Abstract
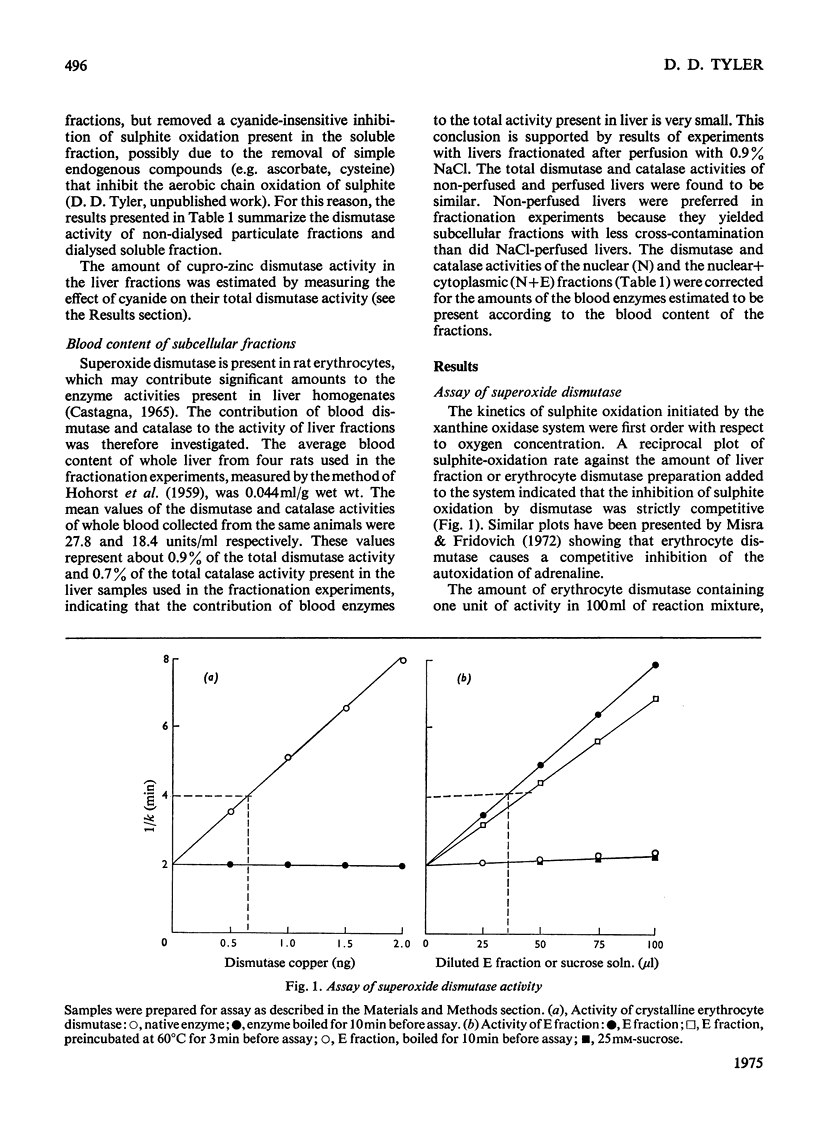
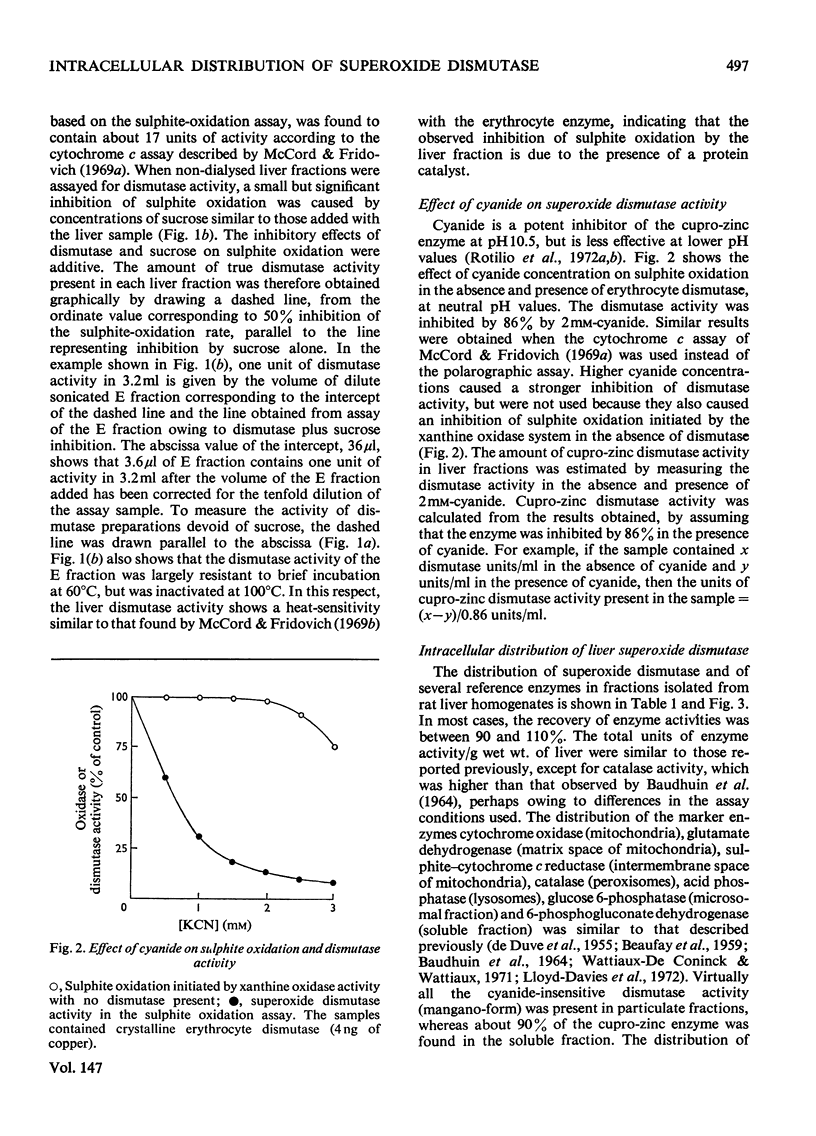
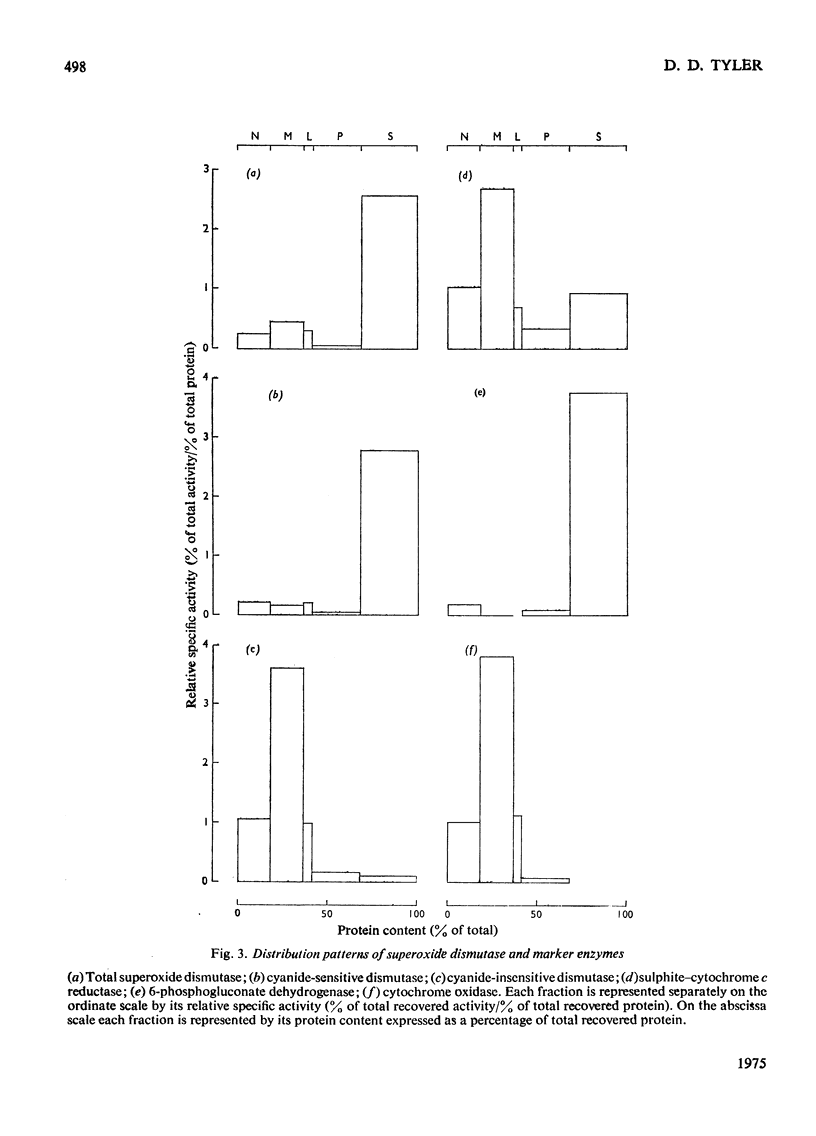
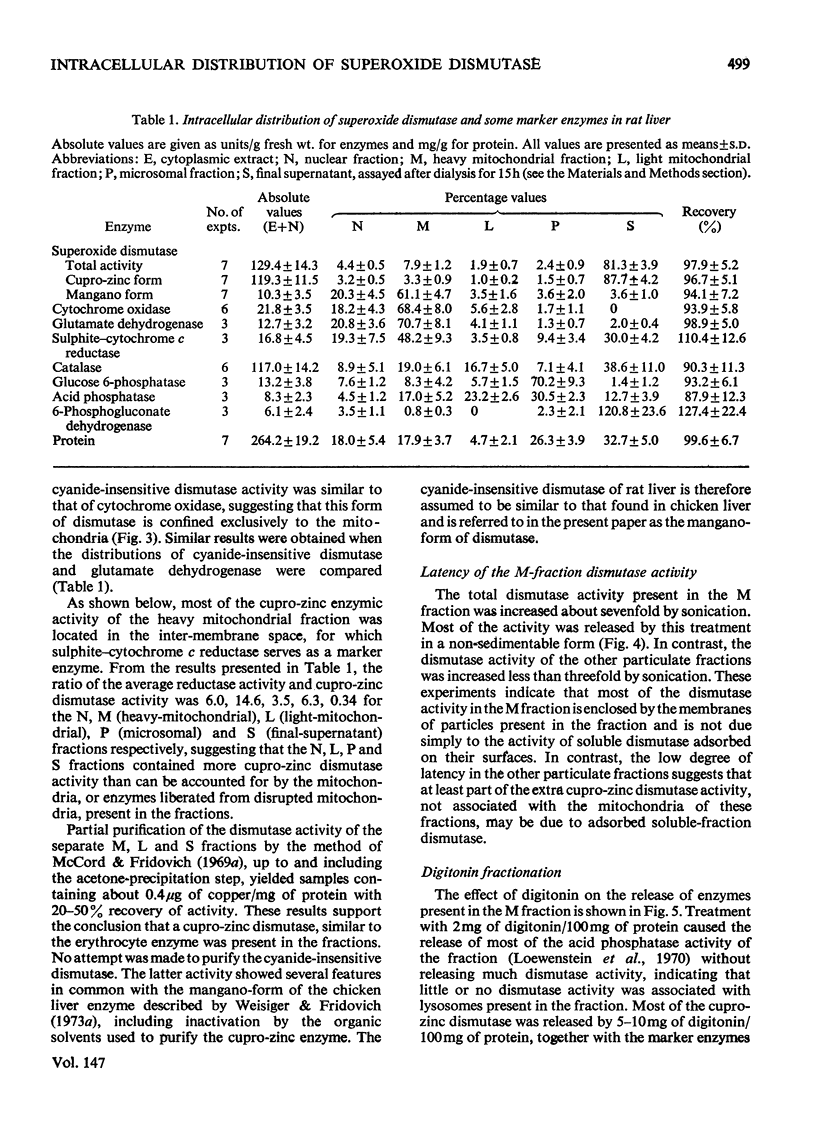
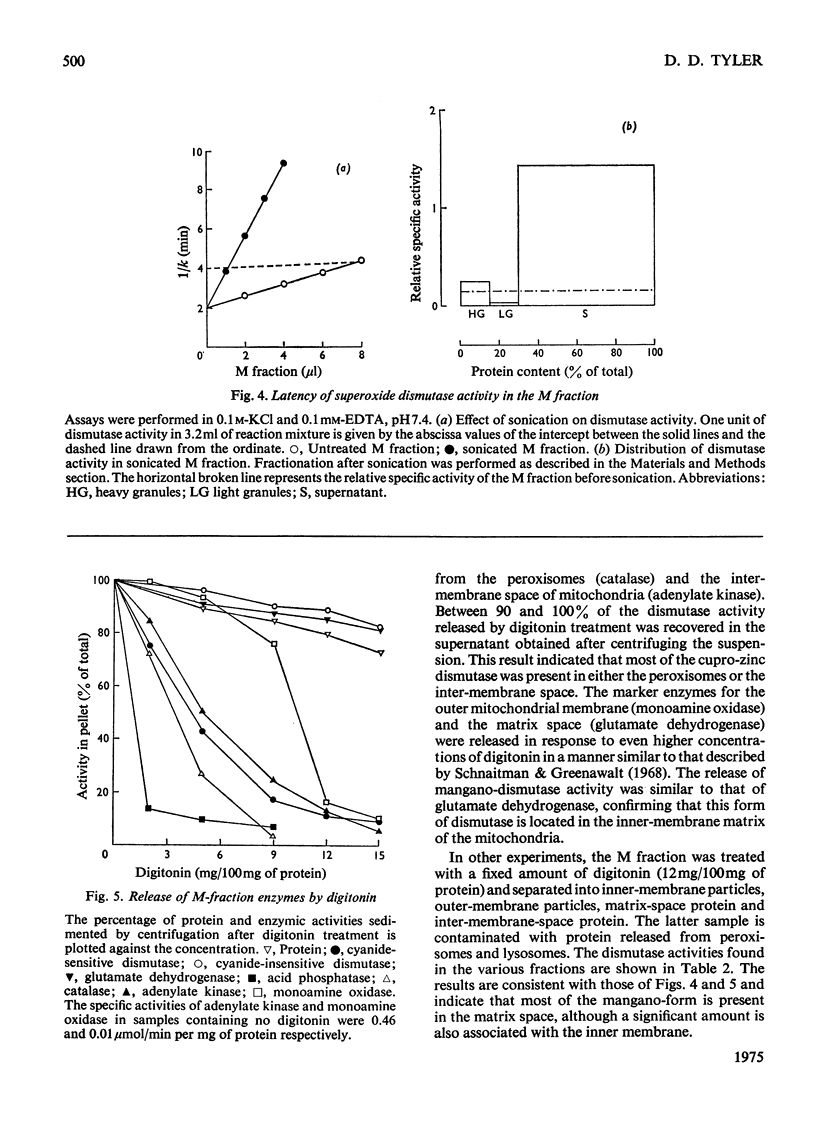
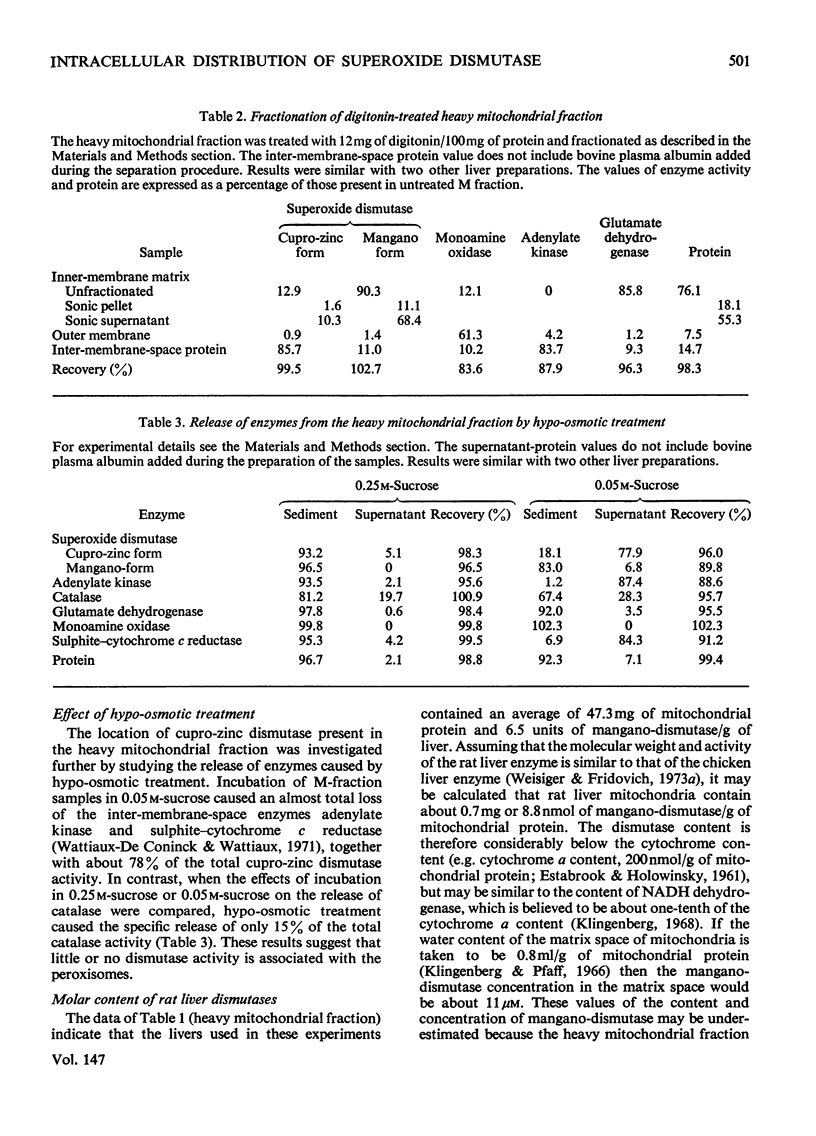
1. A polarographic assay of superoxide (O2--) dismutase (EC 1.15.1.1) activity is described, in which the ability of the enzyme to inhibit O2---dependent sulphite oxidation, initiated by xanthine oxidase activity, is measured. The assay was used in a study of the intracellular distribution of superoxide dismutase in rat liver. Both cyanide-sensitive cupro-zinc dismutase (92% of the total activity) and cyanide-insensitive mangano-dismutase (8%) were measured. 2. Rat liver homogenates contained both particulate (16%y and soluble (84%) dismutase activity. The particulate activity contained both types of dismutase, whereas nearly all the soluble dismutase was a cupro-zinc enzymes. The distribution pattern of mangano-dismutase was similar to that of cytochrome oxidase and glutamate dehydrogenase, indicating that the enzyme was probably present exclusively in the mitochondria. 3. Superoxide dismutase activity in the heavy-mitochondrial (M) fraction was latent and was activated severalfold and largely solubilized by sonication. Treatment of the M fraction with digitonin or a hypo-osmotic suspending medium indicated that most of the cupro-zinc dismutase was located in the mitochondrial intermembrane space, whereas the mangano-enzyme was located in the inner-membrane and matrix space. 4. A small amount of dismutase activity appeared to be present in the nuclei and microsomal fraction, but little or no activity in the lysosomes or peroxisomes. 5. The results are discussed in relation to the intracellular location of known O2---generating enzymes, the possible role of superoxide dismutase activity in intracellular H2O2 formation, and to current views on the physiological function of the enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Kiso K. Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur J Biochem. 1973 Mar 1;33(2):253–257. doi: 10.1111/j.1432-1033.1973.tb02677.x. [DOI] [PubMed] [Google Scholar]
- BEAUFAY H., BENDALL D. S., BAUDHUIN P., DE DUVE C. Tissue fractionation studies. 12. Intracellular distribution of some dehydrogenases, alkaline deoxyribonuclease and iron in rat-liver tissue. Biochem J. 1959 Dec;73:623–628. doi: 10.1042/bj0730623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico R. J., Deutsch H. F. The presence of zinc in human cytocuprein and some properties of the apoprotein. J Biol Chem. 1970 Feb 25;245(4):723–727. [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W., HOLOWINSKY A. Studies on the content and organization of the respiratory enzymes of mitochondria. J Biophys Biochem Cytol. 1961 Jan;9:19–28. doi: 10.1083/jcb.9.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Detection of free radicals generated during enzymic oxidations by the initiation of sulfite oxidation. J Biol Chem. 1961 Jun;236:1836–1840. [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973 Sep;158(1):396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Fried R., Fried L. W., Babin D. R. Biological role of xanthine oxidase and tetrazolium-reductase inhibitor. Eur J Biochem. 1973 Mar 15;33(3):439–445. doi: 10.1111/j.1432-1033.1973.tb02701.x. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS D. E., WHARTON D. C. Studies of the electron transport system. XXXV. Purification and properties of cytochrome oxidase. J Biol Chem. 1961 Jun;236:1850–1856. [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Further characterization of bovine superoxide dismutase and its isolation from bovine heart. J Biol Chem. 1971 May 10;246(9):2875–2880. [PubMed] [Google Scholar]
- Lavelle F., Michelson A. M., Dimitrijevic L. Biological protection by superoxide dismutase. Biochem Biophys Res Commun. 1973 Nov 16;55(2):350–357. doi: 10.1016/0006-291x(73)91094-2. [DOI] [PubMed] [Google Scholar]
- Lloyd-Davies K. A., Michell R. H., Coleman R. Glycerylphosphorylcholine phosphodiesterase in rat liver. Subcellular distribution and localization in plasma membranes. Biochem J. 1972 Apr;127(2):357–368. doi: 10.1042/bj1270357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein J., Scholte H. R., Wit-Peeters E. M. A rapid and simple procedure to deplete rat-liver mitochondria of lysosomal activity. Biochim Biophys Acta. 1970 Dec 8;223(2):432–436. doi: 10.1016/0005-2728(70)90201-x. [DOI] [PubMed] [Google Scholar]
- MOHAMED M. S., GREENBERG D. M. Isolation of purified copper protein from horse liver. J Gen Physiol. 1954 Mar;37(4):433–439. doi: 10.1085/jgp.37.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Strickland S., Mayhew S. G., Howell L. G., Engel P. C., Matthews R. G., Schuman M., Sullivan P. A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969 Sep 10;36(6):891–897. doi: 10.1016/0006-291x(69)90287-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969 Nov 25;244(22):6056–6063. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Morgan E. J. The Distribution of Xanthine Oxidase I. Biochem J. 1926;20(6):1282–1291. doi: 10.1042/bj0201282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER H., FOLCH J. Cerebrocuprein I. A copper-containing protein isolated from brain. J Neurochem. 1957;1(3):260–271. doi: 10.1111/j.1471-4159.1957.tb12081.x. [DOI] [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. NADPH-dependen lipid peroxidation catalyzed by purified NADPH-cytochrome C reductase from rat liver microsomes. Biochem Biophys Res Commun. 1972 Aug 21;48(4):789–795. doi: 10.1016/0006-291x(72)90676-6. [DOI] [PubMed] [Google Scholar]
- Peeters-Joris C., Vandevoorde A. M., Baudhuin P. Localisation intracellulaire de la dismutase du radical superoxyde du foie de rat. Arch Int Physiol Biochim. 1973 Dec;81(5):981–981. [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Bray R. C., Fielden E. M. A pulse radiolysis study of superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):605–609. doi: 10.1016/0005-2744(72)90359-2. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Morpurgo L., Giovagnoli C., Calabrese L., Mondovì B. Studies of the metal sites of copper proteins. Symmetry of copper in bovine superoxide dismutase and its functional significance. Biochemistry. 1972 May 23;11(11):2187–2192. doi: 10.1021/bi00761a028. [DOI] [PubMed] [Google Scholar]
- Sato S. Free radicals in NADPH-microsomes-triphenyltetrazolium chloride system as evidenced by initiation of sulfite oxidation. Biochim Biophys Acta. 1967;143(3):554–561. doi: 10.1016/0005-2728(67)90060-6. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. W., Coon M. J. Effect of superoxide generation and dismutation on hydroxylation reactions catalyzed by liver microsomal cytochrome P-450. J Biol Chem. 1971 Dec 25;246(24):7826–7829. [PubMed] [Google Scholar]
- TAPPEL A. L. Unsaturated lipide oxidation catalyzed by hematin compounds. J Biol Chem. 1955 Dec;217(2):721–733. [PubMed] [Google Scholar]
- Wattiaux-de Coninck S., Wattiaux R. Subcellular distribution of sulfite cytochrome c reductase in rat liver tissue. Eur J Biochem. 1971 Apr 30;19(4):552–556. doi: 10.1111/j.1432-1033.1971.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Zimmermann R., Flohé L., Weser U., Hartmann H. J. Inhibition of lipid peroxidation in isolated inner membrane of rat liver mitochondria by superoxide dismutase. FEBS Lett. 1973 Jan 15;29(2):117–120. doi: 10.1016/0014-5793(73)80539-3. [DOI] [PubMed] [Google Scholar]


