Abstract
Background
High-intensity interval training (HIT) does not burn fat during exercise. However, it significantly reduces visceral adipose after long-term training. The underlying mechanism may be related to the elevation of fat consumption during the post-exercise recovery period, which is regulated by the hypothalamus–adipose axis. Lactate is a hallmark metabolite of high-intensity exercise, which could mediate significant neuroplasticity through the brain-derived neurotrophic factor (BDNF) pathway. However, whether HIT could enhance hypothalamus activity and adipose catabolism in the recovery period remains to be elucidated. Also, it is worth exploring whether adding lactate administration to prolonged, continuous submaximal aerobic training (AT) could simulate HIT-induced neuroplastic effects and fat loss.
Methods
First, we compared the influence of 4-week HIT and aerobic training (AT) on the electrophysiology of the ventromedial hypothalamus (VMH), which is deeply involved in the regulation of lipolysis, as well as the 24-hour excess post-exercise oxygen consumption (EPOC), the fat oxidation rate and lipolysis. To further confirm whether excess lactate during AT could reproduce the effect of HIT, we also observed the effects of lactate infusion during AT (AT + Lac) on neuroplasticity and metabolism.
Results
Four-week HIT induced higher BDNF expression and a higher neuronal spike firing rate in VMH than AT, accompanied by elevated EPOC, fat oxidation and visceral fat lipolysis. AT + Lac and HITT could induce similar hypothalamic and metabolic changes. However, power spectral density analysis of local field potentials (LFPs) showed that the AT + Lac group was affected in fewer frequency bands than the HIT group.
Conclusion
HIT-induced reduction of visceral fat was accompanied by increased VMH activity. Adding lactate administration to AT could partially reproduce hypothalamic plasticity and the metabolic effects of HIT. However, different band changes of LFPs implied that the neuronal subpopulations or pathways influenced by these two methods were not entirely consistent.
Keywords: High-intensity interval training, Lactate, Visceral fat, VMH, LFPs
Background
Central obesity, caused by the accumulation of visceral fat, leads to a variety of serious metabolic disorders. Dietary or medical interventions induce larger total-body fat loss; however, exercise has a more pronounced impact on visceral fat and brings higher health benefits [1]. Previously, the prevailing view was that moderate-intensity aerobic training (AT) has the highest fat-burning efficiency since it is performed closest to the maximum fat oxidation intensity (FATmax) [2]. However, compared with AT, a growing body of evidence confirms that high-intensity interval training (HIT) could induce a comparable or even stronger effect on visceral fat loss, especially in studies involving obesity or female subjects [3], despite its reliance on glycolytic metabolism and the minuscule rate of fat oxidation during exercise [4].
The precise mechanism by which HIT could induce fat loss remained unclear. The prevailing speculation was that fat burning occurred post-exercise to meet recovery needs after vigorous training [5]. In the immediate (0–1-hour) recovery period, studies suggested small differences in EPOC between HIT and AT, while HIT could induce larger EPOC on a longer time scale (hours after exercise) [6]. The delayed component of EPOC is related to elevated lipid metabolism during the recovery period, and the sympathetic nervous system (SNS) and catecholamine act as key regulators [7]. Adipose metabolism is finely regulated by a neuroendocrine network, with the hypothalamus-SNS-adrenergic receptor (AR) being the prime regulatory axis [8]. In female rats, lipid metabolism levels were relatively weak during the recovery period of AT [9]; however, our past work confirmed that HIT could cause long-term adaptation to visceral fat in female rats. Long-term HIT increased the protein expression and hormone sensitivity of adipocyte ARs, increasing lipase activation and lipolysis in the recovery period [10]. This implied that, as the more vigorous protocol, HIT could enhance post-exercise lipid metabolism in female rodents by enhancing the visceral adipose response to the ‘brain-adipose’ axis. Unfortunately, it is still unclear whether organs upstream of the axis, especially the hypothalamus, also adapt significantly to HIT.
Due to its reliance on glycolysis, a significant feature of HIT is the generation of high levels of lactate. Lactate was once considered a waste product of anaerobic metabolism, but the prevailing view now agrees that it is a linker between glycolysis and oxidative metabolism that plays the role of an energy source, gluconeogenic precursor and signalling molecule by shuttling among cells, tissues and organs [11–13]. During acute hard exercise, elevated blood lactate induces suppression of adipose lipolysis through adipocyte receptor binding [14]. However, long-term lactate administration reduces adipose tissue inflammation and promotes thermogenesis [15, 16]. Lactate has also been proven to mediate brain functional plasticity by increasing the protein expression of brain-derived neurotrophic factor (BDNF) via several processes (e.g. synaptogenesis, neurogenesis and long-term potentiation) [17]. An elevated blood lactate concentration, whether caused by exercise or lactate infusion, could cause several beneficial or detrimental changes in the motor cortex and hippocampus, suggesting that lactate is an important ‘stress’ or ‘exerkine’ for functional brain plasticity [18–20].
The hypothalamus is the primary centre of metabolic regulation, with several nuclei involved in controlling adipose catabolism [21]. Recent studies revealed that high expression of BDNF within the ventromedial hypothalamic nucleus (VMH) enhanced the SNS output of lipolytic signalling [22]. BDNF could mediate long-term potentiation (LTP) by protein synthesis and neurogenic effects [23], which implies its ability to enhance lipolysis during recovery. As the correlation between lactate and BDNF is well established [23, 24], the high levels of lactate produced during HIT may enhance VMH activity during recovery via the BNDF pathway. Unfortunately, few studies have observed the effects of HIT and lactate on hypothalamus function and the accompanying metabolic changes.
In the present study, we hypothesised that HIT may enhance the electrical activity of VMH and visceral fat catabolism during recovery. Further, we hypothesised that adding lactate infusion to AT could reproduce similar neuroplasticity and fat loss effects to HIT. Female rats fed a high-fat diet (HFD) were involved to ensure lower post-exercise lipid metabolism and sufficient visceral fat accumulation in the animal model. As BDNF can mediate neuroplasticity on time scales of hours to days by various mechanisms, the 4-week AT/HIT intervention was executed to ensure sufficient training adaptation [23]. Changes in neuron electrophysiological activity and BDNF expression in VMH, post-exercise (0–24 h) energy metabolism and lipolytic enzyme phosphorylation of visceral fat were observed. These data could help to explain the mechanisms of HIT-induced fat loss and might provide some evidence to confirm whether lactate is a potential monitoring indicator or nutritional supplement to enhance visceral fat loss in exercise.
Methods
Study design
This study included two parts (Exp. 1 and Exp. 2). The Ethics Committee of Hebei Normal University (No. LLSC2024070) gave ethics approval and consent to participate in all experimental procedures. In our previous studies [3, 25, 26], HIT induced greater visceral fat loss than AT in both women and female rats. Due to the significant sexual dimorphism in fat distribution, female rats were involved as subjects to explore the role of the hypothalamus and lactate in visceral fat loss.
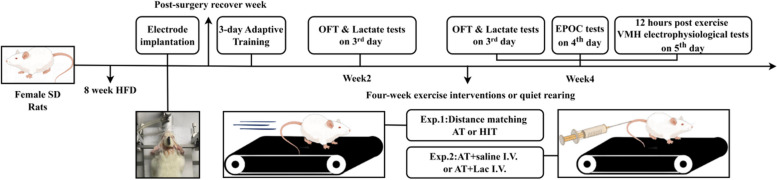
In Exp. 1, to compare the effects of HIT and AT on hypothalamic VMH, EPOC and visceral adipose, 24 rats were involved and divided into Control (C, n = 8), AT (n = 8) and HIT (n = 8) groups using computer-generated random numbers. Rats were fed adaptively for 8 weeks. Then, multichannel electrode implantation surgery of VMH was executed, and the rats were housed for one week to ensure their recovery. The 4-week AT/HIT training intervention was administered following the surgical week and 3-day adaptive training, while the C group was housed under sedentary conditions. Each training week consisted of 5 consecutive training days and 2 rest days (5 − 2 cycle). Open field tests (OFT) and post-exercise blood lactate tests were performed on the third training day of the second and fourth weeks. To avoid interference from circadian rhythms between groups, training was performed at a fixed time (18:00–19:00). Long-range energy metabolism was tested on the fourth training day of the fourth week (20:00 to 19:00 of the next day). VMH electrophysiological tests were performed 11–12 h after the last training session (07:00–08:00), and the animals were sacrificed after anaesthesia (sodium pentobarbital, Solarbio, I. P. ) to collect tissue samples.
In Exp. 2, to verify whether separate blood lactate elevation could induce neuroplasticity and fat loss similar to HIT, 24 rats were divided into Control (C, n = 8), AT with saline I.V. (AT + Saline I.V., n = 8) and AT with lactate I.V. (AT + Lac I.V., n = 8) groups. Animals were housed, trained and tested under the same conditions as in Exp. 1, while the training groups received saline/sodium lactate tail vein injections before each training session (see Fig. 1 for the procedural details).
Fig. 1.
Main experimental procedures (figure generated by Figdraw2.0)
Animals
Due to the distinct differences in fat distribution between the sexes, and to compare our previous study results, which involved all women or rodents [3, 25, 26], female Sprague‒Dawley rats (Changsheng Biotech, China) were selected. The rats were housed in a single cage (23 ± 2 °C, 12-h light/12-h dark cycle, lights on 08:00–20:00) with a high-fat diet throughout all the experiments to ensure significant visceral accumulation. The feed formulations used were described in previous studies [26].
Multi-channel electrode implantation surgery in VMH
The surgical procedure was performed as described in our previous study [26]. First, after being anaesthetized with 5% isoflurane (RWD, China), the animal was fixed on a brain stereotaxic apparatus (RWD, China) with continuous inhalation of 1.5% isoflurane. The ventromedial hypothalamic nucleus (AP: −2.04 mm, ML: 0.4 mm, DV: 8.9 mm) was located with the bregma as the zero point, and the site was marked. The microelectrode was implanted at 50 μm/s, and the electrical signals were collected via a multichannel electrophysiologic recorder (Blackrock Neurotech, USA). The implantation was terminated until the signals were stable with a ≥ 3:1 signal-to-noise ratio of neuronal firing. The surgical site was sealed with biological silicone and reinforced with dental cement. Dexamethasone was administered to mitigate postoperative complications, and a one-week rest period was carried out for postsurgery recovery.
Exercise protocol and lactate administration
Each training group was given AT or HIT 10° uphill treadmill running intervention for 4 weeks. One AT contained 45 min of continuous medium-speed running (approximately 60% VO2max intensity), while each HIT contained numbers of one min high and two min medium-speed cycles. Running speeds were determined according to a graded incremental exercise test (GXT). The maximum running velocity of the HIT equaled the exhaustion speed (ES) of the last stage in the GXT and were adjusted at the end of the 2nd training week to match the increasing running capacity of the rats. To match the training volume, the number of HIT cycles was adjusted to ensure equal average distances between AT and HIT. The running speed of AT increased from 18 to 20 m/min, and the maximum running speed of HIT increased from 28 to 34 m/min throughout the training period. More protocol details have been described in previous studies [10]. In Exp. 1, to identify training-induced lactate elevation, tail-tip blood was collected from rats immediately after exercise and tested with a Biosen C-Line lactate analyser (EKF, Germany). To compare the fatigue levels in HIT vs. AT, the OFT was performed 12 h after exercise, and the horizontal movement distance was calculated via the behavioral analysis software Smart 3.0. In Exp. 2, to achieve high blood lactate concentrations during AT, AT + Lac I.V. subjects received a tail vein injection of sodium L-lactate (Macklin, China; 0.15 g/kg for the 1st and 2nd weeks; 0.2 g/kg for the 3rd and 4th weeks) before each training session. Drug dosages were determined via a preexperiment with reference to a previous study [16]. With this dose, blood lactate concentrations reached 8–10 mmol/L after 25 min of AT and were approximately 4–6 mmol/L at the end of AT.
Post-exercise metabolism monitoring
Post-exercise metabolism was tested by an animal energy monitoring system (CLAMS, Columbus Instruments, USA). Due to the small difference between HIT and AT in the rapid phase (0–1 h) of EPOC and to avoid immediate stress entering the monitoring compartment, the rats were put into the test room approximately 30 min after training, and post-exercise metabolism data (oxygen uptake and respiratory quotient, RQ) were collected only in the slow phase (1–24 h) of EPOC. The fat oxidation rate was calculated via the nonprotein respiratory quotient (fat oxidation rate = 1.695 × VO₂ − 1.701 × VCO₂) expressed in g/hr [27, 28]. To avoid errors in resting metabolic rate changes due to circadian rhythms, all groups were tested during the same post-exercise period (20:00 to 19:00 of the next day).
Serum and visceral adipose glycerol test
Serum and visceral adipose glycerol levels were used to represent lipolysis levels in the whole body and localized fat pads. The samples were tested via the enzyme-labelled microplate method via a glycerol assay kit (F005-1-1 for serum and F005-2-1 for adipose tissue; Jiancheng Bioengineering, China). Twenty samples were randomly selected and tested twice. The intragroup correlation coefficient (ICC) was calculated for quality control and was > 0.85 in both tissues.
Acquisition and analysis of VMH electrophysiological data
The acquisition/analysis methods for these experiments were described in our previous study [29]. Electrical signals from the VMH were collected 12 h after the last training by the CerePlex Direct multichannel electrophysiological data acquisition system (Blackrock Neurotech, USA). The sampling frequency was set at 2 kHz, with a low-pass filter at 250 Hz for data collection. After a 10-minute recording period, a 3-minute segment was randomly intercepted. The number of neuron action potentials (spike firing rate) and local field potentials (LFPs) were analysed by Neuro Explorer (v5.433). Frequency bands were classified into delta (δ, 0.5–4 Hz), theta (θ, 4–8 Hz), alpha (α, 9–12 Hz), beta (β, 12–30 Hz), and gamma (γ, 30–100 Hz) bands.
Western blotting
Periuterine fat was collected to represent visceral fat. Adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) are the most important rate-limiting enzymes for triglyceride hydrolysis. Since ATGL is influenced by both SNS and other proteins such as comparative gene identification-58 (CGI-58), while HSL activity is directly regulated by the SNS-cAMP-PKA pathway [30], the ser660 phosphorylation of hormone sensitive lipase (HSL, a key rate-limiting lipolyase) was selected to assess lipolysis. Adipose tissue samples were processed in a lysis mixture (Solarbio, China) containing a phosphatase inhibitor (Thermo Fisher Scientific, USA). The protein mixture was obtained through shearing, homogenization, ultrasonic disruption, and low-temperature centrifugation. The protein concentration was determined via a BCA kit (Solarbio, China). The target protein was separated on a 10% SDS‒polyacrylamide gel and transferred to a PVDF membrane. After blocking, the membranes were sequentially incubated with primary antibody against HSL-ser660 (1:2000, Cell Signaling Technology, USA) and secondary antibody (1:10000, Solarbio, China). To observe the total amount of activated lipolyase, while similar AT/HIT could not alter the protein expression of HSL in our previous studies [25, 26], ser660 phosphorylation was normalized to that of β-actin. The protein bands were detected via the Fusion FX imaging system (VILBER LOURMAT, France), and the optical densities were analyzed via Image J (v1.54 g).
Immunohistochemical (IHC) analysis of BDNF
After perfusion with saline, the brains were reperfused with 4% paraformaldehyde and fixed for 48 h. The fixed tissue was gradually dehydrated via a sucrose gradient, embedded in OCT and sectioned with a cryostat (Leica, Germany) at a thickness of 10 μm. After heating and blocking, the sections were incubated overnight with BDNF primary antibody (1:200, ET1606-42, HuaBio, China) and then incubated for 50 min with a secondary antibody (1:200, AS014, Abcam, UK). DAPI staining was performed at room temperature for 4 min, followed by washing with PBS. Finally, the sections were mounted with anti-fade mounting medium, and the images were observed and captured via an upright microscope and panoramic tissue cell quantitative analyzer (Tissue Gnostics, Vienna, Austria). The DAPI-stained nuclei appeared blue, while BDNF was visualized in red. The cell density was automatically determined via Image J (v1.54 g).
Statistical analyses
The data are presented as the means ± SDs and were analyzed via SPSS 25.0. Trends between groups over time were analyzed by repeated measures two-way ANOVA. A post hoc test was used for pairwise comparisons when a cross effect (time × group, P < 0.05) was found. Between-group differences on the same time transect were analyzed via one-way ANOVA. The sample size of the present experiment was eight, which is larger than the minimum sample size requirement in previous studies (n = 4, α = 0.05, power = 0.8) [26].
Results
Body weight, food intake, visceral fat, OFT and lactate changes during exp. 1
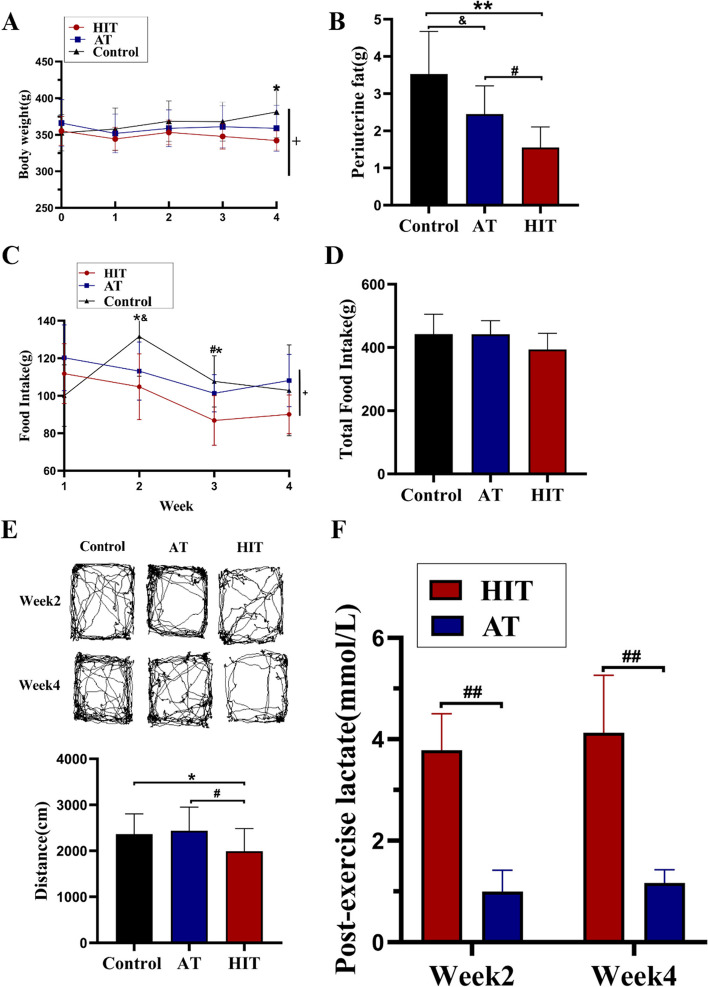
After 4 weeks of training, the body weight of the HIT group was significantly lower than that of the control group, while there was no significant difference between the AT and control groups (Fig. 2A, time × group P < 0.05). Although food intake varied among the groups during the training period (Fig. 2C, time × group P < 0.05), no difference in total food intake among the groups was found (Fig. 2D). The visceral fat mass of the HIT group was significantly lower than that of the AT and control groups (Fig. 2B). The immediate post-exercise blood lactate level of the HIT group was approximately four times greater than that of the AT group at both the 2nd and 4th weeks (Fig. 2F). The OFT was performed after the 2nd and 4th weeks of exercise, and the HIT group moved significantly shorter distances than the control and AT groups did, while no difference between the AT and control groups was found (Fig. 2E).
Fig. 2.
Body weight, food intake, VAT and lactate during and after Exp. 1. A Body weight changes during the 4-week training; B Periuterine fat mass (representing visceral fat); C Changes in food intake during the 4-week training; D Total food intake; E OFT trajectory and horizontal moving distance; F Lactate levels immediately after training. +: Time×Group, P<0.05; *: HIT vs Control, P<0.05; **: HIT vs control, P<0.01; #: HIT vs AT, P<0.05; ##: HIT vs AT, P<0.01; &&: AT vs Control group, P<0.01
Post-exercise metabolic activity and lipase phosphorylation after 4 weeks of training in Exp. 1
After the 4-week intervention, a significant interaction effect on oxygen uptake was detected between the groups (time × group, P < 0.05; Fig. 3A). HIT induced a 1–12-hour and 12–24-hour increase in EPOC, which was significantly greater than that of AT at 1–12 h post-training (P < 0.05, Fig. 3B‒D). Compared with AT and the control, HIT induced a greater whole-body fat oxidation rate at 1–12 h post-training, whereas AT increased the total fat oxidation rate at 24 h (Fig. 3E‒H). The RQ of both training groups decreased and was lower in the HIT group than in the AT group at 5–7 h post-exercise. The RQ of both training groups decreased and was lower in the HIT group than in the AT group at 5–7 h post-exercise. At the time at which the animals were sacrificed (12th hour after the last training), the level of serum glycerol was lower in the AT group than in the training group, whereas the level of visceral fat glycerol was significantly greater in the training group (HIT > AT > Control, P < 0.05; Fig. 3J‒L). Additionally, HSL-ser660 phosphorylation levels were increased in both the HIT and AT groups (P < 0.05, Fig. 3L). In summary, these data suggest that the pro-lipolysis and fat-oxidizing effects of HIT occur mainly within 1–12 h post-exercise, which mediate increased visceral (but not visceral) fat catabolism.
Fig. 3.
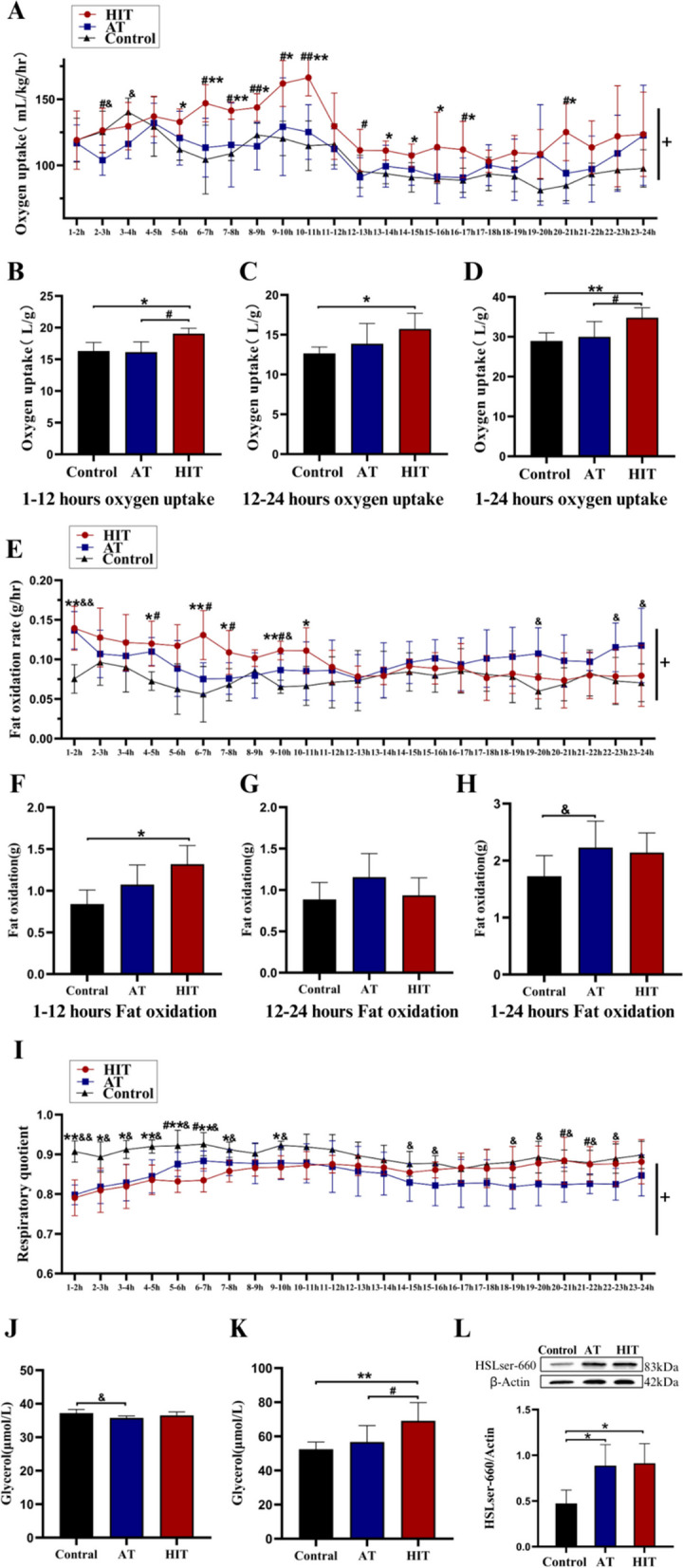
1–24-Hour EPOC and HSL-ser660 phosphorylation in Exp. 1. A Changes in oxygen uptake within 24 hours post-exercise; B, C and D Total oxygen uptake during 1–12, 12–24 and 1–24 hours post-exercise; E Changes in fat oxidation rate within 24 hours post-exercise; F, G and H Total fat oxidation during 1–12, 12–24 and 1–24 hours post-exercise; I Respiratory quotient (RQ) within 24 hours post-exercise; J Serum glycerol concentration; K Glycerol concentration of periuterine fat; L HSL-ser660 phosphorylated expression. +: Time×Group, P<0.05; *: HIT vs Control, P<0.05; **: HIT vs Control, P<0.01; #: HIT vs AT, P<0.05; ##: HIT vs AT, P<0.01; &: AT vs control, P<0.05
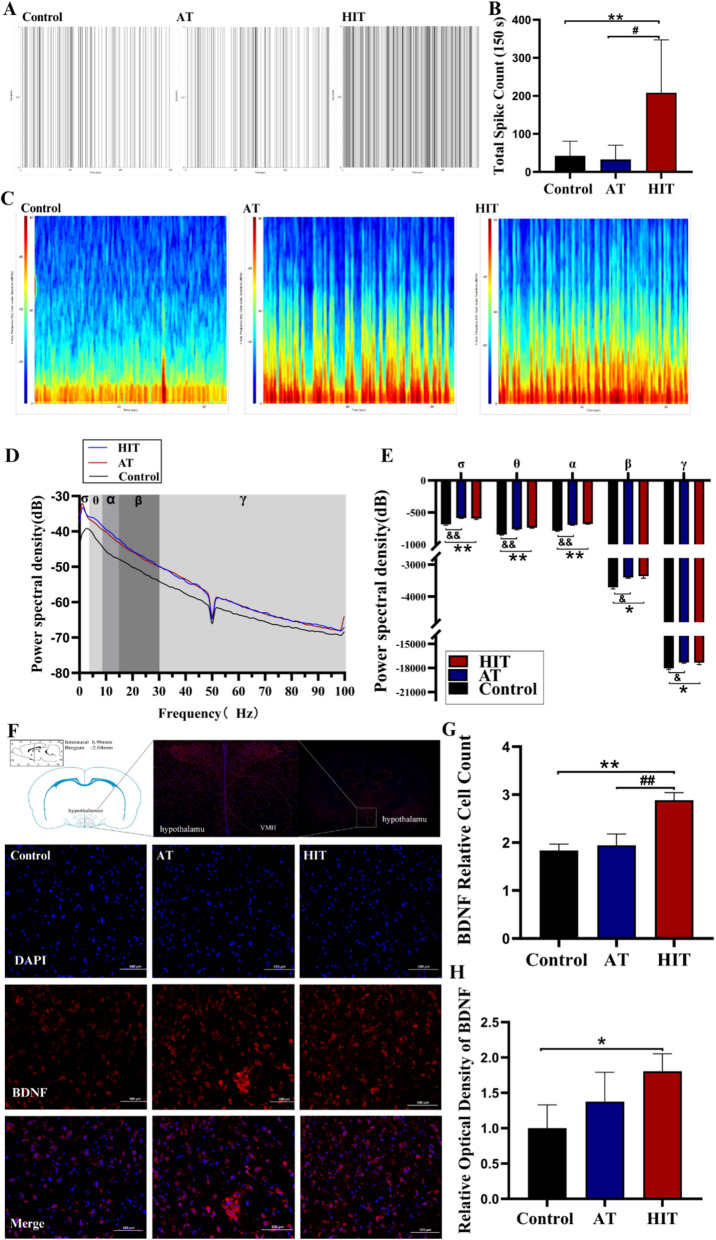
Group differences in VMH electrophysiology and BDNF expression in Exp. 1
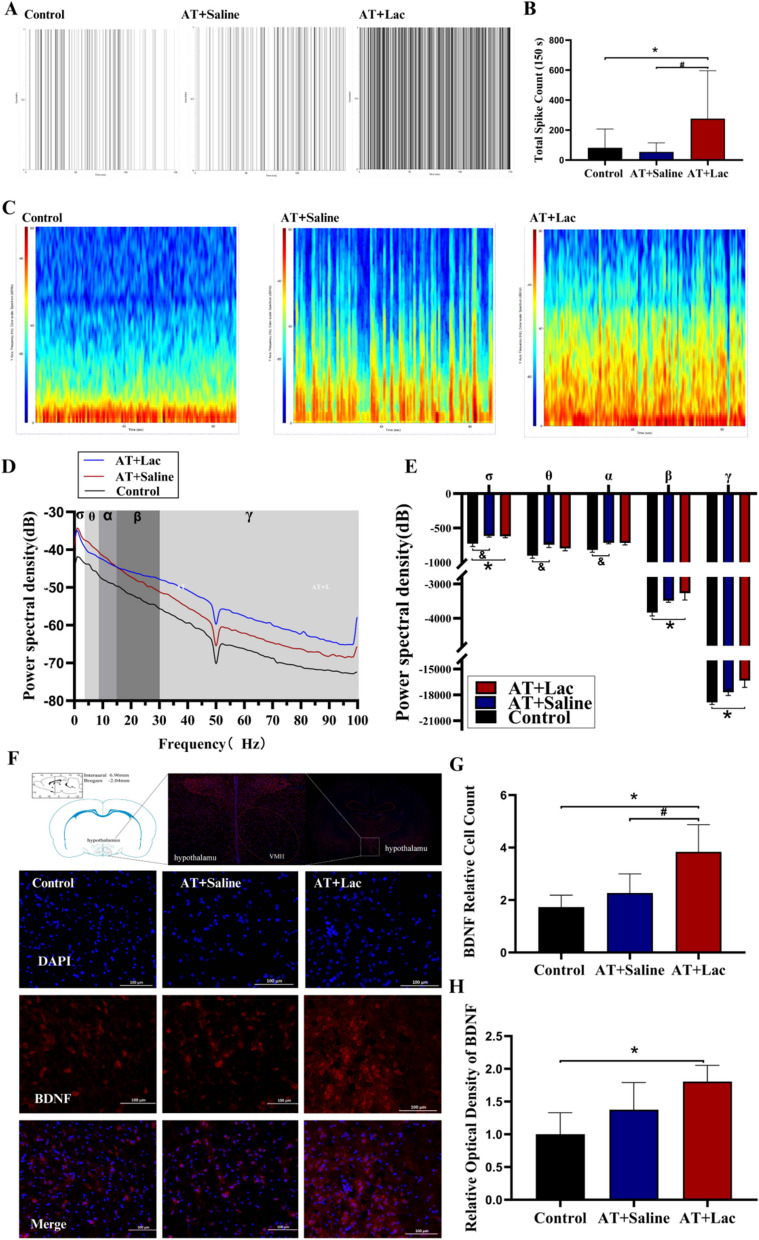
At the end of Exp. 1, LFPs were significantly changed in both exercise groups. Similar to HSL, both training protocols increased the power spectral density of the δ, θ, α, β and γ bands (P < 0.05 or P < 0.01, Fig. 4C, D, E), whereas no difference between HIT and AT was found. However, the spike firing rate of HIT increased significantly (nearly 4 times higher than that of the control/AT, Fig. 4A, B). Like Spike, HIT significantly elevated BDNF expression and the number of BDNF positive cells in the VMH (P < 0.01, Fig. 4F, G, H), suggesting that high-intensity exercise might increase the number of action potentials via BDNF induced neuroplasticity.
Fig. 4.
LFPs, spike firing rate, and BDNF expression of VMH in Exp. 1. A Spike firing point for 150 s; B Spike firing counts; C LFPs spectrogram of the VMH for 3 min; D-E Power spectral density distribution and integration of δ-γ bands; F IHC images of BDNF in the VMH (red: BDNF, blue: DAPI); G Number of BDNF positive cells; H Optical density of BDNF. *: HIT vs Control, P<0.05. **: HIT vs Control, P<0.01; #: HIT vs AT, P<0.05; ##: HIT vs AT, P<0.01; &: AT vs Control, P<0.05; &&: AT vs Control, P<0.01
Body weight, food intake and visceral fat changes during exp. 2
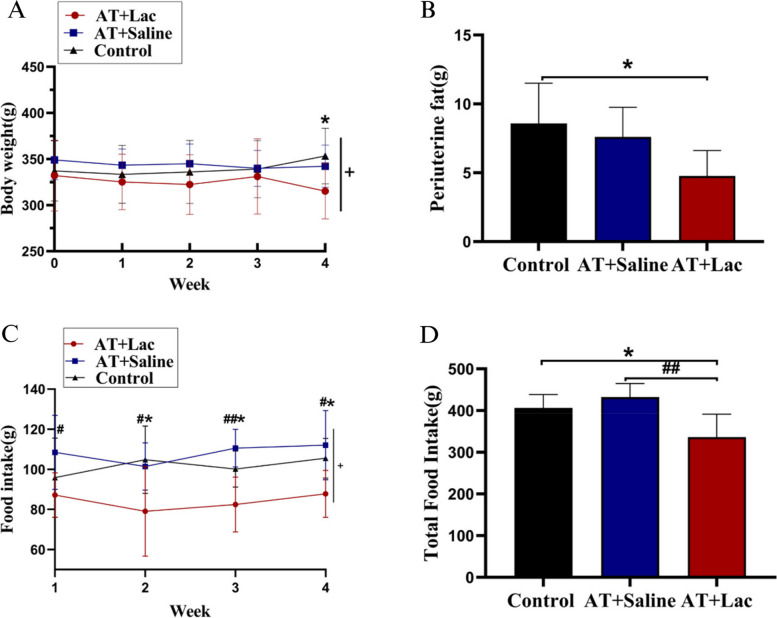
In Exp. 2, lactate infusion prior to AT induced a significant decrease in body weight (time × group, P < 0.05, AT + Lac I.V. vs. control, P < 0.05; Fig. 5A) and visceral fat mass (P < 0.05; Fig. 5B), which suggested that elevated levels of isolated blood lactate could induce fat loss similar to HIT. In addition, lactate infusion induced a significant reduction in food intake, an effect that HIT did not have. Notably, unlike in Exp. 1, the 4-week AT in Exp. 2 did not significantly affect visceral fat mass or food intake, which may have been caused by the different growth curves of the animals in Exp. 1 and 2, which were not strictly executed at the same time.
Fig. 5.
Body weight and food intake during and after Exp. 2. A Body weight changes during the 4-week training; B Periuterine fat mass (representing visceral fat); C Changes in food intake during the 4-week training; D Total food intake. +: Time×Group, P<0.05; *: AT+Lac vs Control, P<0.05; #: AT+Lac vs AT+saline, P<0.05; ##: AT+Lac vs AT+saline, P<0.01
Post-exercise metabolic activity and lipase phosphorylation after 4 weeks of training in Exp. 2
Post-training metabolic monitoring revealed that lactate infusion produced a long-term increase in EPOC in the AT + Lac group (time × group, P < 0.05; Fig. 6A), similar to HIT in Exp. 1. In particular, the sum of the 1–12 h EPOC in the AT + Lac group was significantly greater than that in the control group (P < 0.05, Fig. 6B), whereas the AT + Saline group did not show an increase in oxygen uptake at any time point. Compared with AT, lactate infusion into AT induced lower total EPOC (12–24 h and 1–24 h P > 0.05, Fig. 6C, D) and fewer time points of elevation. Moreover, lactate infusion into AT induced a greater whole-body fat oxidation rate at 1–12 h post-training than AT or the control (Fig. 6E‒H), but no change of RQ in both training groups was found. At the time when the animals were sacrificed (12 h after the last training), the serum glycerol concentration was reduced in the AT + Saline group, whereas the visceral fat glycerol concentration was significantly increased in the AT + Lac group (P < 0.05, Fig. 6J-K). HSL-Ser660 phosphorylation in the AT + Lac group was greater than that in the AT + Saline group (P < 0.05, Fig. 6L), which was consistent with the glycerol content of visceral fat. In summary, these data suggest that lactate infusion into AT enhances visceral fat catabolism 1–12 h after exercise.
Fig. 6.
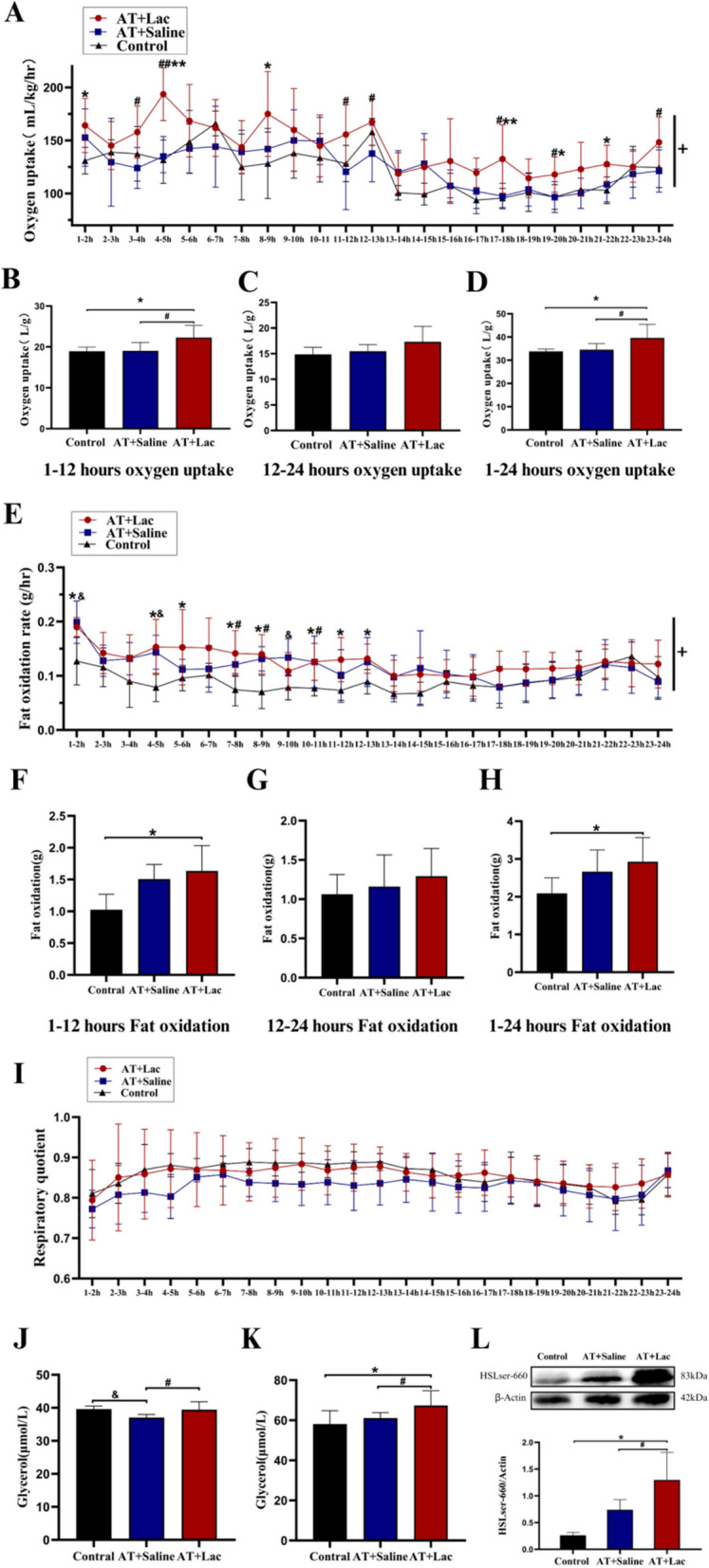
1–24-Hour EPOC and HSL-ser660 phosphorylation in Exp. 2. A Changes in oxygen uptake within 24 hours post-exercise; B, C and D Total oxygen uptake during 1–12, 12–24 and 1–24 hours post-exercise; E Changes in fat oxidation rate within 24 hours post-exercise; F, G and H Total fat oxidation during 1–12, 12–24 and 1–24 hours post-exercise; I Respiratory quotient (RQ) within 24 hours post-exercise; J Serum glycerol concentration; K Glycerol concentration of periuterine fat; L HSL-ser660 phosphorylated expression. +: Time×Group, P<0.05; *: AT+Lac vs Control, P<0.05; **: AT+Lac vs Control, P<0.01; #: AT+Lac vs AT+Saline, P<0.05; ##: AT+Lac vs AT+Saline, P<0.01
Effects of lactate infusion on VMH electrophysiology and BDNF expression in Exp. 2
Electrophysiological data at 12 h after the last training session revealed that lactate infusion significantly increased the post-exercise spike firing rate in the VMH (P < 0.05, Fig. 7A, B), which was similar to HIT. However, unlike Exp. 1, lactate infusion into AT increased the power spectral density of only the δ, β and γ bands of LFPs (P < 0.05; Fig. 7C, D, E), implying that fewer neuronal subpopulations were affected than in HIT. Lactate infusion into the AT also increased BDNF expression and the number of BDNF-positive cells in the VMH (P < 0.05, Fig. 7F, G, H), which was similar to the findings of HIT, suggesting that HIT partially reproduced HIT-induced VMH plasticity.
Fig. 7.
LFPs, spike firing rate and BDNF expression of VMH in Exp. 2. A Spike firing point for 150 seconds; B Spike firing counts; C LFP spectrogram of the VMH for 3 minutes; D-E Power spectral density distribution and integration of δ-γ bands; F IHC images of BDNF in the VMH (red: BDNF, blue: DAPI); G Number of BDNF positive cells; H Optical density of BDNF. *: AT+Lac vs Control, P<0.05; #: AT+Lac vs AT-saline, P<0.05; &: AT+Saline vs Control, P<0.05
Discussion
Although several studies have confirmed that HIT-induced fat catabolism occurs during the post-exercise recovery period [6], the underlying mechanisms have not been clarified. Most pertinent studies to date have focused on the adaptive changes of peripheral adipose tissue to HIT. Enhanced sensitivity to lipolytic hormones [26], increased browning thermogenesis [31] and lactylation of fatty acid synthase [32] have been demonstrated to be involved in the post-exercise fat loss process associated with HIT. However, since a neuroendocrine network finely regulates fat metabolism, how HIT or extra lactate elevation during AT affects the hypothalamus in the post-exercise recovery period remains uncertain.
In the present study, the two main novel findings were as follows: (a) Compared to AT with the same running distance, HIT mediated the enhancement of BDNF expression, increased the spike firing rate and altered the power density distribution of LFPs in hypothalamic VMH, which was accompanied by elevated EPOC, the whole-body fat oxidation rate and visceral fat lipolysis during 1–12 h post-exercise. (b) Lactate infusion combined with AT reproduced similar alterations in hypothalamus and adipose metabolism to HIT, although it only mediated LFP changes in a narrower range among different bands.
Several systematic reviews and meta-analyses have demonstrated that HIT effectively reduces body weight and fat [3, 33, 34]. In some studies involving female participants, HIT has been shown to cause higher EPOC over an extended period after exercise [6] and result in a more pronounced reduction in visceral fat compared to AT [3, 35]. Female participants exhibit lower levels of lipid metabolism during recovery from AT [9]. As a stronger stress, HIT may enhance post-exercise fat catabolism in this group. The present study confirmed that visceral fat weight decreased more in the HIT group (Fig. 2A and B). Although there was fluctuation during the training period, no significant difference in total food intake between the exercise and control groups was found (Fig. 2C and D). OFT data showed that the horizontal distance travelled was significantly lower in the HIT group and unchanged in the AT group in the second and fourth weeks (Fig. 2E), which suggested that HIT does not increase the animal’s voluntary physical activity during the recovery period. Rather, the increased exercise intensity produces greater fatigue and reduces the animal’s desire to exercise voluntarily. The immediate post-exercise blood lactate concentration in the HIT group was approximately four times that in the AT group (Fig. 2F), which confirmed its lactate-raising effect. In summary, these data reconfirmed that HIT reduced visceral fat by more than AT without changing food intake or voluntary physical activity in female rats.
EPOC was typically observed to encompass ‘the rapid component’ within the initial 0–1 h after exercise and ‘the slow component’ within several subsequent hours [6]. The classical ‘oxygen debt’ hypothesis attempted to link EPOC with lactate metabolism during recovery; however, numerous studies have demonstrated a dissociation between them [36]. The rapid component of EPOC is associated with the administration of oxygen, restoration of body temperature, circulation and ventilation and the resynthesis of ATP-CP, while the slow component is linked to the synthesis of glycogen and the repair of tissues that are more relevant to fat catabolism [6]. As much as half of the slow component is caused by the increasing energy cost of lipid metabolism [37], suggesting the importance of long-lasting EPOC for fat loss. HIT augments EPOC to a greater extent than AT, in both the fast and the slow component periods. However, due to the limited duration, the absolute value of the fast component was much lower, equalling approximately one-tenth of that observed in the slow component [38]. Post-exercise fat oxidation is more closely associated with the slow component of EPOC, as it has a larger total volume and is more dependent on fat as the substrate. The present study confirmed that the slow component of EPOC, as well as the fat oxidation rate during the same period, was significantly higher in the HIT vs. the AT group. The post-exercise RQ decreased in both training groups, while it was lower in the HIT group than in the AT group at 5–7 h, which suggested a higher whole-body percentage of fat oxidation. These data confirmed prior study of differential respiratory quotient responses to exercise, with high-intensity exercise induce a greater reduction in RQ [39, 40]. Additionally, enhanced visceral, but not whole-body, adipose lipolysis (increased glycerol and HSL phosphorylation) was found 12 h after HIT. Free fatty acids (FFA) represent a crucial energy source for repairing skeletal muscle tissue [5], and lactate or glycerol is the primary carbon source for gluconeogenesis [41]. Enhanced visceral fat lipolysis could meet the needs of FFA and glycerol for rigorous exercise-induced tissue repair and gluconeogenesis, explaining why HIT effectively reduced visceral fat, consistent with data from previous studies [26, 42, 43].
Due to the strong correlation between adrenaline (E) and norepinephrine (NE) secretion and exercise intensity, the traditional view is that the enhanced lipolysis observed after exercise was caused by elevated catecholamine secretion [44]. However, HIT-induced catecholamine elevation usually returns to normal levels within 1–3 h after exercise [45]. Such a short-term catecholamine elevation cannot explain the increased EPOC and fat catabolism over 12 h. Besides the circulation, another source of NE for adipose is neurotransmitter release from sympathetic nerve endings. Long-duration lipid metabolism might be related to hypothalamic regulation. Our previous study elucidated a portion of the mechanism from the standpoint of receptor sensitivity. The findings revealed that long-term HIT could enhance the protein expression of the β3-type adrenergic receptor (β3-AR) in visceral adipocytes, subsequently elevating their sensitivity to catecholamines. Moreover, isolated isoprenaline stimulation was demonstrated to induce a more pronounced degree of triglyceride hydrolysis in visceral adipocytes of the HIT group, suggesting enhanced hormone sensitivity [26]. Nevertheless, despite the enhanced sensitivity to NE, TG hydrolysis would not occur without the ‘brain-adipose axis’. VMH is a key nucleus of the energy homeostasis regulatory system in the hypothalamus, which is deeply involved in the SNS output and lipolysis regulation [46]. To test whether the activity of VMH was enhanced after HIT, we employed in vivo multichannel electrophysiological techniques to observe electrophysiological changes directly. The results demonstrated that the number of single-neuron action potentials (spike firings) of VMH was markedly increased in the HIT group. Additionally, the power spectral densities in the δ, θ, α, β and γ bands of LPFs exhibited a notable increase. These data indicated that the VMH exhibited increased activity during the recovery period of HIT.
The potential mechanism by which HIT enhances hypothalamic electrical activity might be related to the BDNF pathway. BDNF is the most abundant neurotrophic factor in the brain, mediating a variety of neuroplasticity changes, and the correlation between lactate and BDNF is well established [23]. It has been confirmed that BDNF can significantly enhance the electrical activity of neurons in the VMH [47], thereby altering energy homeostasis. Deletion of the BDNF gene in the VMH resulted in hyperphagia and obesity [48]. Conversely, injection of BDNF into the VMH was observed to increase the metabolic rate and reduce visceral adiposity [49, 50], which indicates that BDNF plays a key regulator of lipid metabolic control of VMH. Additionally, BDNF is a crucial exerkine in the enhancement of cognitive abilities, memory and other cerebral functions. A recent network meta-analysis confirmed that HIT could induce greater BDNF production than AT [51]. However, most previous studies investigating the relationship between HIT and increased BDNF focused on blood and the cortical and hippocampal regions, but few studies examined this relationship in the hypothalamus. We speculated that HIT-induced VMH electrophysiological changes might also be achieved by increasing BDNF expression. In the present study, IHC data validated the above speculation by demonstrating that HIT resulted in a more pronounced BDNF increase in VMH (Fig. 4F and H).
It should be noted that BDNF mediates neuroplasticity via different mechanisms and on distinct time scales. Within hours, BDNF could enhance protein synthesis and long-term potentiation (LTP), and on longer time scales, BDNF could help neurons of newborns join neuronal circuits by promoting neurogenesis [23]. The present study confirmed that HIT increased the neuron firing rate in the VMH, which may relate to enhanced LTP and an increased number of newborn neurons. In our view, BDNF-induced changes in neuroplasticity are like a kind of ‘qualitative change from a quantitative change’. HIT and lactate may induce both acute and chronic effects of BDNF. Although the effects of a single training bout on neural networks may be temporary and easily fade, long-term interventions may lead to more persistent changes in hypothalamic plasticity, which can mediate more significant metabolic changes. To ensure a significant accumulation of training effects, the present study executed a long-term intervention. Therefore, it could not be determined from the current data whether the HIT-mediated enhancement of VMH activity and fat catabolism was an acute effect of a single training session or a long-term adaptation. The different mechanisms by which BDNF mediates neuroplasticity on different time scales may be an entry point for future research.
A more probing question is thus posed: by which specific stimulus signal did HIT achieve elevated EPOC and lipolysis over 12 h? HIT has been demonstrated to enhance numerous exercise factors, including IL-6, irisin, leptin, adiponectin, HIF-1, etc., which have all been linked to improvements in obesity. HIT comprises two core components: ‘glycolytic metabolism’ during the high-intensity period and ‘aerobic oxidative metabolism’ during the intervals. An intriguing study [52] demonstrated that, akin to HIT, vigorous-intensity continuous training (VCT, > 85% VO2max) also elicited heightened EPOC and post-exercise fat oxidation in comparison to AT. This indicates that glycolysis, which produces high-dose lactate, may play a more significant role. Lactate is a hallmark product of high-intensity exercise. During acute hard exercise (RQ ≥ 1.0), significant elevation of blood lactate inhibits lipolysis through the hydroxycarboxylic acid receptor 1 (HCAR1, also called GPR81, a lactate-specific G protein-coupled receptor) binding [14]. However, HIT has been demonstrated to facilitate the browning of adipose tissue [53] and inhibit fat synthesis via lactate [32]. Even when not engaged in exercise, intravenous or oral lactate administration could still relieve adipose tissue inflammation by G protein-coupled receptor 132 (GPR132)-AMPKα1 pathway and promote adipocyte browning by GPR81-p38 MAPK pathway [16, 54]. In summary, these studies suggested that lactate could mediate both immediate lipolytic inhibition and long-term pro-lipolysis adaptative changes in adipose tissue.
Lactate is also intimately associated with exercise adaptations in brain function, whereby it provides signals that modulate neuronal functions, including excitability, plasticity and memory consolidation [55]. In addition, as an energy substrate, lactate affects brain function as a signal mainly through the hydroxycarboxylic acid receptor 1 (GPR81) and N-methyl-D-aspartate receptor (NMDAR) pathways. In the brain, GPR81 is located primarily at excitatory synapses that inhibits neuronal excitability via cAMP when bound to lactate, which suggested lactate could exacerbate central fatigue by GPR81 during acute exercise [56]. On longer time scales, lactate can alter neuroplasticity via the NMDAR-BDNF pathway: lactate entering neurons can induce NMDAR opening and calcium influx, which in turn promotes ERK phosphorylation and BNDF transcription and expression [57]. Therefore, we hypothesise that lactate, as an exerkine, is key to increasing VMH electrical activity and fat catabolism over 12 h after HIT. Exp. 2 was conducted to ascertain whether lactate, rather than other HIT-related signals, could independently influence the hypothalamus and fat catabolism during exercise. The results showed that pre-exercise lactate administration to the AT group could mediate higher BDNF expression (Fig. 7F and H) and neuron firing in VMH. Furthermore, an elevated slow component of EPOC, fat oxidation, visceral fat lipolysis and fat mass loss were also found. Unlike Exp.1, lactate infusion did not alter the post-exercise RQ. Nevertheless, a direct comparison with different experiments was difficult due to differences in factors affecting RQ (diet, pre-training meal, age, sex, or weather conditions) [40]. However, even in the same experiment, adding lactate infusion to AT did not reduce RQ. One possible reason is that adding lactate to AT did not increase glycogen consumption during exercise as HIT did. Therefore, AT + Lac also could not inhibit post-exercise glucose oxidation (increase RQ). More experiments are needed to compare the differences between the effects of HIT and lactate infusion on whole-body glucose metabolism after exercise. In summary, the results of Exp. 2 suggested that the enhanced lactate concentration during AT could partially reproduce the hypothalamic plasticity and visceral fat loss effects of HIT.
It should be noted that a recent study found that lactate administration without exercise could also promote catabolism of peripheral fat pads via the GPR132 pathway [16]. Unfortunately, due to the limited number of channels in the test instrument, Exp. 2 did not involve a ‘Lac + sedentary’ group. Therefore, it was still unclear whether the hypothalamic adaptation was induced by AT combined with lactate or lactate infusion alone. Further studies are needed to compare the neuroplasticity effects of lactate signalling alone and lactate combined with exercise. It is also important to note, however, that lactate administration did not represent an identical analogue of HIT-induced training adaptation. Lactate infusion alone resulted in a power increase in the δ, β and γ bands in the LFPs of VMH (Fig. 7D-E). Nevertheless, HIT had a broader impact on LFPs (power increase of all δ to γ bands, Fig. 4D-E), indicating that HIT might affect a wider variety of neuron subpopulations than lactate administration combined with AT. Additionally, lactate markedly diminished the animals’ overall food intake throughout the training period, whereas HIT was only noted to exert this effect during the second and third weeks. A recent study revealed that lactate reduced appetite via N-lactoyl-phenylalanine (Lac-Phe) and exerted neuroplastic effects through the BDNF pathway [58]. Food intake reduction in the AT + Lac I.V. group might be related to the higher blood lactate peaks and associated Lac-Phe synthesis caused by I.V. lactate infusion before training. Thus, although AT combined with lactate administration induced similar VMH plasticity, EPOC and visceral fat reduction effects to HIT, the underlying mechanisms were not entirely identical, and more studies are needed in the future to explore the differences between these two interventions.
In conclusion, the present study confirmed for the first time that HIT could increase BDNF expression and electrical activity in the hypothalamic VMH, accompanied by an enhancement of the slow component of EPOC, whole-body fat oxidation and visceral fat lipolysis. Although the underlying mechanisms might not be entirely consistent, lactate administration combined with AT produced effects similar to those of HIT in neuroplasticity and metabolism during recovery. These results may provide two extra hints: firstly, as elevated blood lactate during exercise could enhance fat catabolism, how to monitor and maintain high concentrations of lactate may be an important issue for fat loss; secondly, lactate nutritional supplementation prior to or during exercise may be a potentially promising means of augmenting the visceral fat loss effects of aerobic exercise.
Limitations and prospects
Two limitations existed in this study: first, only female animals were involved in the experiments to ensure continuity with previous studies, and the applicability of the current results to male rodents or women is yet to be explored. Second, due to the limitations of the channel numbers of the metabolic and electrophysiologic monitoring equipment, Exp. 1 and Exp. 2 were not performed simultaneously, and the two data sets could only be compared qualitatively but not quantitatively. In addition, also due to the limited number of test channels, Exp. 2 did not involve a ‘Lac + sedentary’ group, so it is still unclear whether HIT-induced hypothalamic adaptations are replicated by lactate alone or AT combined with lactate. In the future, the specific neuron types and associated signaling pathways within the VMH that can be affected by HIT or lactate should be further identified to provide valuable targets for exercise to control obesity.
Acknowledgements
Not applicable.
Abbreviations
- HIT
High-intensity interval training
- AT
Aerobic training
- AT+Saline I.V.
AT with saline I.V.
- AT+Lac I.V.
AT with lactate I.V.
- FATmax
Maximum fat oxidation Intensity
- BDNF
Brain-derived neurotrophic factor
- VMH
Ventromedial hypothalamic nucleus
- LFPs
Local field potentials
- EPOC
Excess post-exercise oxygen consumption
- HFD
Female rats with high fat diet
- SNS
Sympathetic nervous system
- GXT
Graded incremental exercise test
- LTP
Long-term potentiation
- AR
Adrenergic receptor
- TG
Triglyceride
- OFT
Open field tests
- ES
Exhaustion speed
- RQ
Respiratory quotient
- ICC
Intragroup correlation coefficient
- HSL
Hormone sensitive lipase
- IHC
Immunohistochemical
- FFA
Free fatty acids
- E
Edrenaline
- NE
Norepinephrine
- β3-AR
β3-Adrenergic receptor
- VCT
Vigorous-intensity continuous training
- GPR81
Hydroxycarboxylic acid receptor 1, also called HCAR1
- GPR132
G protein-coupled receptor 132
- ATGL
Adipose triglyceride lipase
- CGI58
Comparative gene identification-58
- NMDAR
N-methyl-D-aspartate receptor
- Lac-Phe
N-lactoyl-phenylalanine
Authors' contributions
Authorship contribution statement. BC and JD designed the training protocol and drafted the manuscript. BC, JD, TS and ZZ performed the surgery and training procedure. YL and BC analyzed the experimental data. WC and YL designed the experiment. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32071171) and the Natural Science Foundation of Hebei Province (No. C2024205024).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Ethics approval and consent to participate in all experimental procedures were approved by the Ethics Committee of Hebei Normal University (No. LLSC2024070).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baishuo Cheng and Jinchan Du contributed to the work equally and should be regarded as co-first authors.
References
- 1.Muscella A, Stefàno E, Lunetti P et al. The regulation of Fat Metabolism during Aerobic Exercise. Biomolecules, 2020,10. [DOI] [PMC free article] [PubMed]
- 2.Schwindling S, Scharhag-Rosenberger F, Kindermann W, et al. Limited benefit of Fatmax-test to derive training prescriptions. Int J Sports Med. 2014;35:280–5. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Tong TK, Kong Z, et al. Exercise training-induced visceral fat loss in obese women: the role of training intensity and modality. Scand J Med Sci Sports. 2021;31:30–43. [DOI] [PubMed] [Google Scholar]
- 4.Dupuit M, Maillard F, Pereira B, et al. Effect of high intensity interval training on body composition in women before and after menopause: a meta-analysis. Exp Physiol. 2020;105:1470–90. [DOI] [PubMed] [Google Scholar]
- 5.Harris MB, andKuo. CH Scientific challenges on Theory of Fat Burning by Exercise. Front Physiol. 2021;12:685166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moniz SC, Islam H, andHazell TJ. Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand J Med Sci Sports. 2020;30:638–51. [DOI] [PubMed] [Google Scholar]
- 7.Børsheim E, Knardahl S, Høstmark AT, et al. Adrenergic control of post-exercise metabolism. Acta Physiol Scand. 1998;162:313–23. [DOI] [PubMed] [Google Scholar]
- 8.Stefanidis Aneta M, Wiedmann Nicole S, Adler Elaine, et al. Hypothalamic control of adipose tissue. Best Practice & Research Clinical Endocrinology & Metabolism; 2014. [DOI] [PubMed] [Google Scholar]
- 9.Henderson GC, Fattor JA, Horning MA, et al. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol. 2007;584:963–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Yang D, Gaofang, Zhao, Xiaobo, et al. Post-exercise effects and Long-Term training adaptations of hormone sensitive lipase Lipolysis Induced by High-Intensity Interval Training in adipose tissue of Mice. Frontiers in Physiology; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierre JM. andAllaman Igor Lactate in the brain: from metabolic end-product to signalling molecule. Nature Reviews Neuroscience; 2018. [DOI] [PubMed] [Google Scholar]
- 12.Joshua DR. andEnerbäck Sven Lactate: the ugly duckling of energy metabolism. Nat Metabolism, 2020. [DOI] [PMC free article] [PubMed]
- 13.Brooks GA. The Science and translation of Lactate Shuttle Theory. Cell Metabol. 2018;27:757–85. [DOI] [PubMed] [Google Scholar]
- 14.Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35:101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z, Liang S, Chen J et al. A Combination of Exercise and Yogurt Intake Protects Mice against Obesity by Synergistic Promotion of Adipose Browning. Journal of agricultural and food chemistry, 2024,null. [DOI] [PubMed]
- 16.Cai H, Wang X, Zhang Z, et al. Moderate l-lactate administration suppresses adipose tissue macrophage M1 polarization to alleviate obesity-associated insulin resistance. J Biol Chem. 2022;298:101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller Patrick D, Yves, Leßmann, Volkmar et al. Lactate and BDNF: key mediators of Exercise Induced Neuroplasticity?. J Clin Med, 2020. [DOI] [PMC free article] [PubMed]
- 18.Coco Marinella B, Andrea R, Tiziana, et al. Influences of blood lactate levels on cognitive domains and Physical Health during a sports stress. Brief Review. International Journal of Environmental Research and Public Health; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Choi Y, Jeong E, et al. Physiological significance of elevated levels of lactate by exercise training in the brain and body. J Biosci Bioeng. 2023;135:167–75. [DOI] [PubMed] [Google Scholar]
- 20.Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Reviews Endocrinol. 2022;18:273–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. Rossi Mark Control of energy homeostasis by the lateral hypothalamic area. Trends in Neurosciences, 2023. [DOI] [PMC free article] [PubMed]
- 22.Wang Putianqi H, Loh Ken W. Michael A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature, 2020. [DOI] [PubMed]
- 23.Müller P, Duderstadt Y, Lessmann V et al. Lactate and BDNF: key mediators of Exercise Induced Neuroplasticity?. J Clin Med, 2020,9. [DOI] [PMC free article] [PubMed]
- 24.Brooks GA, Osmond AD, Arevalo JA et al. Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism. Journal of applied physiology (Bethesda, Md: 1985), 2023,134:529–548. [DOI] [PMC free article] [PubMed]
- 25.Liu Y, Dong G, Zhao X, et al. Post-exercise effects and Long-Term training adaptations of hormone sensitive lipase Lipolysis Induced by High-Intensity Interval Training in adipose tissue of Mice. Front Physiol. 2020;11:535722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Li Y, Cheng B, et al. Comparison of visceral fat lipolysis adaptation to high-intensity interval training in obesity-prone and obesity-resistant rats. Diabetol Metab Syndr. 2022;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Péronnet F. andD. Massicotte Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–9. [PubMed] [Google Scholar]
- 28.Makino A, Yamaguchi K, Sumi D, et al. Comparison of energy expenditure and substrate oxidation between walking and running in men and women. Phys Activity Nutr. 2022;26:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wei L, Tan M, et al. Aerobic exercise improves motor dysfunction in Parkinson’s model mice via differential regulation of striatal medium spiny neuron. Sci Rep. 2024;14:12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frühbeck G, Méndez-Giménez L, Fernández-Formoso JA, et al. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014;27:63–93. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Zhang Q, Yang D et al. HIIT promotes M2 macrophage polarization and sympathetic nerve density to induce adipose tissue Browning in T2DM Mice. Biomolecules, 2024,14. [DOI] [PMC free article] [PubMed]
- 32.Chen X, Huang W, Zhang J, et al. High-intensity interval training induces lactylation of fatty acid synthase to inhibit lipid synthesis. BMC Biol. 2023;21:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nightingale TE, Metcalfe RS, Vollaard NB, et al. Exercise guidelines to promote Cardiometabolic Health in spinal cord injured humans: time to raise the intensity?. Arch Phys Med Rehabil. 2017;98:1693–704. [DOI] [PubMed] [Google Scholar]
- 34.Sabag A, Barr L, Armour M, et al. The effect of high-intensity interval training versus moderate-intensity continuous training on liver fat: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism; 2021. [DOI] [PubMed] [Google Scholar]
- 35.Dupuit M, Rance M, Morel C, et al. Moderate-intensity continuous training or high-intensity interval training with or without Resistance Training for altering body composition in Postmenopausal Women. Med Sci Sports Exerc. 2020;52:736–45. [DOI] [PubMed] [Google Scholar]
- 36.Gaesser GA. andBrooks GA metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc. 1984;16:29–43. [PubMed] [Google Scholar]
- 37.Bahr R, Hansson P. andSejersted OM Triglyceride/fatty acid cycling is increased after exercise. Metab Clin Exp. 1990;39:993–9. [DOI] [PubMed] [Google Scholar]
- 38.Panissa VLG, Fukuda DH, Staibano V, et al. Magnitude and duration of excess of post-exercise oxygen consumption between high-intensity interval and moderate-intensity continuous exercise: a systematic review. Obes Reviews: Official J Int Association Study Obes. 2021;22:e13099. [DOI] [PubMed] [Google Scholar]
- 39.Júdice PB, Sardinha LB. andSilva AM Variance in respiratory quotient among daily activities and its association with obesity status. International journal of obesity (2005), 2021,45:217–224. [DOI] [PubMed]
- 40.Carrillo-Arango HA, Atencio-Osorio MA, López-Álban CA, et al. Metabolic responses to acute sprint interval exercise training performed after an oral 75-gram glucose load in individuals with overweight/obesity. Physiological Rep. 2023;11:e15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fournier PA, Bräu L, Ferreira LD, et al. Glycogen resynthesis in the absence of food ingestion during recovery from moderate or high intensity physical activity: novel insights from rat and human studies. Mol Integr Physiol. 2002;133:755–63. Comparative biochemistry and physiologyPart A. [DOI] [PubMed] [Google Scholar]
- 42.Jiang L, Zhang Y, Wang Z, et al. Acute interval running induces greater excess post-exercise oxygen consumption and lipid oxidation than isocaloric continuous running in men with obesity. Sci Rep. 2024;14:9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun L, Li FH, Li T, et al. Effects of high-intensity interval training on adipose tissue lipolysis, inflammation, and metabolomics in aged rats. Pflugers Arch. 2020;472:245–58. [DOI] [PubMed] [Google Scholar]
- 44.Hazell TJ, Olver TD, Hamilton CD, et al. Two minutes of sprint-interval exercise elicits 24-hr oxygen consumption similar to that of 30 min of continuous endurance exercise. Int J Sport Nutr Exerc Metab. 2012;22:276–83. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Jiao J, Liu Y, et al. The release of Lipolytic Hormones during various high-intensity interval and moderate-intensity continuous training regimens and their effects on Fat Loss. J Sports Sci Med. 2024;23:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balkan B, Van Dijk G, Strubbe JH, et al. Exercise-induced sympathetic FFA mobilization in VMH-lesioned rats is normalized by fasting. Am J Physiol. 1992;262:R981–985. [DOI] [PubMed] [Google Scholar]
- 47.Ameroso D, Meng A, Chen S, et al. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat Metabolism. 2022;4:627–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, An JJ, Sun C et al. Regulation of Energy Balance via BDNF Expressed in Nonparaventricular Hypothalamic Neurons. Molecular endocrinology (Baltimore, Md), 2016,30:494–503. [DOI] [PMC free article] [PubMed]
- 49.Wang C, Bomberg E, Billington CJ, et al. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010;1336:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maekawa F, Fujiwara K, Toriya M, et al. Brain-derived neurotrophic factor in VMH as the causal factor for and therapeutic tool to treat visceral adiposity and hyperleptinemia in type 2 diabetic Goto-Kakizaki rats. Front Synaptic Neurosci. 2013;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Gutiérrez E, Torres-Costoso A, Saz-Lara A, et al. Effectiveness of high-intensity interval training on peripheral brain-derived neurotrophic factor in adults: a systematic review and network meta-analysis. Scand J Med Sci Sports. 2024;34:e14496. [DOI] [PubMed] [Google Scholar]
- 52.Islam H, Townsend LK. Hazell excess Postexercise Oxygen Consumption and Fat utilization following Submaximal continuous and supramaximal interval Running. Res Q Exerc Sport. 2018;89:450–6. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y, Bai M, Wang S, et al. Lactate is a key mediator that links obesity to Insulin Resistance via modulating cytokine production from adipose Tissue. Diabetes. 2022;71:637–52. [DOI] [PubMed] [Google Scholar]
- 54.Yao Z, Yan Y, Zheng X, et al. Dietary lactate supplementation protects against obesity by promoting Adipose Browning in Mice. J Agric Food Chem. 2020;68:14841–9. [DOI] [PubMed] [Google Scholar]
- 55.Magistretti PJ. andAllaman I lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–49. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Xia Y, Xu H, et al. Activation of brain lactate receptor GPR81 aggravates exercise-induced central fatigue. Am J Physiol Regul Integr Comp Physiol. 2022;323:R822–31. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Ruchti E, Petit JM, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci USA. 2014;111:12228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li VL, He Y, Contrepois K, et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature. 2022;606:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.