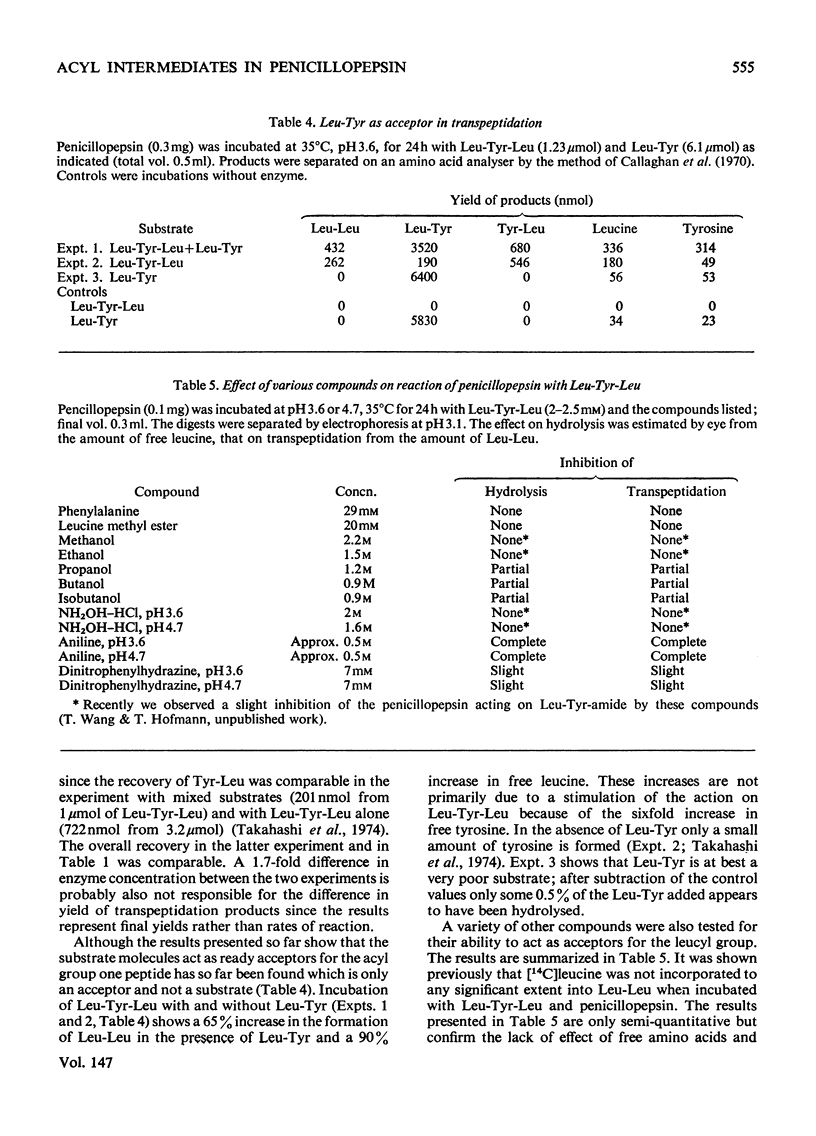
Abstract
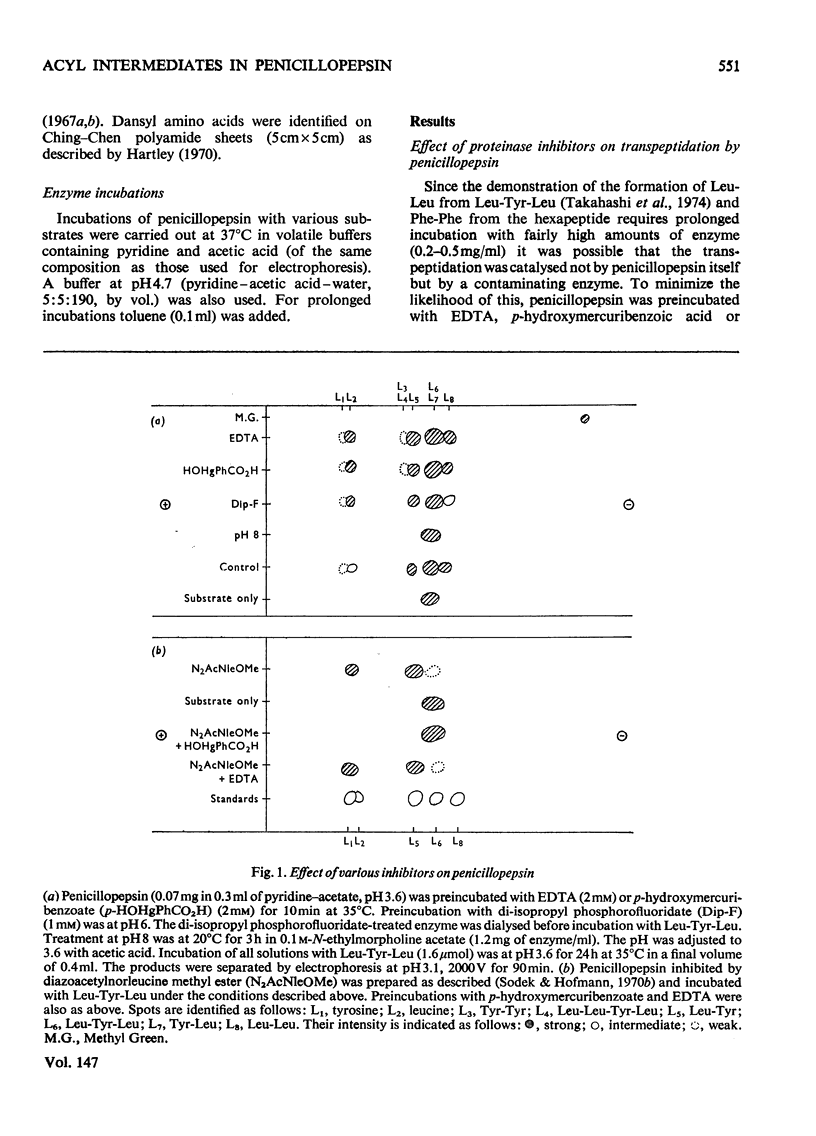
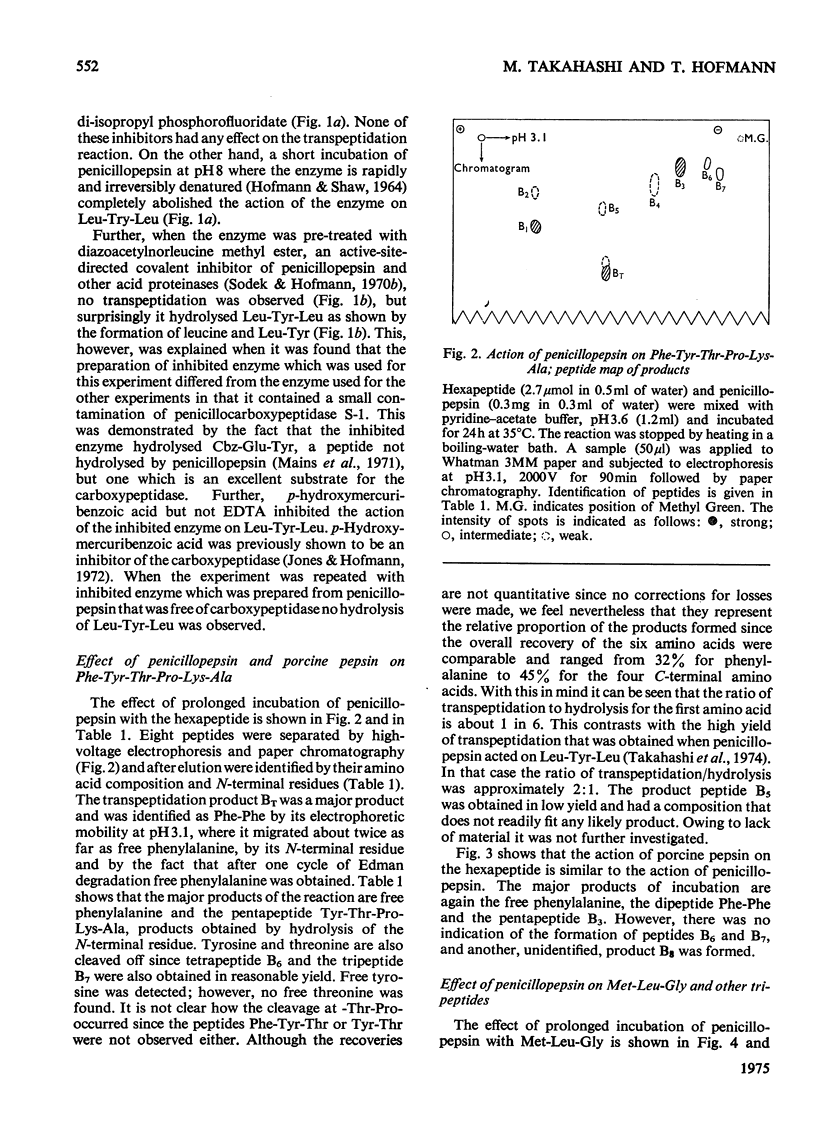
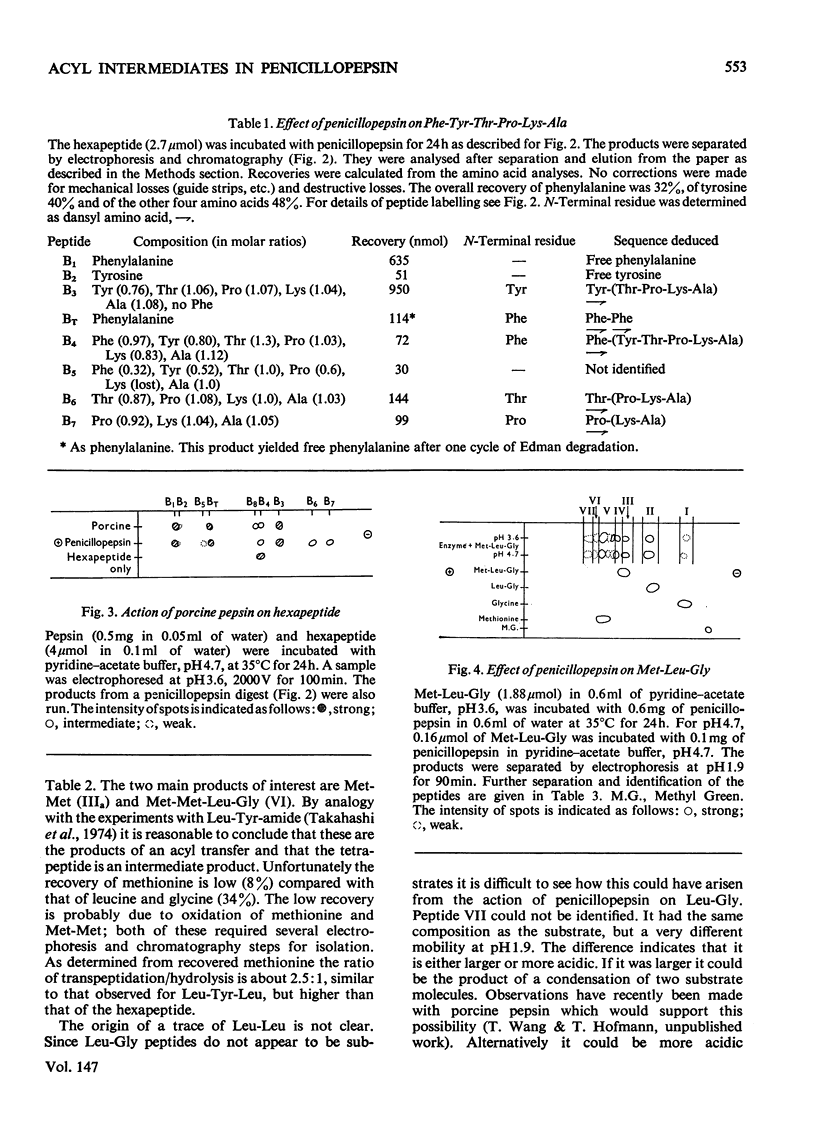
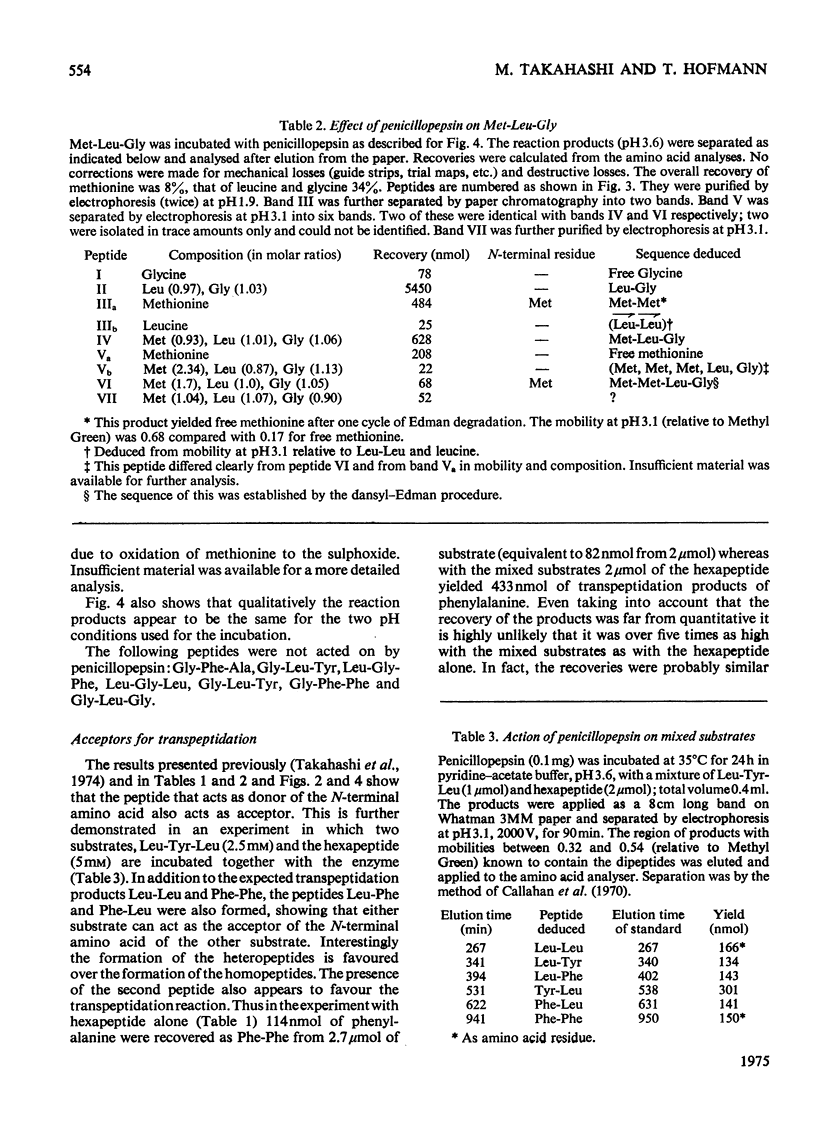
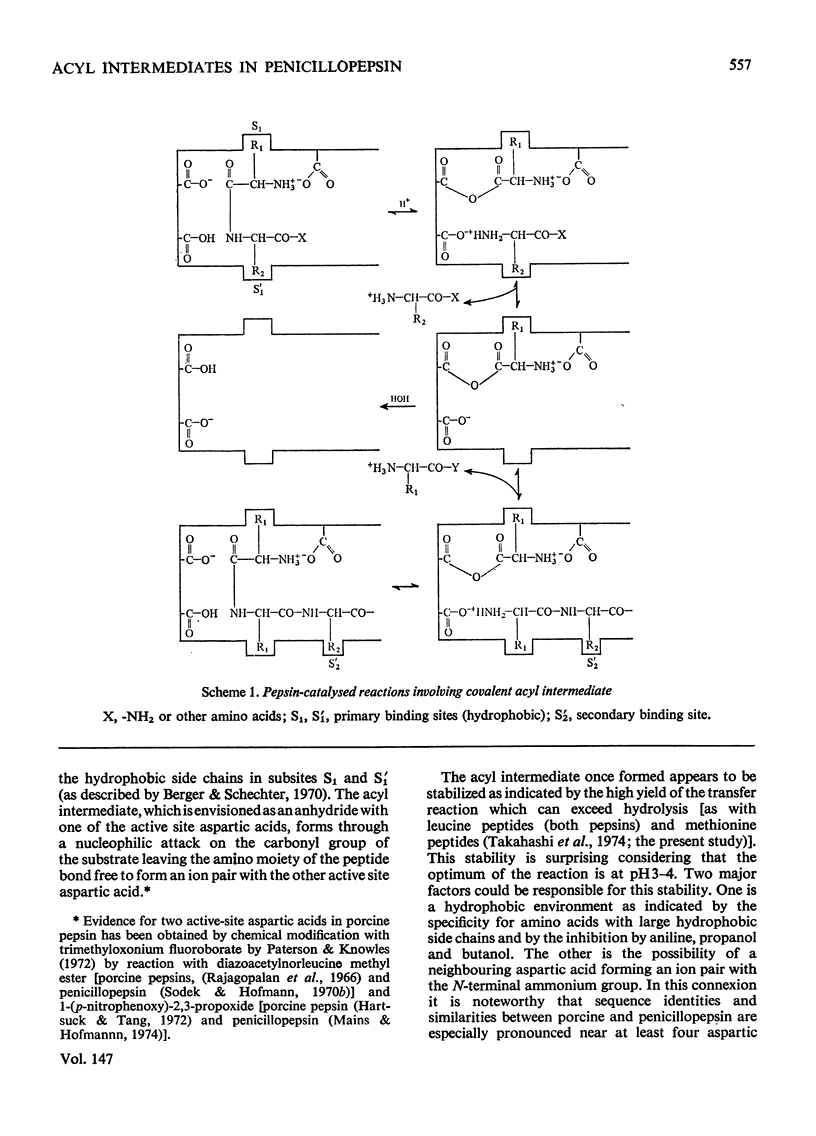
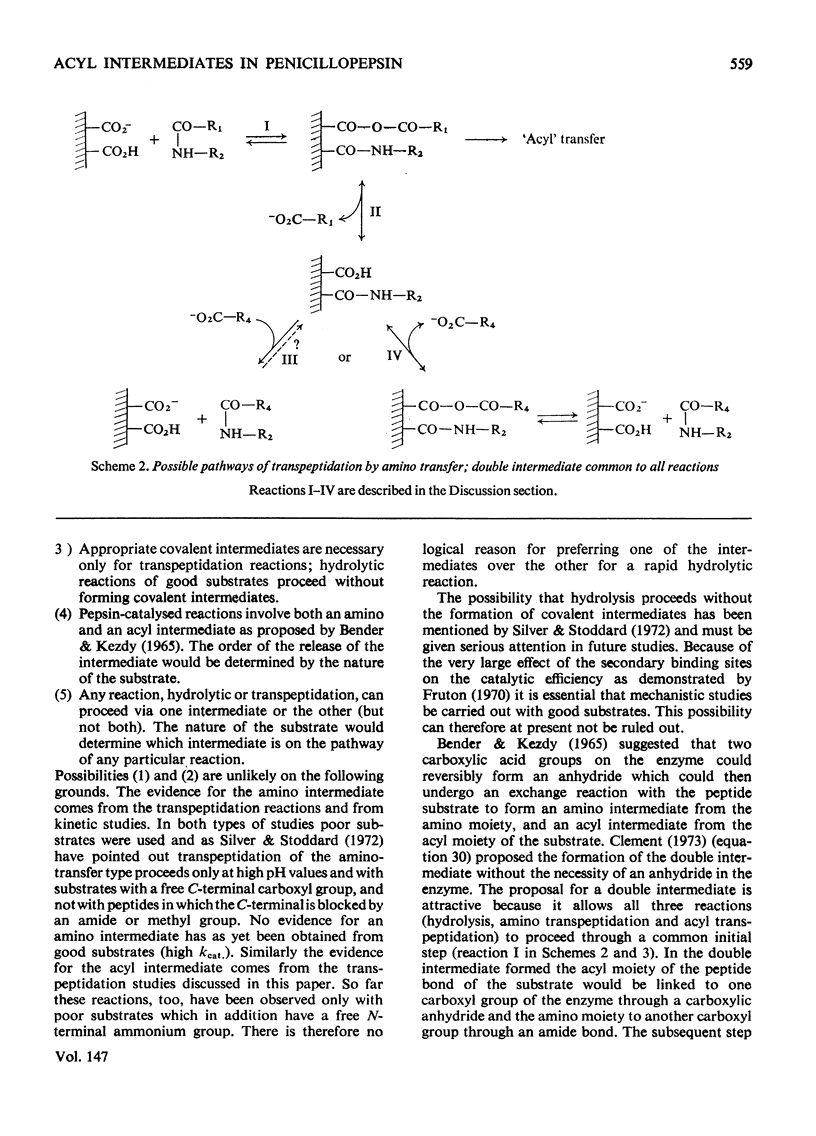
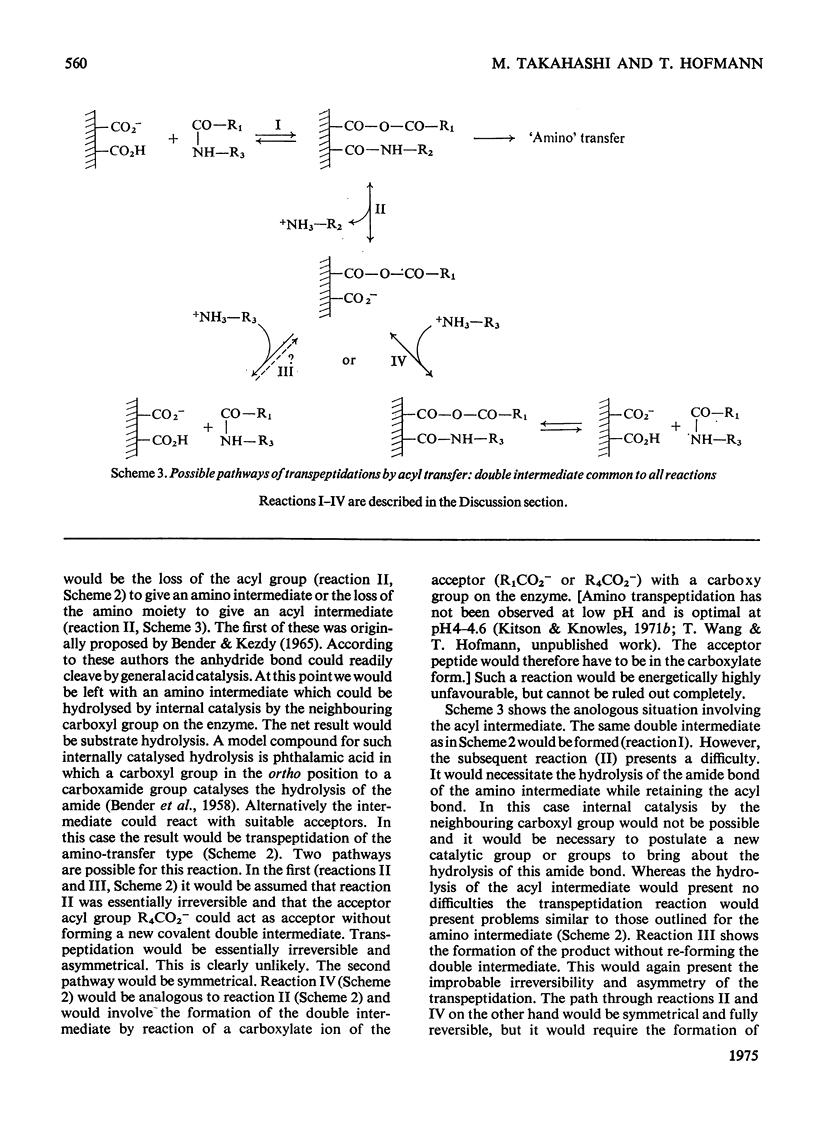
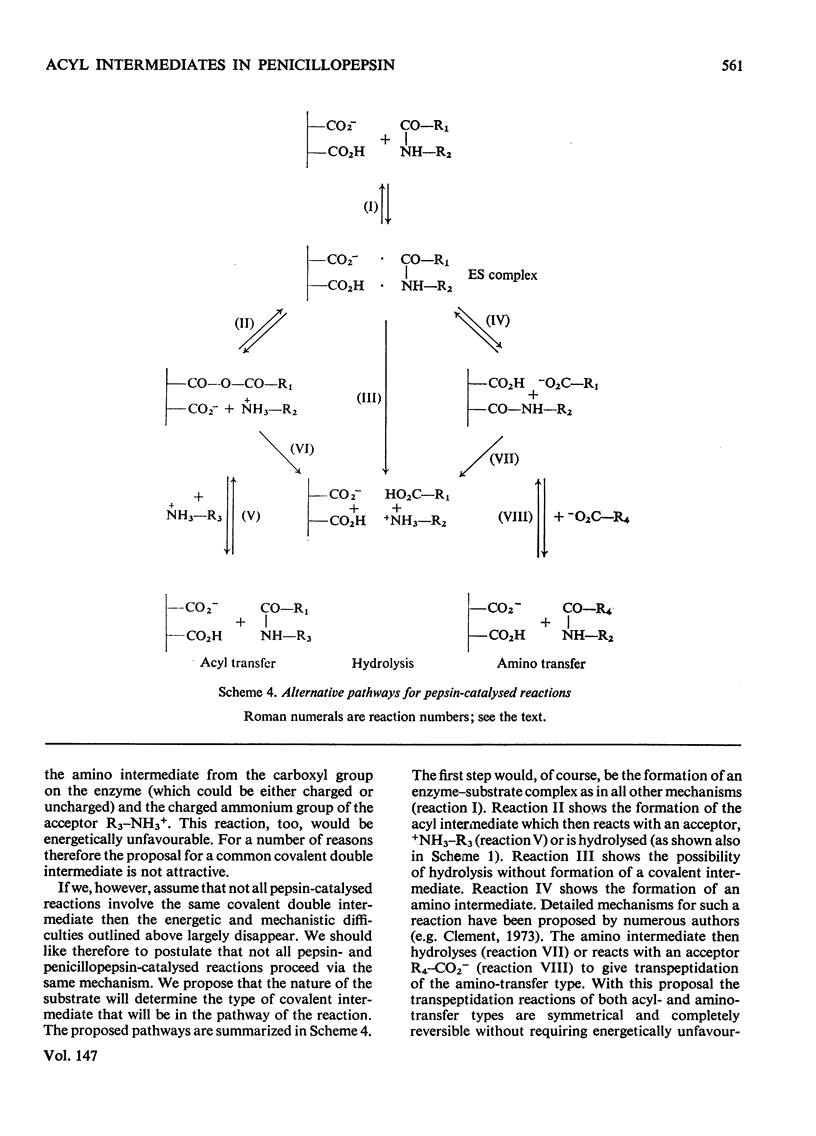
Penicillopepsin catalyses transpeptidation reactions involving the transfer of the N-terminal amino acids of suitable substrates via covalent acyl intermediates to acceptor peptides, usually the substrate. The major products obtained when Phe-Tyr-Thr-Pro-Lys-Ala and Met-Leu-Gly were used as substrates were Phe-Phe and Met-Met respectively. With Met-Leu-Gly the tetrapeptide Met-Met-Leu-Gly was observed as probable intermediate. Co-incubation of Leu-Tyr-Leu and Phe-Tyr-Thr-Pro-Lys-Ala led to the formation of Leu-Phe and Phe-Leu as well as Leu-Leu and Phe-Phe. No reaction was observed with tripeptides in which the first or second amino acid is glycine. It appears that two amino aicds with large hydrophobic residues are needed for the transpeptidation reaction. Nucleophilic compounds other than peptides, such as hydroxylamine, aliphatic alcohols and dinitrophenylhydrazine, were not acceptors for the acyl group. Leucine, phenylalanine and leucine methyl ester also had no effect on the reaction. The transpeptidation reaction proceeded readily at pH 3.6 and 4.7. At pH 6.0 the reaction was slow and at pH 1.9 little or no transpeptidation was observed. Porcine pepsin catalyses similar transpeptidation reactions. Sequence studies show that porcine pepsin and penicillopepsin are homologous. The present study also suggests that they have a very similar mechanism. Evidence available at this time indicates that the mechanism of these enzymes is complex and may be modulated by secondary substrate-enzyme interactions. A hypothesis is presented which proposes that pepsin-catalysed reactions proceed via different covalent intermediates (amino-intermediates or acylintermediates) depending on the nature of the substrate. The possibility that some reactions do not involve covalent intermediates is also discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Callahan P. X., Shepard J. A., Reilly T. J., McDonald J. K., Ellis S. Separation and identification of dipeptides by paper and column chromatography. Anal Biochem. 1970 Dec;38(2):330–356. doi: 10.1016/0003-2697(70)90457-4. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. J., Greenwell P., Knowles J. R. The rate-determining step in pepsin-catalysed reactions, and evidence against an acyl-enzyme intermediate. Biochem J. 1969 Jun;113(2):369–375. doi: 10.1042/bj1130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre G. R., Fruton J. S. Inactivation of pepsin by diphenyldiazomethane. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1161–1167. doi: 10.1073/pnas.54.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUTON J. S., FUJII S., KNAPPENBERGER M. H. The mechanism of pepsin action. Proc Natl Acad Sci U S A. 1961 Jun 15;47:759–761. doi: 10.1073/pnas.47.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruton J. S. The specificity and mechanism of pepsin action. Adv Enzymol Relat Areas Mol Biol. 1970;33:401–443. doi: 10.1002/9780470122785.ch9. [DOI] [PubMed] [Google Scholar]
- Greenwell P., Knowles J. R., Sharp H. The inhibition of pepsin-catalysed reactions by products and product analogues. Kinetic evidence for ordered release of products. Biochem J. 1969 Jun;113(2):363–368. doi: 10.1042/bj1130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HOFMANN T., SHAW R. PROTEOLYTIC ENZYMES OF PENICILLIUM JANTHINELLUM. I. PURIFICATION AND PROPERTIES OF A TRYPSINOGEN-ACTIVATING ENZYME (PEPTIDASE A). Biochim Biophys Acta. 1964 Dec 23;92:543–557. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsuck J. A., Tang J. The carboxylate ion in the active center of pepsin. J Biol Chem. 1972 Apr 25;247(8):2575–2580. [PubMed] [Google Scholar]
- Hollands T. R., Fruton J. S. On the mechanism of pepsin action. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1116–1120. doi: 10.1073/pnas.62.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INWARD P. W., JENCKS W. P. THE REACTIVITY OF NUCLEOPHILIC REAGENTS WITH FUROYL-CHYMOTRYPSIN. J Biol Chem. 1965 May;240:1986–1996. [PubMed] [Google Scholar]
- Jones S. R., Hofmann T. Penicillocarboxypeptidase-S, a nonspecific SH-dependent exopeptidase. Can J Biochem. 1972 Dec;50(12):1297–1310. doi: 10.1139/o72-175. [DOI] [PubMed] [Google Scholar]
- Kitson T. M., Knowles J. R. The inhibition of pepsin-catalysed reactions by structural and stereochemical product analogues. Biochem J. 1971 Apr;122(2):241–247. doi: 10.1042/bj1220241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson T. M., Knowles J. R. The pathway of pepsin-catalysed transpeptidation. Evidence for the reactive species being the anion of the acceptor molecule. Biochem J. 1971 Apr;122(2):249–256. doi: 10.1042/bj1220249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R. On the mechanism of action of pepsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):135–146. doi: 10.1098/rstb.1970.0016. [DOI] [PubMed] [Google Scholar]
- Mains G., Hofmann T. The inactivation of penicillopepsin with 1,2-epoxy-3-(p-nitrophenoxy) propane, an active-site directed reagent. Can J Biochem. 1974 Nov;52(11):1018–1023. doi: 10.1139/o74-142. [DOI] [PubMed] [Google Scholar]
- Mains G., Takahashi M., Sodek J., Hofmann T. The specificity of penicillopepsin. Can J Biochem. 1971 Oct;49(10):1134–1149. doi: 10.1139/o71-164. [DOI] [PubMed] [Google Scholar]
- NEUMANN H., LEVIN Y., BERGER A., KATCHALSKI E. Pepsincatalysed transpeptidation of the amino-transfer type. Biochem J. 1959 Sep;73:33–41. doi: 10.1042/bj0730033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. K., Knowles J. R. The number of catalytically essential carboxyl groups in pepsin. Modification of the enzyme by trimethyloxonium fluoroborate. Eur J Biochem. 1972 Dec 18;31(3):510–517. doi: 10.1111/j.1432-1033.1972.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Moore S., Stein W. H. Pepsin from pepsinogen. Preparation and properties. J Biol Chem. 1966 Nov 10;241(21):4940–4950. [PubMed] [Google Scholar]
- SHARON N., GRISARO V., NEUMANN H. Pepsin-catalyzed exchange of oxygen atoms between water and carboxylic acids. Arch Biochem Biophys. 1962 Apr;97:219–221. doi: 10.1016/0003-9861(62)90070-x. [DOI] [PubMed] [Google Scholar]
- Shkarenkova L. S., Ginodman L. M., Kozlov L. V., Orekhovich V. N. Vkliuchenie O18 iz H2O18 v karboksil'nye gruppy aktivnogo tsentra pepsina. Biokhimiia. 1968 Jan-Feb;33(1):154–161. [PubMed] [Google Scholar]
- Silver M. S., Stoddard M. Amino-enzyme intermediates in pepsin-catalyzed reactions. Biochemistry. 1972 Jan 18;11(2):191–200. doi: 10.1021/bi00752a008. [DOI] [PubMed] [Google Scholar]
- Sodek J., Hofmann T. Amino acid sequence around the active site aspartic acid in penicillopepsin. Can J Biochem. 1970 Sep;48(9):1014–1016. doi: 10.1139/o70-158. [DOI] [PubMed] [Google Scholar]
- Sodek J., Hofmann T. Large-scale preparation and some properties of penicillopepsin, the acid proteinase of Penicillium janthinellum. Can J Biochem. 1970 Apr;48(4):425–431. doi: 10.1139/o70-069. [DOI] [PubMed] [Google Scholar]
- Stevenson K. J. Use of visual dye markers on high-voltage paper ionophoresis. Anal Biochem. 1971 Mar;40(1):29–34. doi: 10.1016/0003-2697(71)90080-7. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Wang T. T., Hofmann T. Acyl intermediates in pepsin and penicillopepsin catalyzed reactions. Biochem Biophys Res Commun. 1974 Mar 15;57(1):39–46. doi: 10.1016/s0006-291x(74)80354-2. [DOI] [PubMed] [Google Scholar]
- Terada S., Yoshida S., Izumiya N. Action of pepsin on synthetic substrates. I. Hydrolysis and transpeptidation of peptides ranging from glycyl-L-tyrosyl-L-tyrosine to triglycyl-L-tyrosine. J Biochem. 1971 Jul;70(1):133–142. doi: 10.1093/oxfordjournals.jbchem.a129609. [DOI] [PubMed] [Google Scholar]
- Wang T. T., Dorrington K. J., Hofmann T. Activation of the action of penicillopepsin on leucyl-tyrosyl-amide by a non-substrate peptide and evidence for a conformational change associated with a secondary binding site. Biochem Biophys Res Commun. 1974 Apr 8;57(3):865–869. doi: 10.1016/0006-291x(74)90626-3. [DOI] [PubMed] [Google Scholar]


