Abstract
Phosphoenolpyruvate carboxykinase (GTP) was induced by a combination of dibutyryl cyclic AMP, theophyline and dexamethasone in Reuber H35 hepatoma cells under conditions where an amino acid in the medium was replaced by an appropriate analogue. 2. With canavanine replacing arginine or with 5-fluorotryptophan or 6-fluorotryptophan replacing tryptophan the induced enzyme had a lower catalytic activity-relative to antibody reactivity. 3. These aberrant enzyme molecules were heat-labile in vitro. 4. Measurements of enzyme degradation in vivo indicated that the canavanine-containing enzyme and the 6-fluorotryptophan-containing enzyme were degraded more rapidly than the enzyme containing all natural amino acids.
Full text
PDF

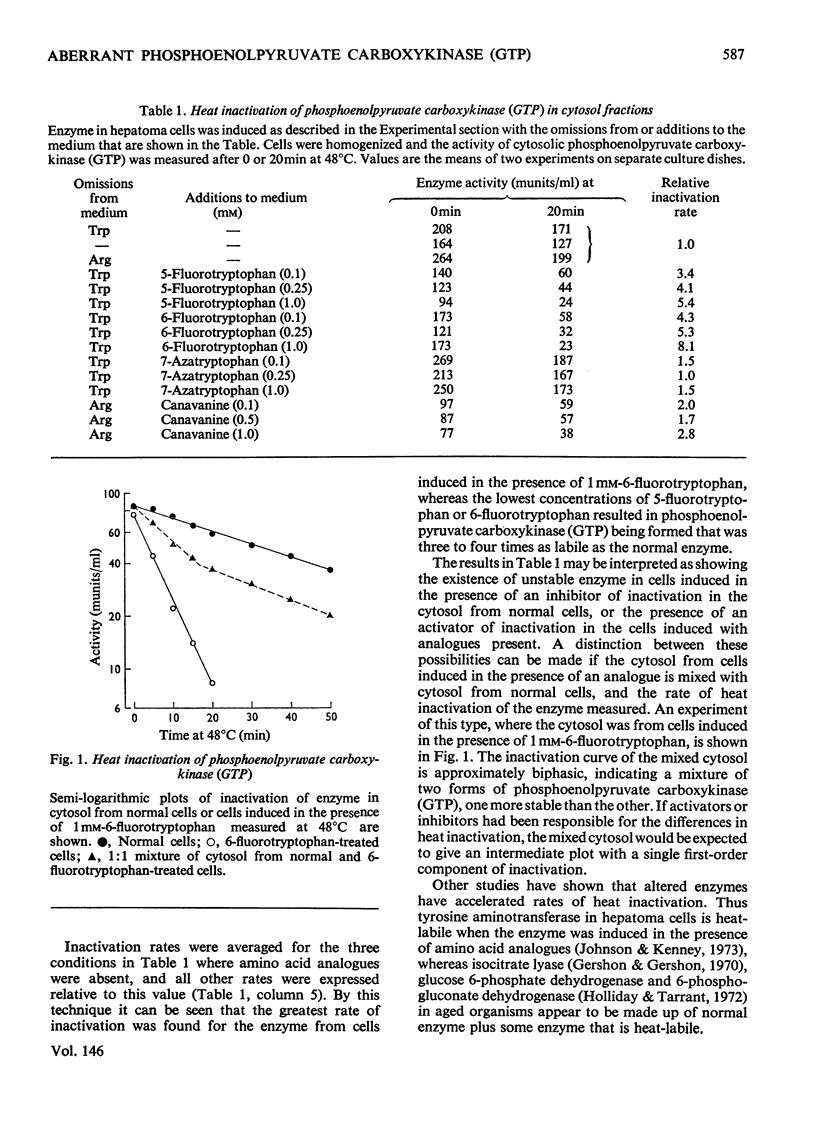
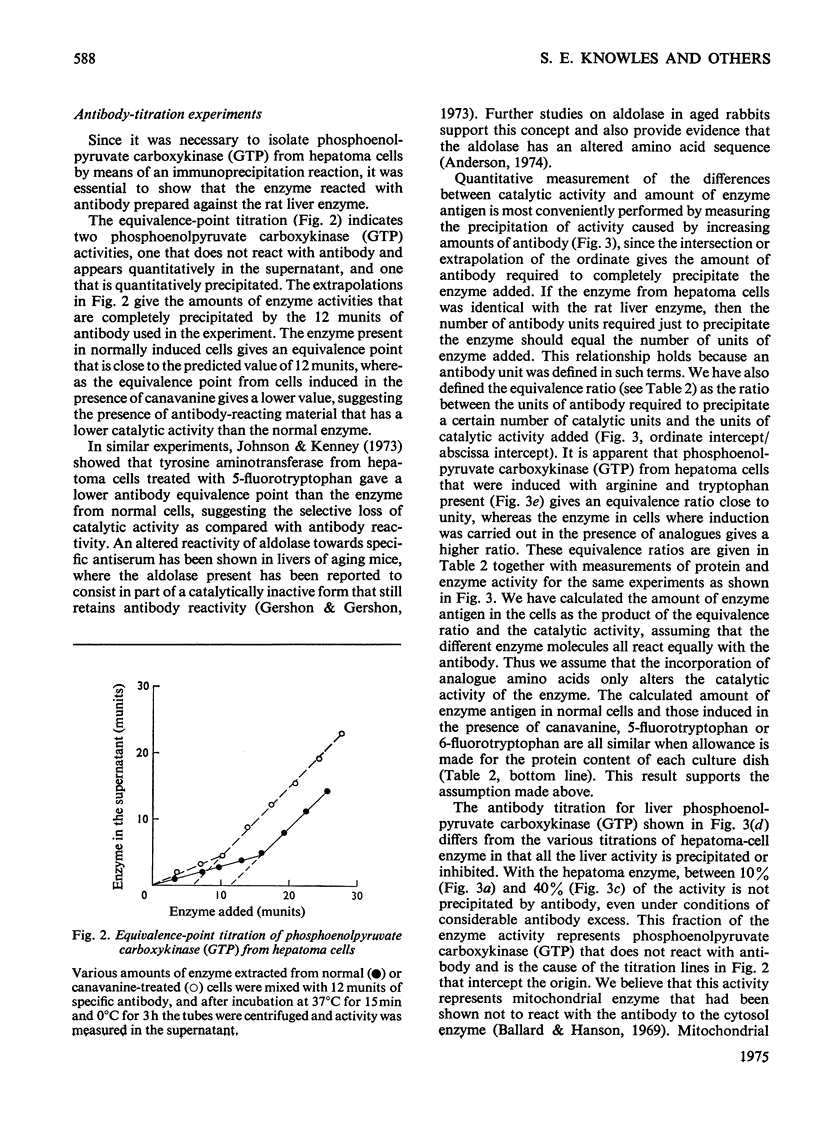
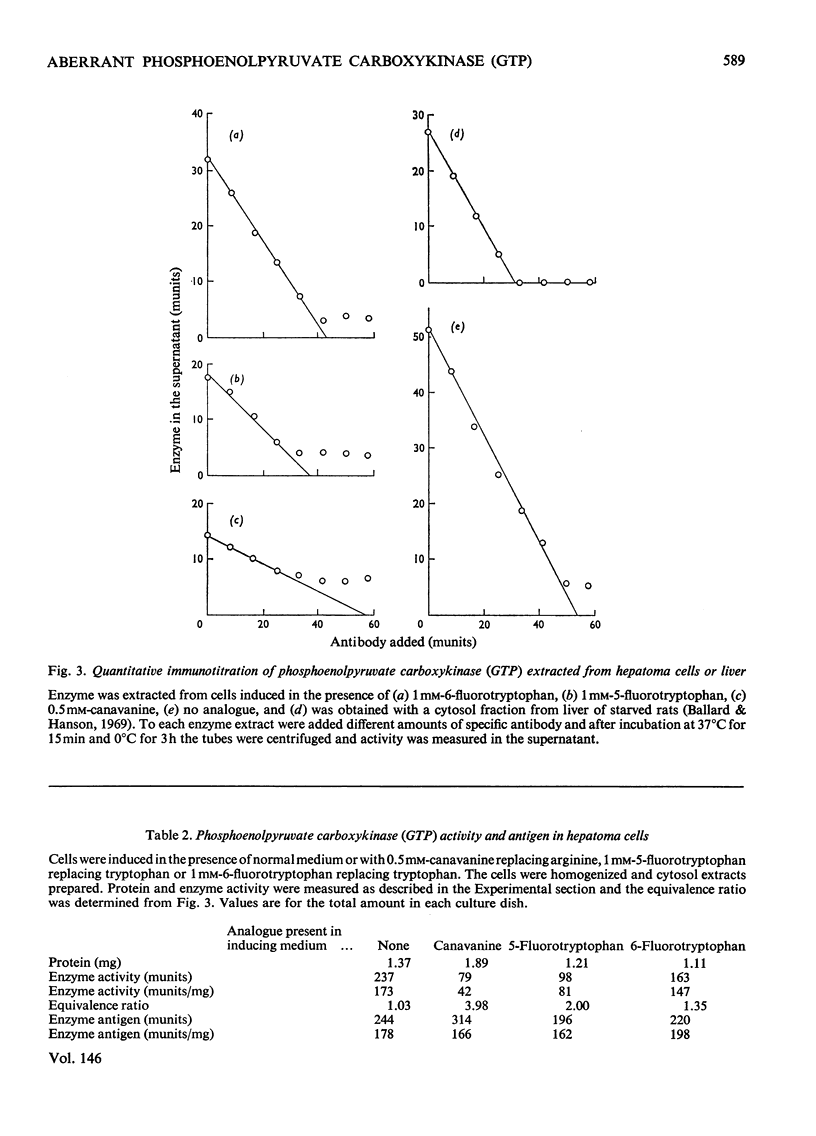
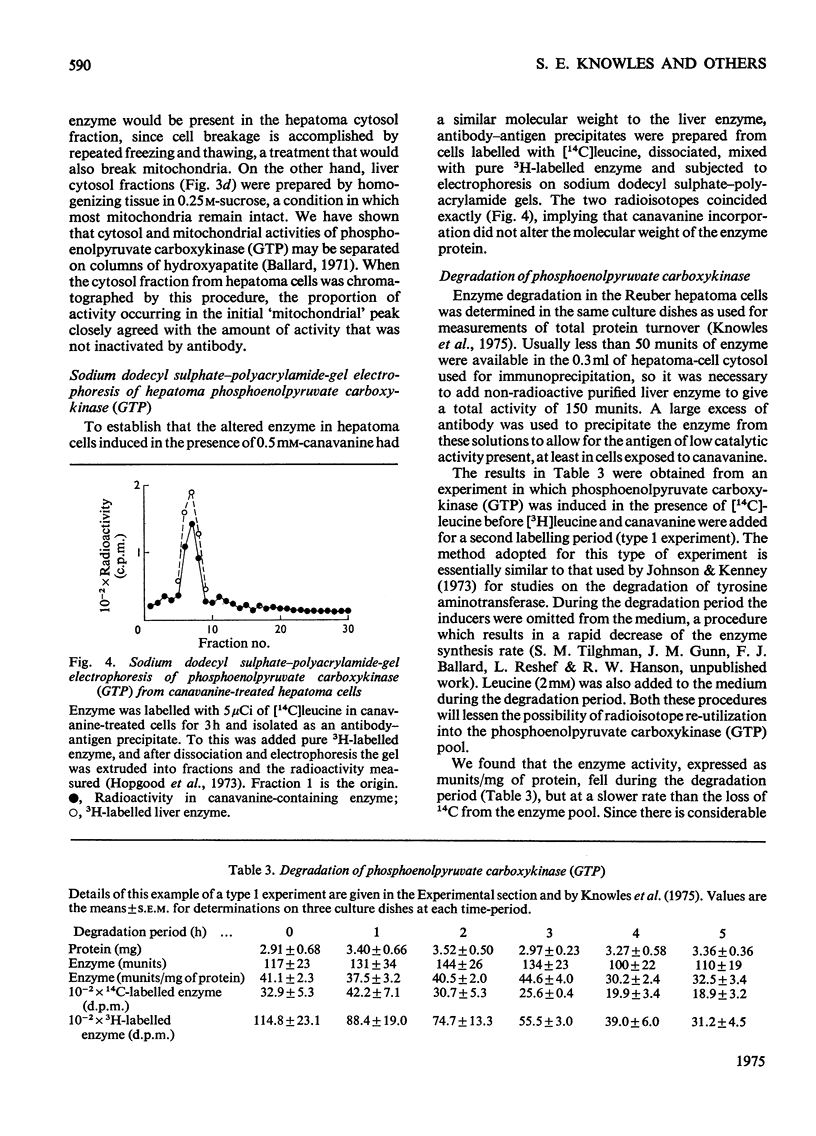


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. Aging effects on the liver aldolase of rabbits. Biochem J. 1974 May;140(2):341–343. doi: 10.1042/bj1400341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. Electrophoretic and chromatographic separation of phosphoenolpyruvate carboxykinases. Biochim Biophys Acta. 1971 Aug 20;242(2):470–472. doi: 10.1016/0005-2744(71)90239-7. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F. Phosphopyruvate carboxylase induction by L-tryptophan. Effects on synthesis and degradation of the enzyme. Biochem J. 1973 Oct;136(2):259–264. doi: 10.1042/bj1360259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F., Reshef L., Hanson R. W. Degradation of phosphoenolpyruvate carboxykinase (guanosine triphosphate) in vivo and in vitro. Biochem J. 1974 Jun;140(3):531–538. doi: 10.1042/bj1400531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon H., Gershon D. Detection of inactive enzyme molecules in ageing organisms. Nature. 1970 Sep 19;227(5264):1214–1217. doi: 10.1038/2271214a0. [DOI] [PubMed] [Google Scholar]
- Gershon H., Gershon D. Inactive enzyme molecules in aging mice: liver aldolase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):909–913. doi: 10.1073/pnas.70.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Holliday R., Tarrant G. M. Altered enzymes in ageing human fibroblasts. Nature. 1972 Jul 7;238(5358):26–30. doi: 10.1038/238026a0. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Ballard F. J. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. Biochem J. 1973 Jun;134(2):445–453. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. W., Kenney F. T. Regulation of tyrosine aminotransferase in rat liver. XI. Studies on the relationship of enzyme stability to enzyme turnover in cultured hepatoma cells. J Biol Chem. 1973 Jul 10;248(13):4528–4531. [PubMed] [Google Scholar]
- Knowles S. E., Gunn J. M., Hanson R. W., Ballard F. J. Increased degradation rates of protein synthesized in hepatoma cells in the presence of amino acid analogues. Biochem J. 1975 Mar;146(3):595–600. doi: 10.1042/bj1460595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


