Abstract
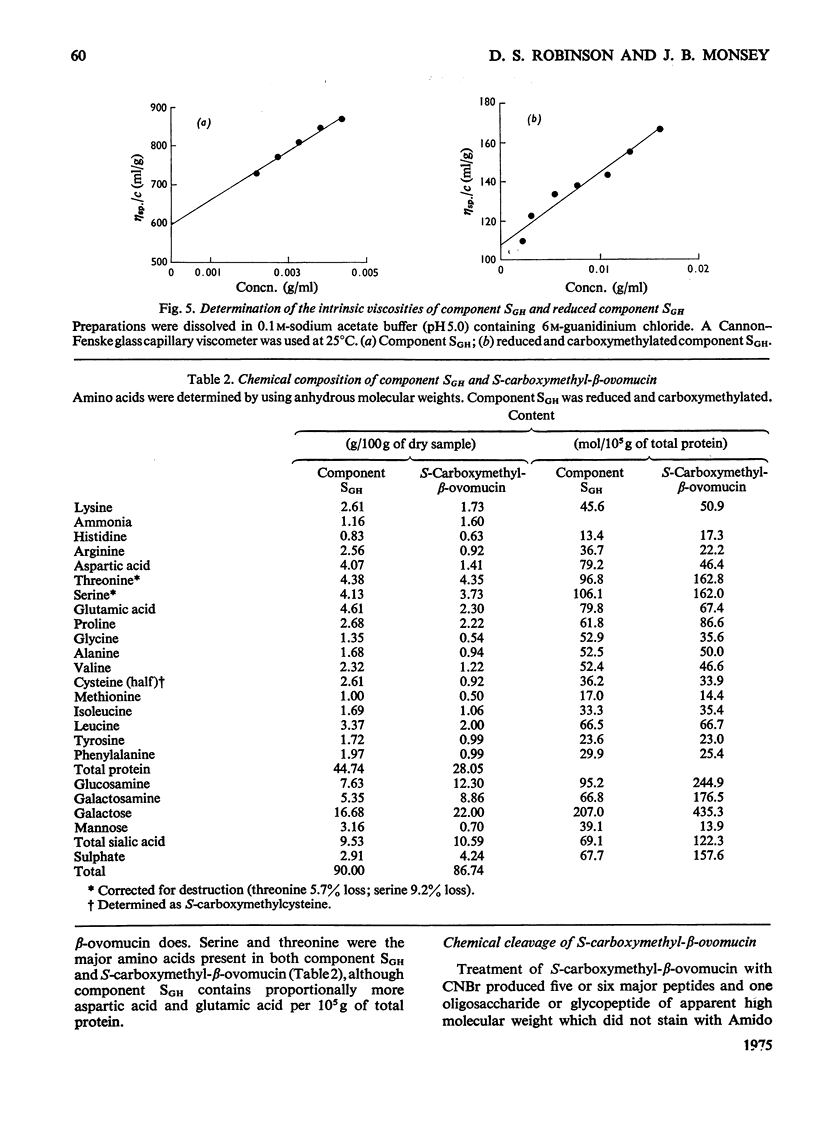
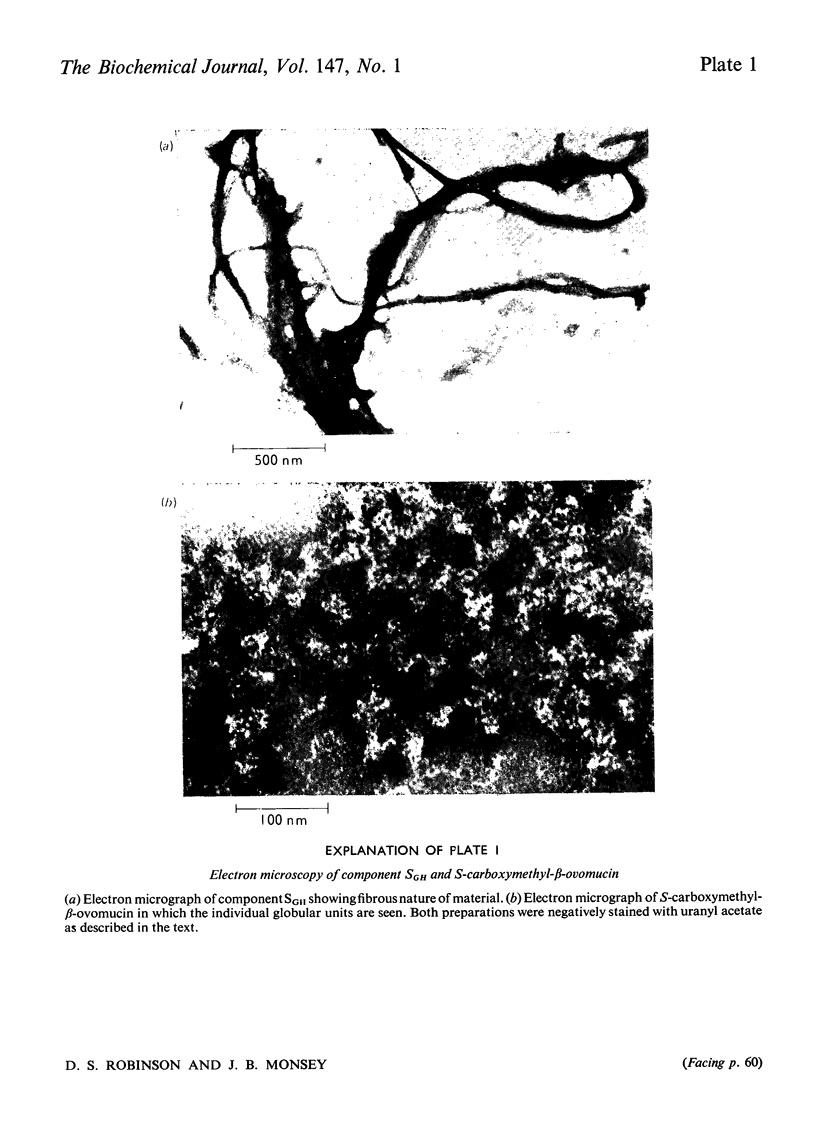
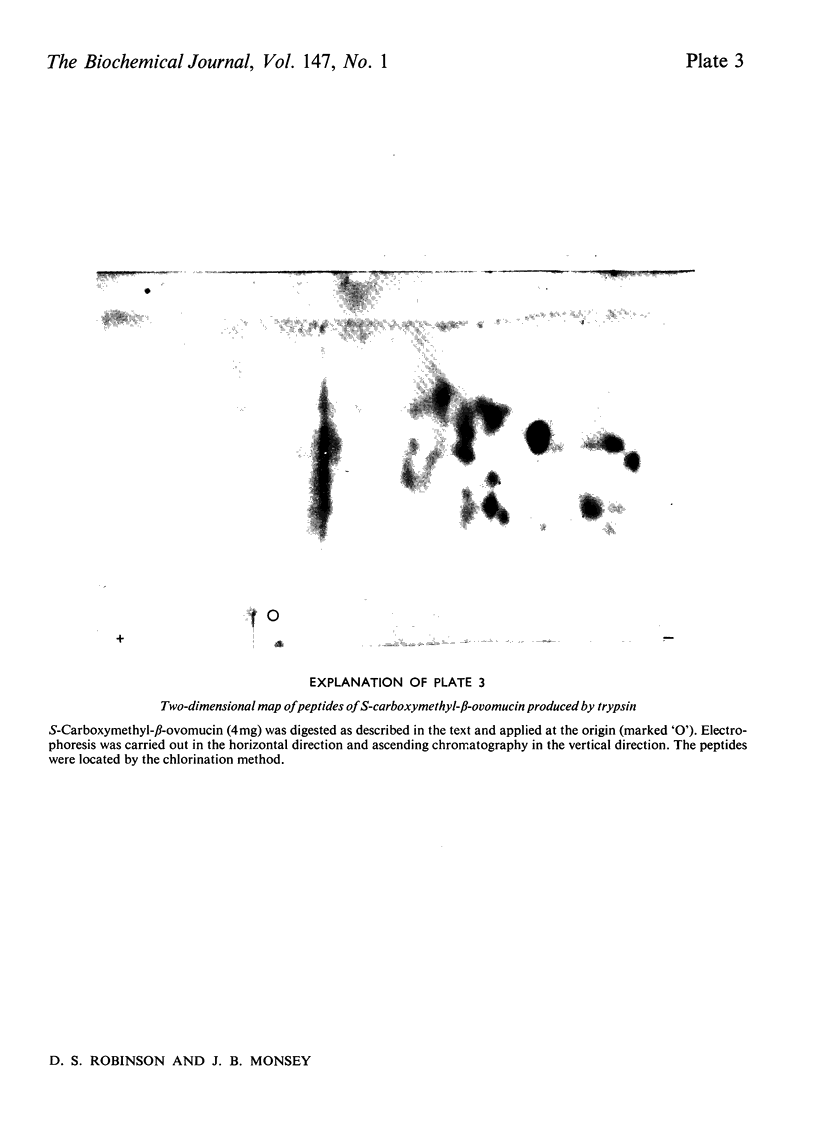
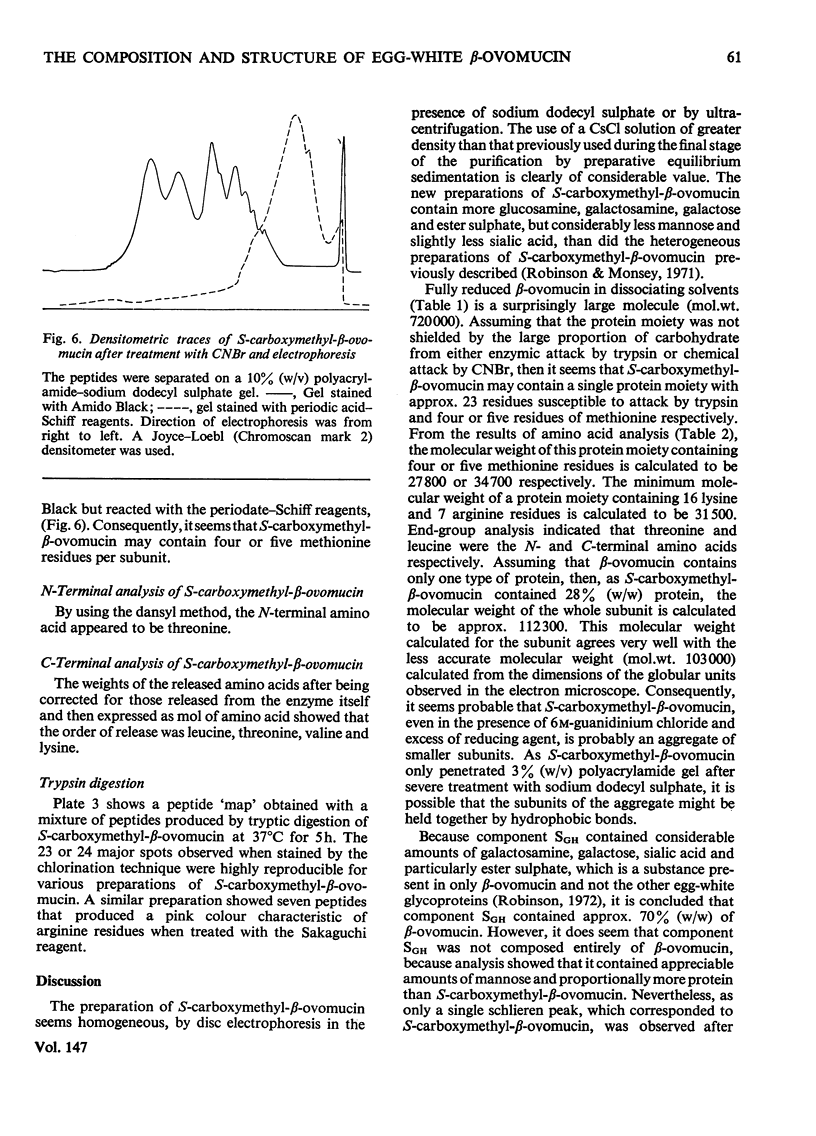
1. New preparations of reduced carboxymethylated beta-ovomucin (S-carboxymethyl-beta-ovomucin) were homogeneous by sedimentation analysis, analytical sedimentation to equilibrium in CsCl gradients, and disc electrophoresis in sodium dodecyl sulphate. 2. Degradation of S-carboxymethyl-beta-ovomucin with either CNBr or trypsin indicated the presence of a subunit (approx. mol. wt. 112300). 3. Electron microscopy showed that S-carboxymethyl-beta-ovomucin consisted of chains of globular units (approx. mol. wt. 103 000). IN 6M-guanidinium chloride S-carboxymethyl-beta-ovomucin existed mainly as an aggregate (mol. wt. 720 000). 4. S-Carboxymethyl-beta-ovomucin contained ester sulphate (4.24%, W/W) and carbohydrate (60%, W/W), which consisted of large amounts of galactose (22%, W/W), galactosamine (8.9%, W/W) and sialic acid (10.6%, W/W). 5. An unreduced soluble fibrous component (component SGH) extracted from crude ovomucin precipitate with 5M-guanidinium chloride contained beta-ovomucin (approx. 70%, W/W). By using the Scheraga-Mandelkern equation the molecular weight of component SGH was calculated to be 11.5 times 10(6).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CROCKER C. Réactions colorées spécifiques de l'arginine et de la tyrosine réalisées après chromatographie sur papier. Biochim Biophys Acta. 1952 Dec;9(6):704–705. doi: 10.1016/0006-3002(52)90236-9. [DOI] [PubMed] [Google Scholar]
- Kobylka D., Carraway K. L. Proteins and glycoproteins of the milk fat globule membrane. Biochim Biophys Acta. 1972 Nov 2;288(2):282–295. doi: 10.1016/0005-2736(72)90249-0. [DOI] [PubMed] [Google Scholar]
- O'Malley J. J., Weaver J. L. Subunit structure of glucose oxidase from Aspergillus niger. Biochemistry. 1972 Sep 12;11(19):3527–3532. doi: 10.1021/bi00769a006. [DOI] [PubMed] [Google Scholar]
- Rees M. W., Short M. N., Kassanis B. The amino acid composition, antigenicity, and other characteristics of the satellite viruses of tobacco necrosis virus. Virology. 1970 Mar;40(3):448–461. doi: 10.1016/0042-6822(70)90188-1. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Monsey J. B. Changes in the composition of ovomucin during liquefaction of thick egg white. J Sci Food Agric. 1972 Jan;23(1):29–38. doi: 10.1002/jsfa.2740230105. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Monsey J. B. Studies on the composition of egg-white ovomucin. Biochem J. 1971 Feb;121(3):537–547. doi: 10.1042/bj1210537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F. K., Kent P. W. Subunits of Tamm-Horsfall glycoprotein. Biochem J. 1970 Mar;116(5):791–796. doi: 10.1042/bj1160791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YANG J. T. The viscosity of macromolecules in relation to molecular conformation. Adv Protein Chem. 1961;16:323–400. doi: 10.1016/s0065-3233(08)60032-7. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]