Abstract
Background
The impact of night shift work on the incidence of type 2 diabetes mellitus (T2DM) is not well understood. This meta-analysis assesses the association between night shift work and the risk of developing T2DM and explores this relationship across various subgroups.
Methods
We systematically searched PubMed, Web of Science, EBSCO, and the Cochrane Library from their inception until February 2024. We employed hazard ratios (HR) and 95% confidence intervals (95%CI) to quantify the association between night shift work and T2DM risk.
Results
Our analysis synthesized data from 9 articles encompassing 10 cohort studies. Overall, night shift workers exhibited a 30% increased incidence of T2DM compared to their daytime counterparts (HR = 1.30, 95% CI: [1.18, 1.43], P < 0.001). Among females, night shift workers had a higher incidence of T2DM (HR = 1.28, 95% CI: [1.16, 1.41]); however, in males, the association was not statistically significant (95% CI: [0.89, 2.63]). For individuals with a body mass index (BMI) > 30 kg/m2, night shift work was associated with an increased T2DM risk (HR = 1.14, P = 0.007), whereas there was no significant association for those with a BMI ≤ 30 kg/m2 (P = 0.255). Further, the risk of T2DM increased with longer durations of night shift work; workers with more than 10 years of night shift work faced a higher T2DM risk than those with 10 years or fewer (HR for > 10 years = 1.17, 95% CI: [1.10, 1.24]; HR for ≤ 10 years = 1.06, 95% CI: [1.03, 1.10]).
Conclusion
Findings suggest potential link between night shift work and T2DM risk. Longer durations of night shift work may increase the risk of T2DM. There may be gender differences (greater harm in women, but the male sample size is small) and obesity differences.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-024-01808-w.
Keywords: Night shift work, Type 2 diabetes mellitus, Gender, BMI, Risk, Meta-analysis
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by elevated blood sugar levels, insulin resistance, or deficiency. It is closely linked to various comorbidities including atherosclerosis, cardiovascular disease, myocardial infarction, heart failure, and stroke [1, 2]. As the global population ages and expands, the prevalence of T2DM has dramatically increased, the global age-standardized total diabetes prevalence increased by 90.5% from 1990 to 2021 [3]. The number of affected people rose from 108 million in 1980 to 422 million in 2014 [4]. In 2021, there were approximately 529 million T2DM patients. As early as 2017, diabetes had already ranked as the ninth - leading cause of death. It is even predicted that more than 1.31 billion people will have T2DM in 2050 [3]. The medical expenses of T2DM patients without complications are approximately 3.2 times the average medical expenditure, and those of patients with microvascular and macrovascular complications can be up to 9 times the average [5]. T2DM represents a substantial challenge to public health security. Beyond genetic factors, the potential risk factors for T2DM include obesity, dietary habits, occupation, lifestyle, and psychological stress. Conversely, intake of 66 g of fruits and/or vegetables per day may reduce the risk of T2DM by a quarter over time [6].
Numerous studies have identified night shift work as a contributing factor to adverse health outcomes, such as increased incidences of T2DM, hypertension, and dyslipidemia [7, 8]. Over the past decade, several meta-analyses have examined the link between night shift work and T2DM. A 2015 meta-analysis found that shift work significantly elevates the risk of T2DM, particularly among females compared to males [9]. However, this study may have overestimated the impact of night shift work on T2DM risk, as it included some participants categorized as prediabetic. A more recent study in 2020 confirmed the positive association between shift work and T2DM risk but noted that less than half of the included studies were cross-sectional, offering weaker evidence [10]. In response to these findings, our current study exclusively focuses on published cohort studies to further investigate the association between night shift work and the risk of T2DM.
Methods
Search Strategy
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The review was registered with PROSPERO (ID: CRD42024520037). We searched the electronic databases PubMed, Web of Science, EBSCO, and the Cochrane Library from inception through February 2024. Search terms used included MeSH terms “Shift Work Schedule” and “Diabetes Mellitus, Type 2”, supplemented with keywords “Night shift”, “Shift work”, and “T2DM”. We also manually screened references to identify relevant studies. Two researchers independently screened all retrieved literature based on pre-defined criteria, with discrepancies resolved through consensus.
Inclusion and exclusion criteria
Inclusion criteria encompassed studies where: (1) participants did not have T2DM, (2) the exposure factor was night shift work (night shift work and shift work that includes night shift work), (3) the control condition was non-night shift work, and (4) the study was an English-language cohort study examining the association between night shift work and T2DM.
Exclusion criteria included: (1) studies where data could not be statistically analyzed, and (2) studies originating from the same cohort, selecting only the most recent or comprehensive articles for inclusion.
Data extraction
Data were extracted by two independent researchers using a standardized form. Extracted data included authors, publication year, country, sample size, participant age and gender, body mass index (BMI), profession, study design, source of population, follow-up duration, adjustment parameters, duration of night shifts, and assessments of prediabetes and T2DM.
Quality Assessment
The quality of cohort studies was assessed independently by two reviewers using the Newcastle-Ottawa Scale (NOS). Four stars were allocated to cohort selection criteria, three to outcome assessment, and two to comparability between groups. Studies were then categorized as low (0–3), moderate (4–6), or high (7–9) quality. Only high-quality studies were included. Any disagreements were resolved by a third investigator.
Statistical analysis
The association between night shift work and T2DM was analysed using hazard ratios (HR) and 95% confidence intervals (95%CI). Given potential heterogeneity due to factors such as shift intensity, dietary habits, ethnicity, and physical activity, a random effects model was employed to calculate weights. Heterogeneity among studies was assessed using the Chi-square and I2 tests, with p < 0.05 and I2 ≥ 50% indicating significant heterogeneity. Original studies with large sample sizes (the results are more reliable) are often given higher weights. The combined results are represented by forest plots. Publication bias was evaluated using Begg’s test. Sensitivity analyses were conducted using the leave-one-out method. Statistical analyses were performed using Stata 12.0, employing a two-sided hypothesis test with significance set at p < 0.05.
Results
Literature search
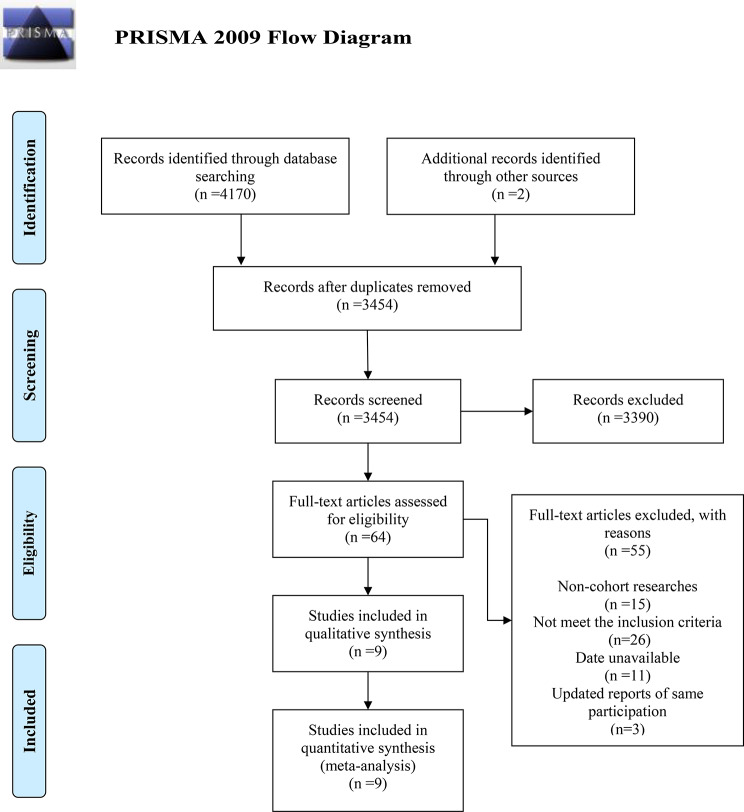
The search and selection processes are depicted in Fig. 1. Initially, 4170 records were retrieved from electronic databases. An additional two studies were included through manual searches of reference lists from relevant studies. After duplicates were removed, 3454 articles remained. Based on title and abstract screening, 3390 articles were excluded. Following a full-text review of 64 articles against the selection criteria, 55 were excluded for the following reasons: non-cohort study design (n = 15), failure to meet inclusion criteria (n = 26), lack of available data (n = 11), and overlapping reports on the same participants (n = 3). Ultimately, 9 articles, comprising 10 cohort studies, were included in the meta-analysis [12–20].
Fig. 1.
Flow diagram of the selection process
Study characteristics
The 9 included articles, published between 1999 and 2021, consisted of one retrospective and nine prospective cohort studies. Geographically, four studies were conducted in Eastern countries and six in Western countries. The detailed characteristics of the included cohort studies are presented in Table 1.
Table 1.
Characteristics of included observational studies in the meta-analysis
| Author | Year | Country | Cohort | No. of participant | Follow-up time (year) | Professions | The type of work shift | Adjust parameters | Study design |
|---|---|---|---|---|---|---|---|---|---|
| Osaki, Y. | 2021 | Japan | J-ECOH | 17,628 | 13 | Workers | Shift work | age, gender, marital status, hypertension, dyslipidemia, family history of diabetes, sedentary work, over time working, job position, alcohol, smoking, sleep duration, physical activity, BMI | Prospective |
| Silva-Costa, A. | 2020 | Brazill | ELSA-Brasil | 8656 | 12 | Civil servants | Night shifts (≥ 5 consecutive hours of night work [22:00–05:00 h] at least four times per month) | age, education, hours of work, BMI, physical activity | Prospective |
| Hanprathet, N. | 2019 | Thailand | NA | 5947 | 7 | Workers | Current shift workers (did permanent night shifts only for at least three night shifts per month or; who rotated between two or three shifts with at least three night shifts per month until the baseline year) | age, marital status, family history of diabetes, BMI, hypertension, DBP, FPG, WBC, TG, HDL-C, WC, education | Retrospective |
| Shan, Z. | 2018 | United States | Nurses’ Health Study | 55,324 | 24 | Nurses | Rotating night shift work | age, calendar year, ethnicity, BMI, marital status, living status, family history of diabetes, menopausal status, oral contraceptive, alcohol, energy intake, smoking, physical activity, AHEI | Prospective |
| Shan, Z. | 2018 | United States | Nurses’ Health Study II | 88,086 | 22 | Nurses | Rotating night shift work | age, marital status, calendar year, BMI, ethnicity, living status, family history of diabetes, menopausal status, oral contraceptive, alcohol, energy intake, smoking, physical activity, AHEI | Prospective |
| Bannai, A. | 2016 | Japan | NA | 3195 | 6 | Civil servants | Shift work | age, marital status, FPG, family history of diabetes, education, job position | Prospective |
| Hansen, Anne B. | 2016 | Denmark | The Danish Nurse Cohort | 19,492 | 19 | Nurses | Night shifts | age, smoking, physical activity, alcohol, fatty meat intake, marital status, job position, acute myocardial infarction, hypertension, fruit and vegetables intake, BMI | Prospective |
| Vimalananda, V. G. | 2015 | USA | BWHS | 28,041 | 8 | NA | Night shift work | age, questionnaire cycle, BMI, family history of diabetes, education, neighborhood socioeconomic status, vigorous activity levels, smoking, alcohol, energy intake, diet, coffee, decaffeinated coffee, soda, diet soda | Prospective |
| Poulsen, K. | 2014 | Denmark | NA | 7305 | 7 | Health care workers | Evening/night shift work | health, work, lifestyle | Prospective |
| Kawakami, N. | 1999 | Japan | NA | 2194 | 8 | Industrial workers | Rotating shift workers engaged in two or three shift work schedules including night shift, with a weekly clockwise rotation | age, BMI, education, alcohol, smoking, physical activity, family history of diabetes | Prospective |
J-ECOH: Japan Epidemiology Collaboration on Occupational Health Study; BWHS: Black Women’s Health Study; No.: number; NA: not available; BMI: body mass index; DBP: diastolic blood pressure; WBC: white blood cell; TG: triglyceride; HDL-C: high density lipoprotein cholesterol; WC: waist circumference; AHEI: Alternate Healthy Eating Index score; FPG: fasting plasma glucose
Assessment of T2DM
T2DM was primarily assessed through fasting plasma glucose levels (FPG) ≥ 126 mg/dL, physician diagnoses, or medication usage. Adjusted parameters commonly included age, gender, marital status, BMI, ethnicity, living status (alone or with others), family history of diabetes, alcohol consumption, and smoking habits. These details are further elaborated in Supplementary Table 1.
Bias risk assessment
The Newcastle-Ottawa Scale (NOS) was employed to objectively assess the quality of the included observational cohort studies. The evaluations revealed that four cohort studies scored 9 points, four scored 8 points, and two scored 7 points. Detailed bias risk assessments are documented in Supplementary Table 2.
Association between Night Shift Work and T2DM
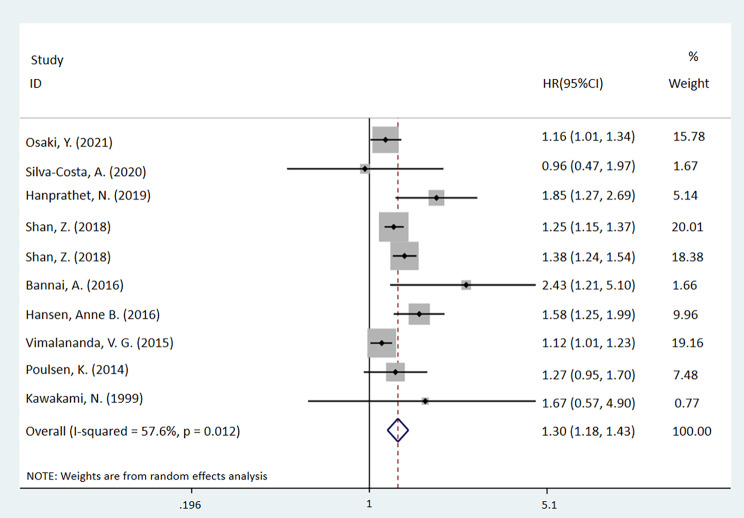
The meta-analysis demonstrated that night shift workers exhibit a higher incidence rate of T2DM compared to non-night shift workers (HR = 1.30, 95%CI: [1.18, 1.43], P < 0.001, I2 = 57.6%). Further details are presented in Fig. 2. All p-values in texts, tables, and captions are derived from HR analysis and all p-values in pictures are from heterogeneity analysis.
Fig. 2.
Forest plot of a meta-analysis assessing the association between night shift work and the risk of T2DM in all participants (p < 0.001)
Subgroup analysis of the association between night shift work and risk of T2DM
Gender association
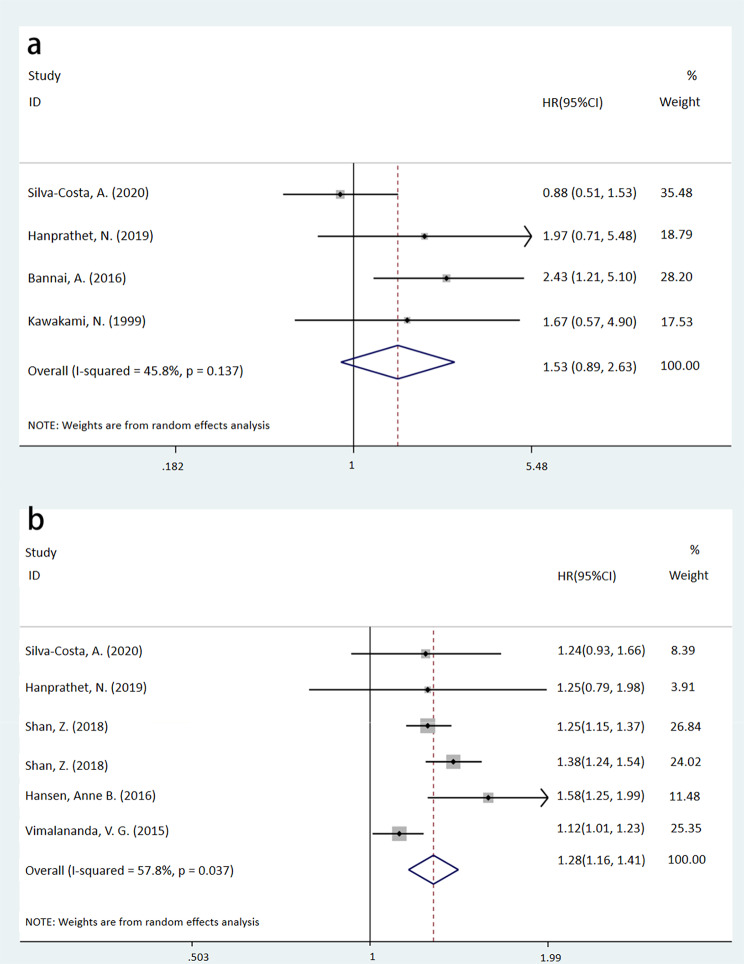
In the female subgroup, night shift work was associated with a higher incidence of T2DM than the non-night shift work group (HR = 1.28, 95%CI: [1.16, 1.41], P < 0.001, I2 = 57.8%). In contrast, no significant association was observed in the male subgroup (HR = 1.53, 95%CI: [0.89, 2.63], P = 0.128, I2 = 45.8%). Further details can be found in Fig. 3.
Fig. 3.
Forest plots of a meta-analysis assessing the association between night shift work and the risk of T2DM in sex-specific subgroups (a: male, p = 0.128; b: female, p < 0.001)
Occupational association
Both medical and non-medical workers exposed to night shifts exhibited a higher risk of T2DM compared to those who were not exposed to night shifts (medical workers: HR = 1.33, P < 0.001; non-medical workers: HR = 1.27, P = 0.007), as detailed in Table 2.
Table 2.
Subgroup analyses assessing the association between night shift work and the risk of T2DM
| Subgroup | No. of researches | No. of non-night shift work | No. of night shift work | HR | 95%CI | p | I2 (%) |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Female | 5 | 89,748 | 104,578 | 1.28 | (1.16,1.41) | < 0.001 | 57.8 |
| Male | 4 | 4340 | 6806 | 1.53 | (0.89,2.63) | 0.128 | 45.8 |
| BMI | |||||||
| ≤ 30 kg/m2 | 2 | 22,549 | 6455 | 1.34 | (0.81, 2.22) | 0.255 | 87.4 |
| > 30 kg/m2 | 2 | 7396 | 4018 | 1.14 | (1.04, 1.25) | 0.007 | 0 |
| Profession | |||||||
| Medical workers | 3 | 70,597 | 93,617 | 1.33 | (1.22, 1.46) | < 0.001 | 33.3 |
| Non-medical workers | 6 | 47,524 | 18,147 | 1.27 | (1.07, 1.52) | 0.007 | 55.2 |
| Night shift duration | |||||||
| ≤ 5 years | 2 | 70,425 | 73,519 | 1.05 | (1.01, 1.10) | 0.024 | 0 |
| > 5 years | 3 | 77,454 | 28,322 | 1.13 | (1.09, 1.18) | < 0.001 | 0 |
| ≤ 10 years | 3 | 89,399 | 77,454 | 1.06 | (1.03, 1.10) | < 0.001 | 0 |
| > 10 years | 3 | 13,264 | 77,454 | 1.17 | (1.10, 1.24) | < 0.001 | 0 |
| Follow-up time | |||||||
| ≤ 10 years | 5 | 30,847 | 15,822 | 1.43 | (1.09, 1.86) | 0.009 | 64.0 |
| > 10 years | 4 | 87,274 | 95,942 | 1.30 | (1.18, 1.43) | < 0.001 | 48.2 |
| Age | |||||||
| ≤ 60 years old | 3 | 56,798 | 92,559 | 1.35 | (1.20, 1.52) | < 0.001 | 44.6 |
NO: number; HR: hazard ratio; 95%CI: 95% confidence intervals; BMI: body mass index; T2DM: type 2 diabetes mellitus
BMI-based association
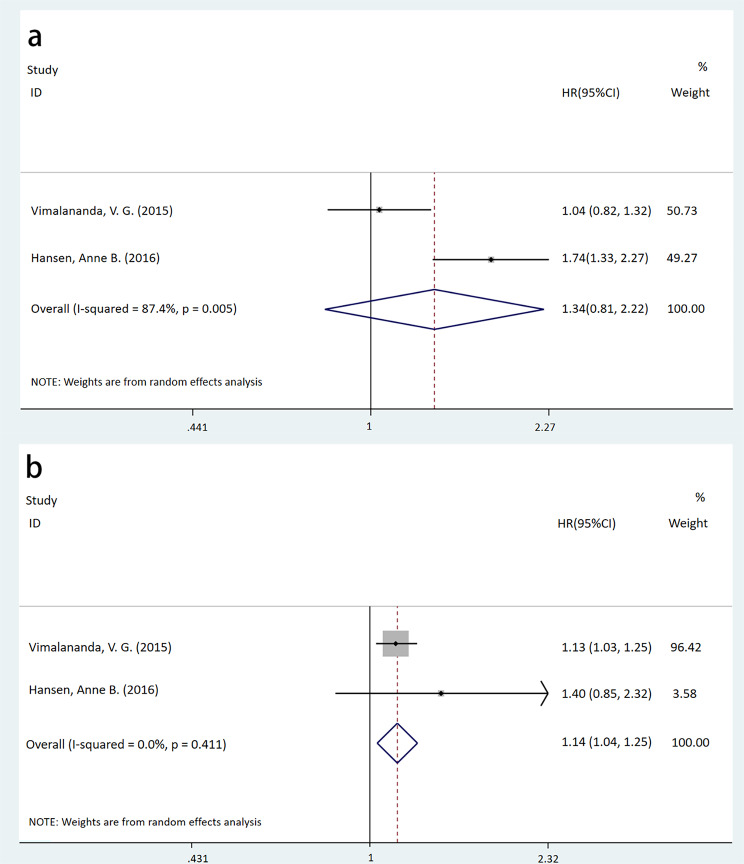
For participants with a BMI ≤ 30 kg/m2, no significant association was found between night shift work and T2DM (HR = 1.34, 95%CI: [0.81, 2.22], P = 0.255, I2 = 87.4%). However, for those with a BMI > 30 kg/m2, night shift workers had a higher incidence of T2DM compared to non-night shift workers (HR = 1.14, 95%CI: [1.04, 1.25], P = 0.007, I2 = 0.0%), as shown in Fig. 4.
Fig. 4.
Forest plots of a meta-analysis assessing the association between night shift work and the risk of T2DM across BMI subgroups (a: BMI ≤ 30 kg/m2, p = 0.255; b: BMI > 30 kg/m2, p = 0.007)
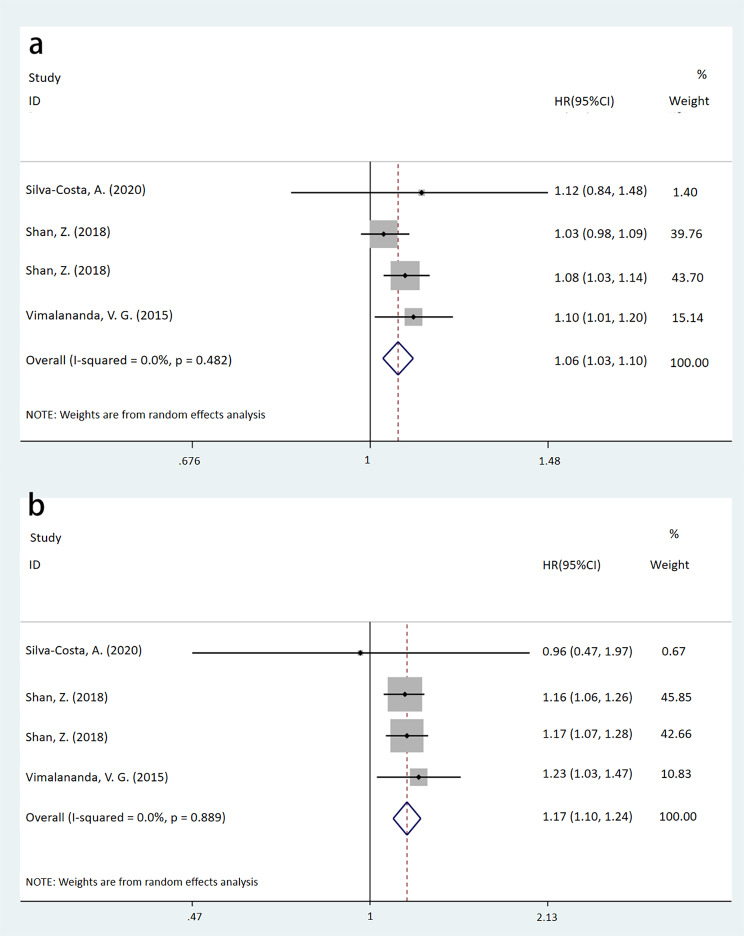
Duration of night shift work
Participants with ≤ 5 years of night shift work had an increased risk of T2DM compared to those with no night shift work (HR = 1.05, 95%CI: [1.01, 1.10]), which was more pronounced in those with > 5 years of exposure (HR = 1.13, 95%CI: [1.09, 1.18]). The risks associated with ≤ 10 years and > 10 years of night shift work were similarly elevated (≤ 10 years: HR = 1.06, 95%CI: [1.03, 1.10]; >10 years: HR = 1.17, 95%CI: [1.10, 1.24]). These findings are detailed in Table 2; Fig. 5.
Fig. 5.
Forest plots of a meta-analysis assessing the association between night shift work and the risk of T2DM across night shift duration subgroups (a: ≤10 years, p < 0.001; b: >10 years, p < 0.001)
Follow-up time
The risk of T2DM was higher in night shift workers compared to non-night shift workers regardless of the follow-up time (≤ 10 years: HR = 1.43, 95%CI: [1.09, 1.86], P = 0.009, I2 = 64.0%; >10 years: HR = 1.30, 95%CI: [1.18, 1.43], P < 0.001, I2 = 48.2%), with details in Table 2.
Age-specific association
For individuals ≤ 60 years old, the incidence of T2DM was significantly higher among night shift workers (HR = 1.35, 95%CI: [1.20, 1.52], P < 0.001, I2 = 44.6%), as reported in Table 2.
Publication bias and sensitivity analysis
The assessment of publication bias and sensitivity regarding the association between night shift work and T2DM is detailed in Supplementary Figs. 1 and 2. The Begg’s test indicated no significant publication bias (P = 0.371). Sensitivity analysis, conducted using the leave-one-out method, confirmed the stability of the statistical results.
Discussion
This meta-analysis sought to investigate the relationship between night shift work and T2DM. The study synthesized data from 9 articles encompassing 10 cohort studies with over 235,800 participants, revealing a significant positive association between night shift work and the risk of developing T2DM. Specifically, the results of this meta-analysis show that night shift work exposed individuals have a 30% increased risk of T2DM compared to non-night shift exposed individuals. This association persisted across subgroup analyses by occupation (including medical and non-medical workers), duration of night shifts, and follow-up duration. Sensitivity analyses confirmed the stability of these results.
The increased risk among night shift workers may stem from disruptions in circadian rhythms. Normally, during the sleep phase under typical circadian conditions, vasopressin neurons in the suprachiasmatic nucleus (SCN) release vasopressin, which promotes the expression of glucose transporter 1 (GLUT1) in the arcuate nucleus (ARC). Night shift work disrupts this mechanism by directly affecting the SCN via the retinohypothalamic tract, leading to subsequent reduction in the activity of vasopressin neurons. This reduction in activity decreases GLUT1 expression in the ARC, thereby impairing glucose uptake, elevating circulating glucose levels, and promoting hyperglycemia [21–26].
Another critical pathway involves the hypothalamic suprachiasmatic nucleus, which releases various hormones that act on the paraventricular nucleus (PVN). The PVN influences the pineal gland to secrete melatonin via the sympathetic nervous system. Melatonin activates M1 and M2 receptors on the α and β cells of pancreatic islets, enhancing insulin secretion, promoting glycogen synthesis and glycolysis, inhibiting liver gluconeogenesis, and stimulating adipogenesis. Night shift work disrupts this pathway by suppressing PVN activation through light stimuli, resulting in reduced melatonin secretion from the pineal gland [27, 28]. This decrease in melatonin leads to lower insulin synthesis, reduced glucose utilization, and elevated circulating glucose levels [29]. Animal studies have demonstrated that melatonin supplementation can improve glucose metabolism in insulin-resistant mice fed high-fat diets [30].
Moreover, meta-analytical evidence from Delpino FM et al. suggests that melatonin may have an inhibitory effect on obesity. Experiments by Aubrecht et al. have shown that male mice exposed to dim light at night (dLAN) in conjunction with a high-fat diet (HFD) experience significant weight gain and impaired glucose tolerance, as well as diminished insulin secretion, indicating a synergistic effect between dLAN and HFD, where dLAN exacerbates the weight gain associated with HFD-induced obesity [31]. In the context of night shift work, many workers tend to consume late-night snacks, often high in fats. Our findings indicate that among those exposed to night shifts, individuals with obesity are at a heightened risk of developing T2DM, likely due to the described mechanisms. Conversely, non-obese individuals do not show an increased risk, possibly owing to their higher insulin sensitivity and more stable glucose levels.
Obese individuals exposed to night shifts experience exacerbated insulin resistance due to the dual impact of obesity and disrupted circadian rhythms. Clinical studies by Al-Sulaiti H et al. have shown that elevated levels of phospholipid metabolites such as choline, glycerophosphoethanolamine, and glycerophosphocholine are positively associated with insulin resistance in obese populations [32]. Obesity, often characterized by an increase in visceral fat, enhances lipid breakdown [33]. Adipose tissue, functioning as an endocrine organ, secretes various adipocyte-derived factors including free fatty acids (FFA), TNF-α, IL-6, leptin, adiponectin, visfatin, and other peptides [34]. FFAs, utilized for energy, reduce glucose uptake, thereby contributing significantly to insulin resistance. Pro-inflammatory cytokines such as TNF-α and IL-6, along with leptin, also promote insulin resistance, with TNF-α specifically inhibiting insulin receptor tyrosine kinase phosphorylation.
Additionally, there is significant evidence of gender disparities in the prevalence of T2DM, with females more frequently affected than males [35]. Our analysis indicates a positive association between night shift work and T2DM prevalence in the female subgroup, while no significant association was found in males. The association between higher BMI and increased T2DM risk is well-established. Systematic reviews have also shown that females are more likely to exhibit higher rates of obesity than males [36], and they have a greater rate of subcutaneous adipose tissue (SAT) formation and a more pronounced increase in waist circumference (WC) [37]. Furthermore, an increased WC is associated with a heightened risk of developing T2DM [38].
Meta-analytical evidence from previous research suggests significant gender association in hormonal profiles related to T2DM. Females generally exhibit higher levels of leptin and adiponectin compared to males of the same age and BMI. Notably, plasma adiponectin levels are inversely correlated with insulin sensitivity, a relationship that is more pronounced in females. Conversely, male testosterone levels, which suppress adiponectin secretion, appear to confer a protective effect against T2DM. Another previous meta-analysis further supports this by showing that men with T2DM have testosterone levels lower by 2.66 nmol/L than those without the disease [39, 40]. In this meta-analysis, it should be emphasized that since most of the cohort studies are nurse cohorts, the proportion of men is small. There are only more than 10,000 men, while there were nearly 200,000 women. The test power of the male subgroup is relatively low, so it is not easy to obtain positive results.
Additionally, women are more vulnerable to the adverse effects of social and occupational stressors, as well as sleep disorders [41]. Research indicates that night shift work exacerbates psychological stress and depression, particularly among women [42]. This stress activates the sympathetic nervous system, which in turn stimulates the hypothalamus to release corticotropin-releasing hormone (CRH), leading to adrenal cortex hormone release. Activating the hypothalamic-pituitary-adrenal axis, this process elevates cortisol levels, which promotes energy storage and mobilizes glucose and lipids into the bloodstream. Such hormonal fluctuations not only increase circulating glucose levels but also diminish insulin sensitivity, ultimately contributing to insulin resistance. Experimental findings by Fransson, L. et al. have shown that removal of the adrenal glands in mice leads to enhanced insulin sensitivity, underscoring the impact of adrenal hormones on glucose metabolism [43].
This study has demonstrated that prolonged exposure to night shifts is associated with an increased risk of developing T2DM, underscoring the detrimental effects linked to extended durations of such work schedules. In addition, the impact of night-shift exposure on the risk of T2DM may also be affected by various factors such as economic status, ethnicity, dietary conditions, and activity intensity. A cohort study has shown that the lower the household income, the higher the risk of diabetes in children or adolescents [44]. This may be related to the consumption of inexpensive and unhealthy food, poor lifestyle habits (smoking, excessive alcohol consumption) and socioeconomic stress among people with lower income. In another study, it has been indicated that a healthy diet and physical activity can reduce the incidence of T2DM [45]. Studies have also demonstrated that individuals of South Asian and African descent seem to be more susceptible to T2DM [46, 47]. This may be associated with genetic factors related to insulin resistance.
Our meta-analysis focused only on cohort studies with high-strength evidence and excluded pre-diabetic individuals. We used HR as the outcome measure for evaluating the risk of T2DM. Therefore, compared with the two previously published meta-analyses [9, 10], we only included 9 cohort studies. For example, the studies by Eriksson, A. K. et al. [48] and Ika, K. et al. [49] used odds ratio (OR) as the evaluation index; the study by Kita, T. et al. [50] was about the association between sleep duration/quality and diabetes risk, and the study by Oberlinner, C. et al. [51] focused on the impact of health examinations on the mortality rate of chronic diseases. None of these studies contained the HR index data of night shift work on the risk of T2DM.
Nevertheless, our study is not without limitations. Since in the included original studies the number of the female population was larger than that of the male, the lack of observed significant impact of night shift work on the prevalence of T2DM in the male subgroup might be related to the small sample size. For the subgroup analysis related to BMI, the ideal approach would be to use a dose-effect model and conduct linear regression analysis to explore the relationship between BMI and night shift work on the incidence of T2DM. Due to a lack of data, we can only perform subgroup analyses for BMI > 30 kg/m2 and BMI ≤ 30 kg/m2. Since there are differences in the adjustment factors of each original study and not all factors can be controlled, these can lead to biases in the results. The absence of detailed original data on variables such as age, intensity of night shift work, start times, and dietary habits restricted our ability to conduct more nuanced and diversified subgroup analyses. It is hoped that there will be more original research data in the future.
Conclusion
In summary, the present study establishes a correlation between night shift work and an elevated risk of T2DM, with the risk increasing as the duration of night shift exposure extends. Notably, this association exhibits gender-specific variations; the data reveal a discernible trend in women, whereas no significant trend is apparent in men. Regarding obesity status, a propensity for a stronger association with increased T2DM risk is observed among obese individuals, in contrast to their non-obese counterparts, where the association is less pronounced. These findings are consistent across various occupations, including medical and non-medical fields, diverse geographical regions, and multiple follow-up periods. While these results align with certain aspects of prior research, they underscore the need for further investigation and validation to elucidate the underlying mechanisms and potential occupational health interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- 95%CI
95% Confidence Interval
- ARC
Arcuate Nucleus
- BMI
Body Mass Index
- CRH
Corticotropin-Releasing Hormone
- dLAN
Dim Light at Night
- FFA
Free Fatty Acids
- FPG
fasting plasma glucose levels
- GLUT1
Glucose Transporter 1
- HR
Hazard Ratio
- HFD
High-Fat Diet
- NOS
Newcastle-Ottawa Scale
- Odds ratio
OR
- PVN
Paraventricular Nucleus
- SAT
Subcutaneous Adipose Tissue
- SCN
Suprachiasmatic Nucleus
- SNS
Sympathetic Nervous System
- T2DM
Type 2 Diabetes Mellitus
- WC
Waist Circumference
Author contributions
FX designed the research process. FX and KSH searched the database for corresponding articles. KSH and RRF extracted useful information from the articles above. YMZ used statistical software for analysis. FX, KSH and KQX drafted the meta-analysis. KSH and JNT polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Funding
No financial support was received for this study.
Data availability
The datasets supporting the conclusions of this article are included within the article. For further details, please contact the corresponding author.
Declaration
Ethics approval and consent to participate
Not applicable, as this paper is based on research from global databases.
Clinical trial number
not applicable.
Consent to Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Einarson TR, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators GBDD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2023;402(10397):203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaboration N. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Maskari F, El-Sadig M, Nagelkerke N. Assessment of the direct medical costs of diabetes mellitus and its complications in the United Arab Emirates. BMC Public Health. 2010;10:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenhill C. Dietary factors in the risk of T2DM. Nat Rev Endocrinol. 2020;16(10):537. [DOI] [PubMed] [Google Scholar]
- 7.Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest, 2021. 131(19). [DOI] [PMC free article] [PubMed]
- 8.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes Mellitus. Endocr Rev. 2016;37(3):278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan Y, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72(1):72–8. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, et al. Association between shift work and risk of type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of observational studies. Chronobiol Int. 2020;37(1):29–46. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanprathet N, et al. Increased risk of type 2 diabetes and abnormal FPG due to Shift Work differs according to gender: a Retrospective Cohort Study among Thai workers in Bangkok, Thailand. Diabetes Metab Syndr Obes. 2019;12:2341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Costa A, et al. Lifetime night work exposure and the risk of type 2 diabetes: results from the longitudinal study of adult health (ELSA-Brasil). Chronobiol Int. 2020;37(9–10):1344–7. [DOI] [PubMed] [Google Scholar]
- 14.Hansen AB, et al. Night shift work and incidence of diabetes in the Danish nurse cohort. Occup Environ Med. 2016;73(4):262–8. [DOI] [PubMed] [Google Scholar]
- 15.Vimalananda VG, et al. Night-shift work and incident diabetes among African-American women. Diabetologia. 2015;58(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami N, et al. Overtime, psychosocial working conditions, and occurrence of non-insulin dependent diabetes mellitus in Japanese men. J Epidemiol Community Health. 1999;53(6):359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bannai A, et al. The risk of developing diabetes in Association with long working hours differs by Shift Work schedules. J Epidemiol. 2016;26(9):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan Z, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363:k4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osaki Y, et al. Shift work and the onset of type 2 diabetes: results from a large-scale cohort among Japanese workers. Acta Diabetol. 2021;58(12):1659–64. [DOI] [PubMed] [Google Scholar]
- 20.Poulsen K, et al. Work, diabetes and obesity: a seven year follow-up study among Danish health care workers. PLoS ONE. 2014;9(7):e103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding C, Magkos F. Oxytocin and Vasopressin Systems in Obesity and metabolic health: mechanisms and perspectives. Curr Obes Rep. 2019;8(3):301–16. [DOI] [PubMed] [Google Scholar]
- 22.Jansen LT, et al. Osmotic stimulation of vasopressin acutely impairs glucose regulation: a counterbalanced, crossover trial. Am J Clin Nutr. 2019;110(6):1344–52. [DOI] [PubMed] [Google Scholar]
- 23.Andres-Hernando A et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. JCI Insight, 2021. 6(1). [DOI] [PMC free article] [PubMed]
- 24.Rodriguez-Cortes B, et al. Suprachiasmatic nucleus-mediated glucose entry into the arcuate nucleus determines the daily rhythm in blood glycemia. Curr Biol. 2022;32(4):796–805. e4. [DOI] [PubMed] [Google Scholar]
- 25.Hurtado-Alvarado G, et al. Suprachiasmatic nucleus promotes hyperglycemia induced by sleep delay. Curr Biol. 2023;33(20):4343–52. e4. [DOI] [PubMed] [Google Scholar]
- 26.Lebedeva S, et al. Metabolic effects of vasopressin in pathophysiology of diabetic kidney disease. Front Endocrinol (Lausanne). 2023;14:1176199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94–106. [DOI] [PubMed] [Google Scholar]
- 28.Xia AY et al. Molecular mechanisms of the melatonin receptor pathway linking circadian rhythm to type 2 diabetes Mellitus. Nutrients, 2023. 15(6). [DOI] [PMC free article] [PubMed]
- 29.Cipolla-Neto J, et al. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–81. [DOI] [PubMed] [Google Scholar]
- 30.Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15(2):105–25. [DOI] [PubMed] [Google Scholar]
- 31.Fonken LK, et al. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 2013;154(10):3817–25. [DOI] [PubMed] [Google Scholar]
- 32.Amin MN, et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab Syndr. 2019;13(2):1213–24. [DOI] [PubMed] [Google Scholar]
- 33.Shinde AB, Song A, Wang QA. Brown Adipose tissue heterogeneity, Energy Metabolism, and Beyond. Front Endocrinol (Lausanne). 2021;12:651763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Sulaiti H, et al. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J Transl Med. 2019;17(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turki A, et al. Gender-dependent associations of CDKN2A/2B, KCNJ11, POLI, SLC30A8, and TCF7L2 variants with type 2 diabetes in (north African) Tunisian arabs. Diabetes Res Clin Pract. 2014;103(3):e40–3. [DOI] [PubMed] [Google Scholar]
- 36.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadaegh F, et al. Gender differences in the impact of 3-year status changes of metabolic syndrome and its components on incident type 2 diabetes mellitus: a decade of follow-up in the Tehran lipid and glucose study. Front Endocrinol (Lausanne). 2023;14:1164771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, et al. Independent and Joint associations of BMI and Waist Circumference with the onset of type 2 diabetes Mellitus in Chinese adults: prospective data linkage study. JMIR Public Health Surveill. 2023;9:e39459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allan CA. Sex steroids and glucose metabolism. Asian J Androl. 2014;16(2):232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harding BN, et al. Changes in melatonin and sex steroid hormone production among men as a result of rotating night shift work - the HORMONIT study. Scand J Work Environ Health. 2022;48(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saksvik IB, et al. Individual differences in tolerance to shift work–a systematic review. Sleep Med Rev. 2011;15(4):221–35. [DOI] [PubMed] [Google Scholar]
- 42.Al-Hrinat J, et al. The impact of night shift stress and sleep disturbance on nurses quality of life: case in Palestine Red Crescent and Al-Ahli Hospital. BMC Nurs. 2024;23(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13(9):547–60. [DOI] [PubMed] [Google Scholar]
- 44.Yen FS, et al. Parental income level and risk of developing type 2 diabetes in Youth. JAMA Netw Open. 2023;6(11):e2345812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemmingsen B, et al. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12(12):CD003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Echouffo-Tcheugui JB, Dagogo-Jack S. Preventing diabetes mellitus in developing countries. Nat Rev Endocrinol. 2012;8(9):557–62. [DOI] [PubMed] [Google Scholar]
- 47.Chew NWS, et al. Cell Metab. 2023;35(3):414–e4283. The global burden of metabolic disease: Data from 2000 to 2019. [DOI] [PubMed]
- 48.Eriksson AK, et al. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care. 2013;36(9):2683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ika K, et al. Shift work and diabetes mellitus among male workers in Japan: does the intensity of shift work matter? Acta Med Okayama. 2013;67(1):25–33. [DOI] [PubMed] [Google Scholar]
- 50.Kita T, et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012;35(2):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberlinner C, et al. Medical program for shift workers–impacts on chronic disease and mortality outcomes. Scand J Work Environ Health. 2009;35(4):309–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article. For further details, please contact the corresponding author.