Abstract
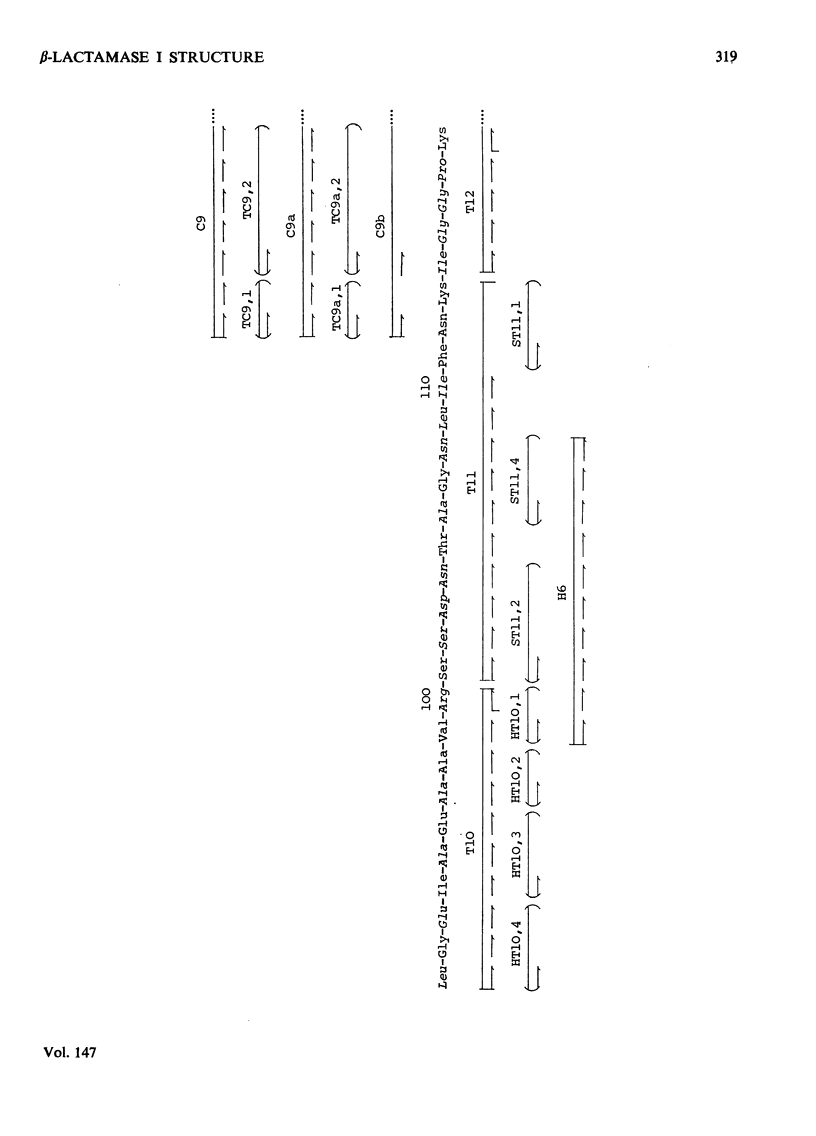
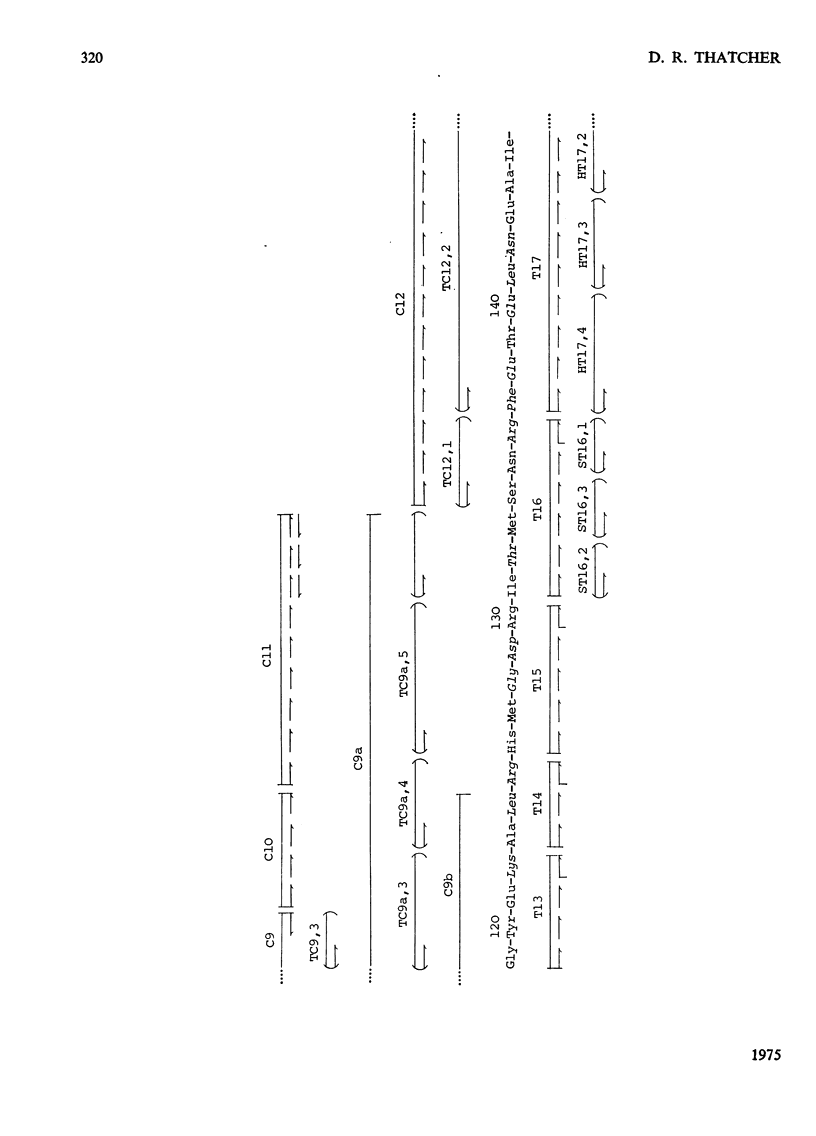
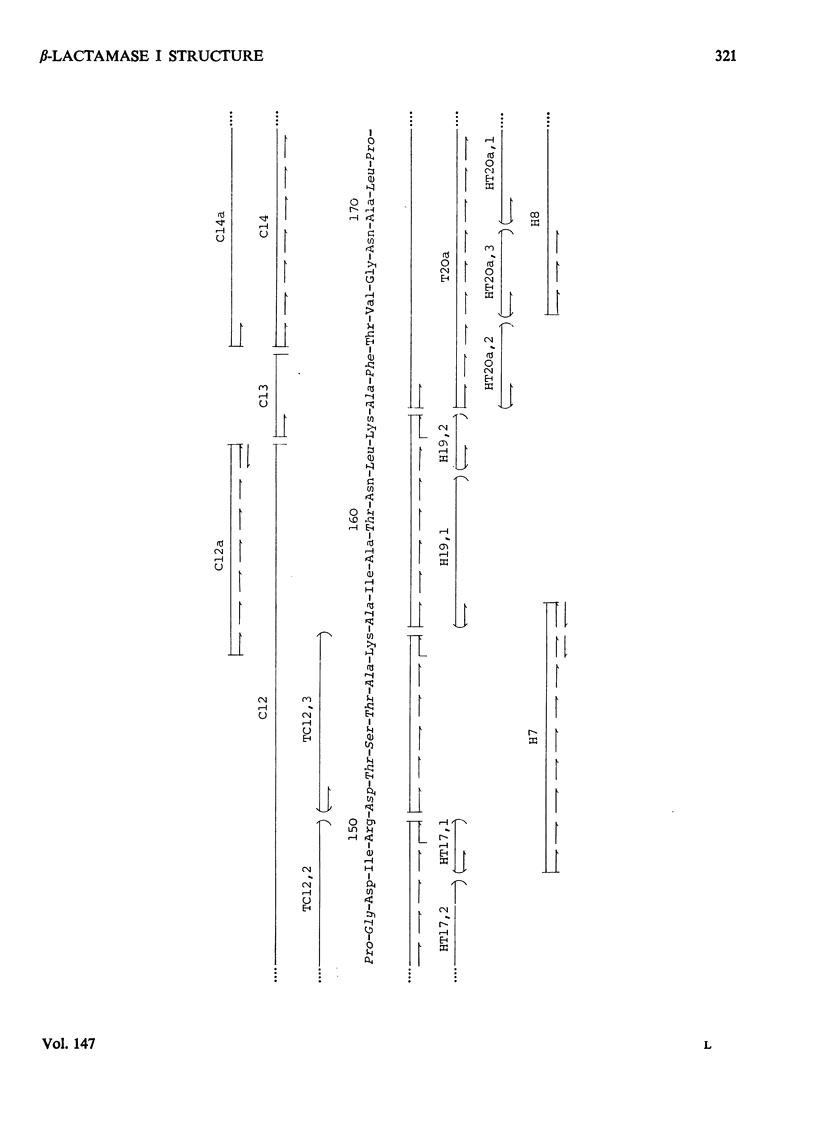
The chemical structure of the extracellular beta-lactamase I of Bacillus cereus 569/H was investigated. Three electrophoretically homogenous charge variants of this enzyme were isolated and amino acid analysis of each revealed no significant differences. However, a degree of N-terminal heterogeneity was found by direct end-group modification of the protein and also on alignment of peptides from tryptic and chymotryptic digestion. The N-terminal heterogeneity observed was great enough to explain the production of the beta-lactamase I isoenzymes which are probably produced by postsynthesis modification of a single gene product. Over 80% of the amino acid sequence of beta-lactamase I was determined by the detailed analysis of peptides derived from tryptic, chymotryptic and thermolytic digests. Five polypeptide fragments were constructed from these data and aligned by comparison with the known amino acid sequences of the penicillinases produced by Bacillus licheniformis and Staphylococcus aureus (Ambler & Meadway, 1969). About 60% of the proposed sequence was identical with that of B. licheniformis penicillinase, whereas the S. aureus enzyme had only about 40% of its residues in common with beta-lactamase I. These results are discussed with reference to the possible evolutionary relationships existing between known beta-lactamases. Detailed evidence for the amino acid sequence proposed has been deposited as Supplementary Publication SUP 50044 (27 pages) at the British Library (Lending Division), Boston Spa, Wetherby, W. Yorks. LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1975), 145, 5.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Meadway R. J. Chemical structure of bacterial penicillinases. Nature. 1969 Apr 5;222(5188):24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Meadway R. J. The use of thermolysin in amino acid sequence determination. Biochem J. 1968 Aug;108(5):893–895. doi: 10.1042/bj1080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P. The amino acid sequence of cytochrome c' from Alcaligenes sp. N.C.I.B. 11015. Biochem J. 1973 Dec;135(4):751–758. doi: 10.1042/bj1350751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER S. A., BOURNE E. J., STACEY M., WARD R. B. Some paper-chromatographic studies with Aspergillus niger '152' transfructosylase. Biochem J. 1958 May;69(1):60–62. doi: 10.1042/bj0690060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON J. V., Jr, PATTERSON J. A. ACCELERATED AUTOMATIC CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS ON A SPHERICAL RESIN. Anal Chem. 1965 Aug;37:1108–1110. doi: 10.1021/ac60228a008. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Conformational changes in the extracellular beta-lactamase I from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):137–141. doi: 10.1042/bj1430137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent C. E. The amino-aciduria in Fanconi syndrome. A study making extensive use of techniques based on paper partition chromatography. Biochem J. 1947;41(2):240–253. doi: 10.1042/bj0410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Aspects of molecular evolution. Annu Rev Genet. 1973;7:343–380. doi: 10.1146/annurev.ge.07.120173.002015. [DOI] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Kuwabara S., Lloyd P. H. Protein and carbohydrate moieties of a preparation of -lactamase II. Biochem J. 1971 Aug;124(1):215–220. doi: 10.1042/bj1240215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Lloyd P. H., Peacocke A. R. Sedimentation-equilibrium studies on the heterogeneity of two beta-lactamases. Biochem J. 1970 Jul;118(3):467–474. doi: 10.1042/bj1180467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K. End group determination. Mol Biol Biochem Biophys. 1970;8:25–90. doi: 10.1007/978-3-662-12834-3_3. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A., Winter W. P. Evolution of structure and function of proteases. Science. 1967 Dec 29;158(3809):1638–1644. doi: 10.1126/science.158.3809.1638. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- Pollock M. R. The function and evolution of penicillinase. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):385–401. doi: 10.1098/rspb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Wang K. T., Huang J. M., Wang I. S. Polyamide layer chromatography of DNP-amino acids. J Chromatogr. 1966 May;22(2):362–368. doi: 10.1016/s0021-9673(01)97109-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


