initial arrest, amounting to 73% (95% CI: 68%–77%) after this period. This corresponds to a 27% probability of re‐arrest within 20 min (1–0.73 = 0.27).
FIGURE 4.
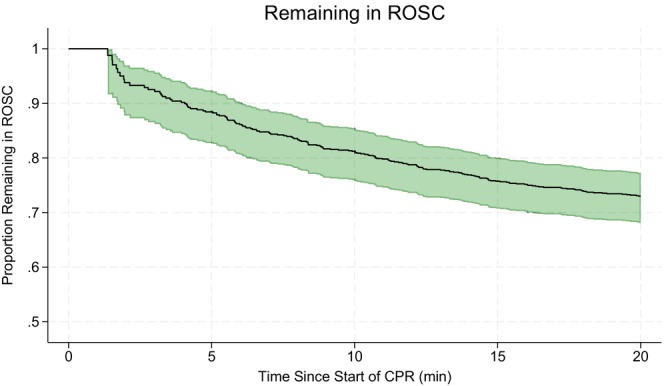
The proportion of patients who maintain return of spontaneous circulation (ROSC) over time after the initial arrest, amounting to 73% after 20 min. Only the first re‐arrest per episode is considered. The green area represents the 95% confidence interval (CI).
4. DISCUSSION
This is the first investigation of the immediate and short‐term risks of re‐arrest from ROSC during IHCA. The main finding is that the immediate risk of re‐arrest was approximately 2% per minute during the first 20 min after the initial arrest, depending on the time since the initial event and the rhythm leading to ROSC. The probability of a re‐arrest per episode was 27% within 20 min and 33% in total. As in OHCA, the rhythm of re‐arrest primarily mirrored the initial rhythm. 10 , 18 , 37
When considering the two analyses provided (immediate vs. total probability of re‐arrest), it is important to note that they provide different kinds of information to the clinician at the bedside. The immediate, 1‐min, perspective is highly dynamic, while the perspective of total probability within 20 min captures the episode as a whole. The latter thus disregards the different dynamics of shockable and non‐shockable rhythms.
The highest risk of re‐arrest was observed for re‐arrest to VF/VT in patients who initially obtained ROSC from VF/VT, and the hazard decreased with time. The probability of re‐arrest from ROSC preceded by PEA/asystole was lower than in the VF/VT group throughout the period of observation. In a clinical context, this reflects the fact that in‐hospital VF/VT often receives early defibrillation, but that the underlying cause, for example, coronary ischemic events, is seldom treated at this point, and thus, they often re‐arrest back to VF/VT. Bhardwaj et al. previously reported that 56% of patients who re‐arrest after an initial arrest with VF/VT present with VF/VT again during their stay. 21 Furthermore, a shockable re‐arrest rhythm is associated with a better outcome in OHCA. 10 , 16 , 38 In arrests with PEA, ROSC may be achieved when an underlying cause has been more or less reversed, and thus, the probability of immediate re‐arrest may be lower. 4 , 39 , 40
This study provides a description of reality and quantifies the risk of re‐arrest after achieving ROSC. The findings correlate well with clinical experience and support the current practice regarding post‐ROSC treatment. 41 Achieving ROSC from PEA indicates that the treatment of the underlying cause is on the right course. Nevertheless, if the patient re‐arrests to PEA, a recent study from our group revealed that the probability of obtaining ROSC again in that scenario is quite high at 26% within the first 3 min. 25
The observed short‐term cumulative risk of re‐arrest at 27% within 20 min is not trivial. Bhardwaj et al. reported that 24% of CA patients re‐arrested during subsequent hospitalization when re‐arrest was defined as the loss of pulse and receipt of CPR after ≥20 min of sustained ROSC. 21 Our finding of a similar re‐arrest proportion within 20 min highlights the very dynamic nature of this early period. It is important to note that the total probability represents an average and is elevated by some patients who experience multiple events of ROSC and re‐arrest. We get an impression of the magnitude of this phenomenon in Figure 1 and how this affects the results by comparing the total number of observed re‐arrests (62%) with the proportion of episodes that contain at least one re‐arrest (35%) and the estimate from the Poisson model (33%).
It is necessary to point out that PEA is a condition that cannot be diagnosed by ECG alone. ROSC and PEA may represent a continuum of increasing severity. Pulse palpation in either the femoral or carotid artery is the current method used to distinguish PEA from ROSC, although previous studies have shown that manual pulse palpation can be inaccurate. 42 , 43 , 44 , 45 Measurement of end‐tidal CO2 (ETCO2) is a helpful supplement, as an abrupt rise indicates ROSC. 46 , 47 , 48 , 49 A recent experimental study shows promising results from applying a non‐invasive carotid Doppler to detect ROSC. 50 The European Resuscitation Council (ERC) recommends that intra‐arterial blood pressure monitoring be established as a part of post‐resuscitation care. 41 Investigating rates of re‐arrest using the above‐mentioned measurements would be an interesting topic for future studies.
5. LIMITATIONS AND STRENGTHS
Due to the lack of objective circulation measurement, our retrospective classification of ROSC may have resulted in an underestimation, as we disregarded very brief periods of ROSC lasting less than 1 min. Furthermore, we included all data available to us, collected over 20 years at four different hospitals. This has two methodological consequences. Firstly, we lack information on all the circumstances of IHCA and do not possess data on missed IHCA cases. Secondly, the guidelines have been updated several times during the period, albeit mainly focusing on optimizing existing protocols and making defibrillators widely available. 13 , 51 , 52 , 53 Furthermore, we have estimated the risk of re‐arrest at the population level rather than for individuals. The latter would require an approach similar to what Norvik and coworkers did when taking the ECG development during PEA into account. 54
The primary strengths of this study include the unique availability of objective, time‐stamped data from defibrillators, which closely reflect the real‐time clinical conditions at the bedside. Additionally, the large sample size from four general hospitals ensures coverage of many different IHCA scenarios.
6. CONCLUSION
The immediate risk of re‐arrest from ROSC during IHCA was approximately 2% per minute. The short‐term risk of re‐arrest was 27% within 20 min and 33% in total. Patients tended to re‐arrest with the same rhythm from which ROSC was initially obtained. The highest risk of re‐arrest was to VF/VT if ROSC was obtained from VF/VT. Different risks of re‐arrest may reflect the degree to which the underlying cause of CA has been addressed.
AUTHOR CONTRIBUTIONS
E. Unneland: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing—Original Draft, Visualization A. Norvik: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing—Review & Editing D. Bergum: Conceptualization, Investigation, Data Curation, Writing—Review & Editing, Supervision D.G. Buckler: Data Curation, Writing—Review & Editing A. Bhardwaj: Writing—Review & Editing T. Eftestøl: Writing—Review & Editing Elisabete Aramendi: Conceptualization, Software, Writing—Review & Editing T. Nordseth: Conceptualization, Investigation, Data Curation, Writing—Review & Editing B. Abella: Conceptualization, Writing—Review & Editing J.T Kvaløy: Methodology, Writing—Review & Editing E. Skogvoll: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing—Review & Editing, Supervision, Project administration, Funding acquisition.
FUNDING INFORMATION
This work received support from the Faculty of Medicine and Health Science at NTNU and the Norwegian Research Council. Additionally, we received support from the Ministerio de Ciencia, Innovación y Universidades through grant RTI2018‐101475‐B100, jointly with the Fondo Europeo de Desarrollo Regional (FEDER). The study was also supported by the Basque Government through grant IT1229‐19. Laerdal Medical Inc. provided the research defibrillator used for data collection in Chicago. The authors received no funding from Laerdal for the current study.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Supporting information
Data S1.
ACKNOWLEDGEMENTS
We would like to extend our gratitude to Helge Myklebust, Director of Research at Laerdal Medical Inc., for providing access to data from the University of Chicago Hospitals. We also appreciate the staff at the Research Unit at St. Olav's University Hospital for their diligent documentation of emergency team callouts. We would also like to thank Arne Henriksen for his help with proofreading. Finally, we honor the invaluable contributions of the late Unai Irusta, Associate Professor at the University of the Basque Country.
Unneland E, Norvik A, Bergum D, et al. Re‐arrest immediately after return of spontaneous circulation: A retrospective observational study of in‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2025;69(1):e14567. doi: 10.1111/aas.14567
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In‐hospital cardiac arrest: a review. JAMA. 2019;321(12):1200‐1210. doi: 10.1001/jama.2019.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chon GR, Lee J, Shin Y, et al. Clinical outcomes of witnessed and monitored cases of in‐hospital cardiac arrest in the general ward of a university hospital in Korea. Respir Care. 2013;58(11):1937‐1944. doi: 10.4187/respcare.02448 [DOI] [PubMed] [Google Scholar]
- 3. Nordseth T, Bergum D, Edelson DP, et al. Clinical state transitions during advanced life support (ALS) in in‐hospital cardiac arrest. Resuscitation. 2013;84(9):1238‐1244. doi: 10.1016/j.resuscitation.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 4. Norvik A, Unneland E, Bergum D, et al. Pulseless electrical activity in in‐hospital cardiac arrest–a crossroad for decisions. Resuscitation. 2022;176:117‐124. doi: 10.1016/j.resuscitation.2022.04.024 [DOI] [PubMed] [Google Scholar]
- 5. Tirkkonen J, Hellevuo H, Olkkola KT, Hoppu S. Aetiology of in‐hospital cardiac arrest on general wards. Resuscitation. 2016;107:19‐24. doi: 10.1016/j.resuscitation.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 6. Tran S, Deacon N, Minokadeh A, et al. Frequency and survival pattern of in‐hospital cardiac arrests: the impacts of etiology and timing. Resuscitation. 2016;107:13‐18. doi: 10.1016/j.resuscitation.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang MT, Huang WC, Yen DHT, Yeh EH, Wu SY, Liao HH. The potential risk factors for mortality in patients after in‐hospital cardiac arrest: a multicenter study. Front Cardiovasc Med. 2021;8:630102. doi: 10.3389/fcvm.2021.630102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamada A, Takeuchi Y, Nishizaki Y, Daida H. Bag‐valve‐mask ventilation with airway adjuncts improves neurological outcomes of in‐hospital cardiac arrest. Intern Med. 2012;51(12):1517‐1521. doi: 10.2169/internalmedicine.51.7015 [DOI] [PubMed] [Google Scholar]
- 9. Hartke A, Mumma BE, Rittenberger JC, Callaway CW, Guyette FX. Incidence of re‐arrest and critical events during prolonged transport of post‐cardiac arrest patients. Resuscitation. 2010;81(8):938‐942. doi: 10.1016/j.resuscitation.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salcido DD, Stephenson AM, Condle JP, Callaway CW, Menegazzi JJ. Incidence of rearrest after return of spontaneous circulation in out‐of‐hospital cardiac arrest. Prehosp Emerg Care. 2010;14(4):413‐418. doi: 10.3109/10903127.2010.497902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon H, Ahn KO, Park JH, Lee SY. Effects of pre‐hospital re‐arrest on outcomes based on transfer to a heart attack centre in patients with out‐of‐hospital cardiac arrest. Resuscitation. 2022;170:107‐114. doi: 10.1016/j.resuscitation.2021.11.012 [DOI] [PubMed] [Google Scholar]
- 12. Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S366‐S468. doi: 10.1161/cir.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 13. Soar J, Böttiger BW, Carli P, et al. European resuscitation council guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115‐151. doi: 10.1016/j.resuscitation.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 14. Han KS, Kim SJ, Lee EJ, Lee SW. The effect of extracorporeal cardiopulmonary resuscitation in re‐arrest after survival event: a retrospective analysis. Perfusion. 2020;35(1):39‐47. doi: 10.1177/0267659119850679 [DOI] [PubMed] [Google Scholar]
- 15. Jung YH, Jeung KW, Lee HY, et al. Rearrest during hospitalisation in adult comatose out‐of‐hospital cardiac arrest patients: risk factors and prognostic impact, and predictors of favourable long‐term outcomes. Resuscitation. 2022;170:150‐159. doi: 10.1016/j.resuscitation.2021.11.037 [DOI] [PubMed] [Google Scholar]
- 16. Woo JH, Cho JS, Lee CA, et al. Survival and Rearrest in out‐of‐hospital cardiac arrest patients with prehospital return of spontaneous circulation: a prospective multi‐regional observational study. Prehosp Emerg Care. 2021;25(1):59‐66. doi: 10.1080/10903127.2020.1733716 [DOI] [PubMed] [Google Scholar]
- 17. Brooke Lerner E, O'Connell M, Pirrallo RG. Rearrest after prehospital resuscitation. Prehosp Emerg Care. 2011;15(1):50‐54. doi: 10.3109/10903127.2010.519820 [DOI] [PubMed] [Google Scholar]
- 18. Shibahashi K, Nonoguchi N, Inoue K, Kato T, Sugiyama K. Incidence, risk factors, and impact of post‐return of spontaneous circulation events in patients with out‐of‐hospital cardiac arrest: a population‐based study in Tokyo, Japan. Resuscitation. 2024;202:110303. doi: 10.1016/j.resuscitation.2024.110303 [DOI] [PubMed] [Google Scholar]
- 19. Kazaure HS, Roman SA, Sosa JA. A population‐level analysis of 5620 recipients of multiple in‐hospital cardiopulmonary resuscitation attempts. J Hosp Med. 2014;9(1):29‐34. doi: 10.1002/jhm.2127 [DOI] [PubMed] [Google Scholar]
- 20. Menon PR, Ehlenbach WJ, Ford DW, Stapleton RD. Multiple in‐hospital resuscitation efforts in the elderly. Crit Care Med. 2014;42(1):108‐117. doi: 10.1097/CCM.0b013e31829eb937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhardwaj A, Ikeda DJ, Grossestreuer AV, et al. Factors associated with re‐arrest following initial resuscitation from cardiac arrest. Resuscitation. 2017;111:90‐95. doi: 10.1016/j.resuscitation.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 22. Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in‐hospital cardiac arrest. Crit Care Med. 2010;38(1):101‐108. doi: 10.1097/CCM.0b013e3181b43282 [DOI] [PubMed] [Google Scholar]
- 23. Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in‐hospital cardiac arrest using a real‐time audiovisual feedback system. Resuscitation. 2007;73(1):54‐61. doi: 10.1016/j.resuscitation.2006.10.027 [DOI] [PubMed] [Google Scholar]
- 24. Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest. JAMA. 2005;293(3):305‐310. doi: 10.1001/jama.293.3.305 [DOI] [PubMed] [Google Scholar]
- 25. Unneland E, Norvik A, Bergum D, et al. Non‐shockable rhythms: a parametric model for the immediate probability of return of spontaneous circulation. Resuscitation. 2023;191:109895. doi: 10.1016/j.resuscitation.2023.109895 [DOI] [PubMed] [Google Scholar]
- 26. Nordseth T, Eftestøl T, Aramendi E, Kvaløy JT, Skogvoll E. Extracting physiologic and clinical data from defibrillators for research purposes to improve treatment for patients in cardiac arrest. Resuscitation Plus. 2024;18:100611. doi: 10.1016/j.resplu.2024.100611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonso E, Ruiz J, Aramendi E, et al. Reliability and accuracy of the thoracic impedance signal for measuring cardiopulmonary resuscitation quality metrics. Resuscitation. 2015;88:28‐34. doi: 10.1016/j.resuscitation.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 28. Ayala U, Eftestøl T, Alonso E, et al. Automatic detection of chest compressions for the assessment of CPR‐quality parameters. Resuscitation. 2014;85(7):957‐963. doi: 10.1016/j.resuscitation.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 29. Cummins RO, Chamberlain D, Hazinski MF, et al. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital ‘Utstein style’. Circulation. 1997;95(8):2213‐2239. doi: 10.1161/01.CIR.95.8.2213 [DOI] [PubMed] [Google Scholar]
- 30. Curtis MJ, Hancox JC, Farkas A, et al. The Lambeth conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther. 2013;139(2):213‐248. doi: 10.1016/j.pharmthera.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 31. Rosner B. Fundamentals of biostatistics. Fundamentals of Biostatistics. Eight ed. Cengage Learning; 2016:825. [Google Scholar]
- 32. Greenwich M. A unimodal hazard rate function and its failure distribution. Stat Pap. 1992;33(1):187‐202. doi: 10.1007/BF02925324 [DOI] [Google Scholar]
- 33. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457‐481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 34. Rabe‐Hesketh & Skrondal . Multilevel and Longitudinal Modeling Using Stata. 3rd ed. Stata Press; 2012. [Google Scholar]
- 35. StataCorp . StataCorp, College Station. 2024. https://www.stata.com. [Google Scholar]
- 36. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2024. [Google Scholar]
- 37. Yamashita A, Kurosaki H, Takada K, et al. Prehospital epinephrine as a potential factor associated with prehospital rearrest. Prehosp Emerg Care. 2020;24(6):741‐750. doi: 10.1080/10903127.2020.1725197 [DOI] [PubMed] [Google Scholar]
- 38. Shin H, Kim G, Lee Y, et al. Can we predict good survival outcomes by classifying initial and Re‐arrest rhythm change patterns in out‐of‐hospital cardiac arrest settings? Cureus. 2020;12(12):e12019. doi: 10.7759/cureus.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabjohns J, Quan T, Boniface K, Pourmand A. Pseudo‐pulseless electrical activity in the emergency department, an evidence based approach. Am J Emerg Med. 2020;38(2):371‐375. doi: 10.1016/j.ajem.2019.158503 [DOI] [PubMed] [Google Scholar]
- 40. Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726‐1734. doi: 10.1056/NEJMra1208943 [DOI] [PubMed] [Google Scholar]
- 41. Nolan JP, Sandroni C, Böttiger BW, et al. European resuscitation council and European Society of Intensive Care Medicine guidelines 2021: post‐resuscitation care. Intensive Care Med. 2021;47(4):369‐421. doi: 10.1007/s00134-021-06368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bahr J, Klingler H, Panzer W, Rode H, Kettler D. Skills of lay people in checking the carotid pulse. Resuscitation. 1997;35(1):23‐26. doi: 10.1016/s0300-9572(96)01092-1 [DOI] [PubMed] [Google Scholar]
- 43. Dick WF, Eberle B, Wisser G, Schneider T. The carotid pulse check revisited: what if there is no pulse? Crit Care Med. 2000;28(11 Suppl):N183‐N185. doi: 10.1097/00003246-200011001-00002 [DOI] [PubMed] [Google Scholar]
- 44. Moule P. Checking the carotid pulse: diagnostic accuracy in students of the healthcare professions. Resuscitation. 2000;44(3):195‐201. doi: 10.1016/s0300-9572(00)00139-8 [DOI] [PubMed] [Google Scholar]
- 45. Ochoa FJ, Ramalle‐Gómara E, Carpintero JM, García A, Saralegui I. Competence of health professionals to check the carotid pulse. Resuscitation. 1998;37(3):173‐175. doi: 10.1016/s0300-9572(98)00055-0 [DOI] [PubMed] [Google Scholar]
- 46. Crickmer M, Drennan IR, Turner L, Cheskes S. The association between end‐tidal CO2 and return of spontaneous circulation after out‐of‐hospital cardiac arrest with pulseless electrical activity. Resuscitation. 2021;167:76‐81. doi: 10.1016/j.resuscitation.2021.08.014 [DOI] [PubMed] [Google Scholar]
- 47. Hartmann SM, Farris RWD, Di Gennaro JL, Roberts JS. Systematic review and meta‐analysis of end‐tidal carbon dioxide values associated with return of spontaneous circulation during cardiopulmonary resuscitation. J Intensive Care Med. 2015;30(7):426‐435. doi: 10.1177/0885066614530839 [DOI] [PubMed] [Google Scholar]
- 48. Idris AH, Staples ED, O'Brien DJ, et al. End‐tidal carbon dioxide during extremely low cardiac output. Ann Emerg Med. 1994;23(3):568‐572. doi: 10.1016/s0196-0644(94)70080-x [DOI] [PubMed] [Google Scholar]
- 49. Paiva EF, Paxton JH, O'Neil BJ. The use of end‐tidal carbon dioxide (ETCO2) measurement to guide management of cardiac arrest: a systematic review. Resuscitation. 2018;123:1‐7. doi: 10.1016/j.resuscitation.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 50. Faldaas BO, Nielsen EW, Storm BS, et al. Hands‐free continuous carotid Doppler ultrasound for detection of the pulse during cardiac arrest in a porcine model. Resusc Plus. 2023;15:100412. doi: 10.1016/j.resplu.2023.100412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deakin CD, Nolan JP, Soar J, et al. European resuscitation council guidelines for resuscitation 2010 section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305‐1352. doi: 10.1016/j.resuscitation.2010.08.017 [DOI] [PubMed] [Google Scholar]
- 52. Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G, European Resuscitation Council . European resuscitation council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation. 2005;67(Suppl 1):S39‐S86. doi: 10.1016/j.resuscitation.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 53. Soar J, Nolan JP, Böttiger BW, et al. European resuscitation council guidelines for resuscitation 2015: section 3. Adult advanced life support. Resuscitation. 2015;95:100‐147. doi: 10.1016/j.resuscitation.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 54. Norvik A, Kvaløy JT, Skjeflo GW, et al. Heart rate and QRS duration as biomarkers predict the immediate outcome from pulseless electrical activity. Resuscitation. 2023;185:109739. doi: 10.1016/j.resuscitation.2023.109739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Research data are not shared.


